Abstract
Endothelial cells play a pivotal role in the initiation and perpetuation of inflammation. C1q, the first component of the classical pathway of complement, is a potent stimulus leading to endothelial cell activation and cytokine production. The specific cellular mechanisms through which endothelial cells are stimulated by C1q are not known. We stimulated human umbilical vein endothelial cells (HUVEC) with either monomeric C1q or C1q-bearing immune complexes (C1q-IC) in the presence or absence of inhibitors of protein tyrosine kinases (PTK) or mitogen-activated protein kinases (MAPK). C1q-IC, but not monomeric C1q, induced IL-8 production in dose- and time-dependent fashion. R3, a cross-linking monoclonal IgM antibody against the126 kD phagocytic C1q receptor (C1qR), also stimulated IL-8 production. IL-8 mRNA accumulation was detected by Northern blot analysis within 2 h of stimulation by the immune complexes and was enhanced by the addition of cycloheximide. Secretion of IL-8 by C1q-IC stimulated HUVEC was completely blocked by the PTK inhibitor, genistein or the MAPK inhibitor, UO126. These experiments demonstrate that C1q-IC-induced production of IL-8 in HUVEC is dependent upon the activation of PTK and MAPK. These findings also support a role for the phagocytic C1qR as an important activator of HUVEC by immune complexes.
Keywords: antigen–antibody complex, C1q, IL-8, endothelium, MAP kinase
INTRODUCTION
Endothelial cells regulate molecular and cellular movement across the vessel wall by serving as the source of multiple factors and mediators that are critical for normal homeostasis [1]. In addition, endothelial cells represent an important part of the immune system, participating in immunoregulatory and inflammatory events by secreting proinflammatory mediators, upregulating adhesion molecules, and expressing receptors for inflammatory stimuli [2,3].
Immune complex accumulation in circulation or tissues is implicated in a broad spectrum of human diseases characterized by acute and/or chronic inflammation, including rheumatoid arthritis [4,5], juvenile rheumatoid arthritis [6,7] and systemic lupus erythematosus [8,9]. Injury to host tissue in immune complex disease is attributed, in part, to the capacity of IgG-containing complexes to activate the complement cascade, resulting in the release of phlogistic C4a and C3a peptides.
Recent studies have shown that complement and other soluble mediators of inflammation can stimulate the inflammatory function of endothelial cells in vitro. For example, endothelial cells express receptors for C5a, which regulate expression of P-selectin [10]. Endothelial cells also interact with complement components C5b-9 [11].
Human umbilical vein endothelial cells (HUVEC), a cell line frequently used for in vitro models of inflammation, possess at least one receptor capable of binding C1q-bearing immune complexes [12]. However, the specific C1q binding proteins that mediate binding of immune complexes to HUVEC remains unclear. There are three potential candidates: one for the globular ‘heads’ of the C1q molecule (‘gC1qR’) [13] and two for the collagen-like ‘stalks’. It has been shown recently, however, that the gC1qR is an intracellular binding protein, not a true ‘receptor’. [14]. Similarly, the 60 kD ‘receptor’ for the C1q collagenous portion now appears to be indistinguishable from calreticulin, an intracellular calcium binding protein [15]. Although both these receptors may be exposed to the extracellular environment in apoptotic blebs [16], neither appears to be a classic-type transmembrane receptor. A third C1q binding protein, the so-called phagocytic C1q receptor (C1qRp), was identified by its capacity to enhance IgG-mediated phagocytosis in leucocytes [17]. This receptor is expressed on human umbilical vein endothelial cells (HUVEC) [18] and contains intracellular motifs compatible with its being a tyrosine-kinase-activated receptor.
Binding of C1q-bearing immune complexes to HUVEC induces the expression of the adhesion molecules E-selectin, ICAM-1 and VCAM-1 [19], and the secretion of chemokines IL-8 and MCP-1 and the cytokine IL-6 [20]. Thus, immune complex stimulation of endothelium is a potentially important pathological phenomenon, and understanding its mechanisms is likely to deepen our understanding of chronic inflammatory disease.
We used C1q-bearing immune complexes to stimulate HUVEC in experiments designed to clarify the mechanisms through which such complexes activate endothelial cells.
MATERIALS AND METHODS
Proteins and antibodies
Bovine serum albumin (BSA) was obtained in fatty acid free form from Sigma (St Louis, MO, USA). Rabbit IgG anti-BSA antibody was obtained from ICN/Capel (Durham, NC, USA). Purified human C1q (glycerol-free) was obtained from Advanced Research Technologies (San Diego, CA, USA). Recombinant human TNFα, monoclonal anti-IL-8, HRP-conjugated polyclonal anti-IL-8 and recombinant IL-8 standards used for ELISA assays were obtained from R&D (Minneapolis, MN, USA). Goat antihuman C1q and goat antimouse IgG antibodies and murine IgM were purchased from Sigma. R3 IgM murine monoclonal antibody to the phagocytic 126 kD C1q receptor (C1qRp) [17] was a kind gift from Dr Andrea Tenner (University of California, Irvine, USA). Antibodies to the p44/42 extracellular signal–regulated (ERK) MAP kinase and E10 MoAb specific for the phosphorylated forms of the 44/42 MAP kinases were purchased from New England Biolabs (Beverly, MA, USA).
Reagents
M199 media, l-glutamine, penicillin, streptomycin and Trizol were purchased from Gibco (Grand Island, NY, USA). Human serum was obtained from Irvine Scientific (Santa Ana, CA, USA). IL-8 and β-actin primers, used to generate probes for Northern blotting experiments, were purchased from ClonTech (Palo Alto, CA, USA). The BCA protein assay kit, which was used for protein quantification using bicinchinic acid, was obtained from Pierce (Rockford, IL, USA). Bacterial lipopolysaccharide (LPS), cycloheximide, genistein, LPS-free gelatin and polymyxin B were purchased from Sigma. Phenylmethylsulphonyl fluoride (PMSF), pepstatin A, sodium orthovanadate, NP-40 and sodium dodecyl sulphate were also purchased from Sigma. UO126, a specific inhibitor of the Erk-1/2 mitogen-activated protein kinases (MAPK), was purchased from Promega (Madison, WI, USA). All other reagents were obtained from Fisher Scientific (Pittsburgh, PA, USA) unless otherwise noted.
Endothelial cell cultures
Primary cultures of human umbilical vein endothelial cells (HUVEC) were established from umbilical cords taken after the delivery of healthy term newborns. Human investigation committee approval was obtained for the use of the umbilical cord specimens. Isolation and characterization of HUVEC was undertaken exactly as described [21]. Briefly, umbilical cord veins were flushed with Hanks's balanced salt solution (HBSS) and incubated with 0·1% collagenase in HBSS for 10 min at 37°C. HUVEC were isolated and cultured in medium 199 containing 20% human serum, 2 mm glutamine, 100 U/ml penicillin and 100 µg/ml of streptomycin as described. Cells were grown to confluence on LPS-free, gelatin-coated tissue culture plastic. Cells were used in the first to fifth cell passages. Pharmacological agents, dissolved in fresh medium, were added to confluent cell monolayers for the time intervals and at the final concentrations indicated in the text. As a control, fresh medium lacking the agent was added to cells.
Immune complex preparation
BSA-anti-BSA immune complexes were formed at 2 × antigen excess in PBS. The equivalence point was measured by a turbidometric assay as described previously [22]. Immune complexes were formed in PBS in the presence of 50 µg/ml purified human C1q at 37°C for 60 min. Insoluble material was removed by centrifugation. The complexes were separated from uncomplexed antigen and antibody by using size-exclusion chromatography on S-300 equilibrated with PBS as described previously [23]. The exclusion volume, representing material ≥ 1·5 × 106 MW was saved and concentrated to 4–6 mg/ml by ultrafiltration. The protein concentrations of immune complex preparations were measured using the BCA protein assay.
Incubation of HUVEC with immune complexes and anti-C1qRp antibody
HUVEC were incubated with C1q-bearing immune complexes at concentrations ranging from 25 to 800 µg/ml and time courses ranging from 24 to 72 h. Immune complex concentrations were within the range of those found in plasma in patients with a broad range of chronic inflammatory diseases, including juvenile rheumatoid arthritis [7] and systemic lupus erythematosus [24]. Medium was collected and stored at −70°C until used for the measurement of IL-8 concentrations. In selected experiments, HUVEC were exposed to the complexes for 2 h, washed with fresh medium, then cultured for an additional 34 h before culture supernatants were collected.
Several other experiments were performed in order to clarify the specific nature of the receptor(s) responsible for biological effects induced by C1q-bearing immune complexes. In one set of experiments, C1q-bearing immune complexes were preincubated with 800 µg/ml polyclonal anti-C1q antibody prior to their being added to the cells. In another set of experiments, murine R3 IgM MoAb to the126 kD C1qRp (10 µg/ml) was used to cross-link the receptor. Cells were then incubated and IL-8 was measured in tissue culture supernatants exactly as described above.
In selected experiments, cells were incubated with inhibitors of protein tyrosine kinase (PTK) (genistein), and MAPK (UO126). Inhibitors were added over a range of concentrations to confirm biological effects.
Negative controls were also undertaken to assure that effects attributed to C1q-bearing immune complex (C1q-IC) were not due to small amounts of contaminating LPS. In these experiments, the C1qIC were heated to 100°F for 5 min prior to their being added to the cells. This procedure completely degrades and inactivates the immune complexes but not heat-stable LPS [25].
Quantification of IL-8 by ELISA
IL-8 antigen in the supernatants was measured by ELISA using a standard ‘sandwich’ technique as described [26]. The sensitivity of the assay is 2·5 pg/ml, and the assay is linear at IL-8 concentrations ranging from 16 to 2000 pg/ml. Differences between experimental and control specimens were analysed by two-tailed Student's t-tests using a commercially available statistical software program (GraphPad Prism, San Diego, CA, USA).
Northern blotting for IL-8 mRNA
HUVEC were cultured and incubated with C1q-bearing immune complexes exactly as described above. In selected experiments, HUVEC were incubated with C1q-bearing immune complexes in the presence of 5 µg/ml cycloheximide (which inhibits protein biosynthesis). Medium was removed and cells lysed with Trizol reagent. Total RNA was extracted exactly as recommended by the manufacturer. Denatured RNA was then subjected to electrophoresis in 1% agarose/0·66 m formaldehyde gels. RNA was transferred overnight in 20× SSC buffer to nylon membranes and cross-linked to the membranes with UV light. The cDNA probes used for IL-8 and β-actin blots were generated by polymerase chain reaction using commercially available primers and DNA templates for IL-8 and β-actin as described previously [25]. The probes were labelled using a commercially available chemiluminescence system (Amersham, Arlington, IL, USA). Prehybridization, hybridization, stringency washes and detection of the bound probe were then performed exactly as recommended by the manufacturer. X-ray films were incubated with membranes for periods varying from 3 min to 3 h prior to the development of luminograms.
Immunoblotting for activated Erk1/2 in HUVEC lysates
HUVEC seeded in 35-mm or 60-mm dishes were stimulated with C1q-IC (400 µg/ml) for time periods ranging from 5 to 30 min at 37°C. Reactions were stopped by washing the cells with cold PBS (4°C) followed by stop buffer containing 2 mm PMSF, 3 mm Na-EDTA, 1 µm pepstatin A and 20 nm iodoacetamide at 4°C. The stop solution was aspirated after 10 min and lysis buffer (2% NP-40, 0·5% SDS, 5 mm EDTA, 10 mm NaF, and vanadate 0·2 mg/ml in TBS buffer, 0·8% NaCl, 0·02% KCl and 0·3% Tris base pH 7·4) was added. Proteins were separated by sodium dodecyl sulphate-polyacrylamide electrophoresis (SDS-PAGE) and transferred electrophoretically to nitrocelulose membranes. Western blotting was performed using MAPK p44/42 antibody and E10 MoAb specific for the phosphorylated for of the p44/42 MAPK exactly as recommended by the manufacturer.
RESULTS
C1q-bearing immune complexes induce IL-8 secretion from HUVEC in a concentration- and time-dependent manner
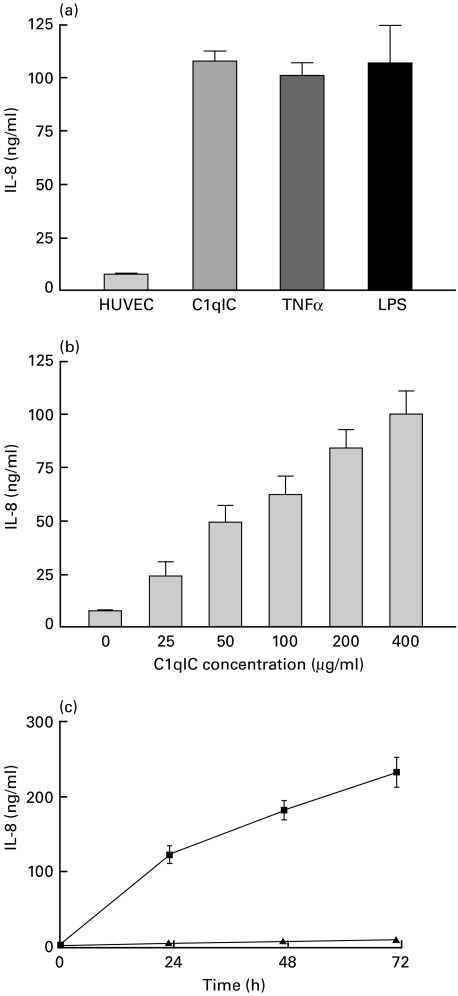
In the first set of experiments, we treated confluent HUVEC monolayers with a single concentration of C1q-IC (400 µg/ml) for 24 h. Unstimulated HUVEC produced a low level of background secretion of IL-8 protein. Stimulation of HUVEC with C1q-IC resulted in a greater than 13-fold increase in IL-8 secretion (7·9 ± 0·9 versus 108·4 ± 7·9 ng/ml). This was within the same range as IL-8 secretion in HUVEC stimulated with 100 U/ml TNFα (101·7 ± 5·6 ng/ml) or 10 ng/ml LPS (107·6 ± 12·7 ng/ml) for 24 h (Fig. 1a).
Fig. 1.
IL-8 secretion after stimulation of HUVEC by C1qIC. (a) Confluent monolayers of HUVEC were treated with 400 µg/ml C1q-IC, 100 U/ml human TNFα or 10 ng/ml LPS for 24 h. Levels of IL-8 secretion achieved with C1qIC were identical to those achieved with either TNFα or LPS. (b) Concentration-dependent secretion of IL-8 by HUVEC incubated with increasing doses of C1qIC for 24 h. (c) Kinetics of IL-8 secretion by HUVEC after incubation with 400 µg/ml (▪) C1qIC or 100 µg/ml BSA (▴) for the indicated times. Detectable levels of IL-8 above background were found in cell culture supernates within 4 h and continued to increase throughout the 72 h incubation period. IL-8 concentrations were measured in cell cuture supernates by ELISA. Results are means ± s.e.m. of three to five independent experiments.
Dose–response effects were then measured using C1q-IC in concentrations ranging from 25 to 400 µg/ml for 24 h. C1q-IC increased the level of IL-8 in a dose-dependent manner, as shown in Fig. 1b. Kinetics studies showed an unusual pattern from that not observed in immune complex stimulated lymphocytes [22] or human choriocarcinoma cells [27]. Increases in IL-8 concentration in the cell culture supernatants were detectable within 4 h after stimulation of the cells, but continued to rise steadily over the entire 72-h period over which the cells were cultured. No significant secretion was detected after incubation with BSA (Fig. 1c). These same results were also obtained when cells were washed and placed in fresh medium 2 h after exposure to the immune complexes (data not shown).
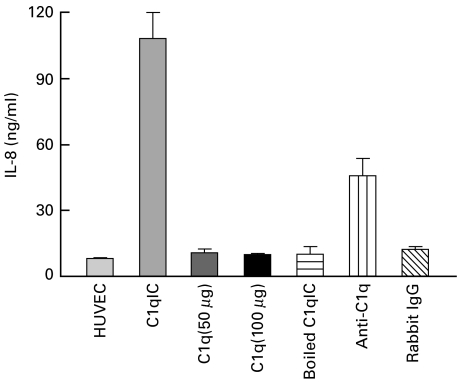
Uncomplexed C1q at doses of 50 and 100 µg/ml did not elicit IL-8 secretion from HUVEC (Fig. 2). Similarly, no increases in IL-8 secretion above background were seen using uncomplexed rabbit IgG anti-BSA antibody. Heating the immune complex preparations completely abrogated their capacity to elicit IL-8 secretion from the cells, demonstrating that IL-8 secretion was not due to LPS contamination of the complexes. Pre-incubating C1q-IC with polyclonal anti-C1q antibody (Fig. 2) significantly reduced their capacity to stimulate IL-8 secretion from the HUVEC (108·4 ± 11·7 versus 45·9 ± 7·9, P < 0·0001).
Fig. 2.
Specificity of C1q immune complexes. Confluent monolayers of HUVEC were treated with C1q-IC (400 µg/ml) or monomeric C1q (50 or 100 µg/ml), boiled C1q-IC (400 µg/ml), anti-C1q antibody (800 µg/ml) mixed with C1q-IC (400 µg/ml) or rabbit IgG anti-BSA antibody (400 µg/ml), respectively, for 24 h. Only C1q-IC stimulated IL-8 secretion from the cells. Preincubating the complexes with polyclonal goat antihuman C1q antibody (‘Anti-C1q’) significantly reduced the capacity of the complexes to induce IL-8 secretion. IL-8 was measured in cell culture supernates by ELISA. Results are means ± s.e.m. of three independent experiments.
Superinduction of IL-8 mRNA
Figure 3a shows a representative Northern blot analysis of IL-8 mRNA expression in HUVEC stimulated with C1q-IC. IL-8 mRNA was not detected in unstimulated HUVEC, but was detectable in C1q-IC-activated HUVEC as early as 2 h after stimulation. IL-8 mRNA accumulation peaked at 4 h, after which levels diminished. However, IL-8 mRNA was still detectable 24 h after stimulation of the cells with immune complexes. IL-8 mRNA expression was not dependent upon prior synthesis of other protein mediators. Cycloheximide, a potent inhibitor of protein synthesis enhanced rather than diminished IL-8 mRNA expression. This pattern of IL-8 induction in HUVEC was identical to that observed in HUVEC stimulated with 100 U/ml TNFα (data not shown).
Fig. 3.
IL-8 mRNA expression after stimulation of HUVEC by C1qIC. (a) Confluent monolayers of HUVEC were treated with 400 µg/ml C1q-IC. After the indicated time points, total RNA was isolated, and 20 µg of RNA was subjected to electrophoresis and transferred to nylon membranes for Northern analysis. The same membrane was sequentially hybridized with IL-8 and β-actin cDNA probes. HUVEC were treated in the presence or absence of 5 µg/ml cycloheximide as shown. IL-8 mRNA was detectable within 2 h in immune complex-stimulated HUVEC and was sustained out to 24 h, the last time period examined. Cycloheximide enhanced IL-8 mRNA accumulation. Cycloheximide-induced enhancement of IL-8 mRNA accumulation was also seen in HUVEC stimulated with TNFα (not shown). Results shown are representative of three independent experiments.
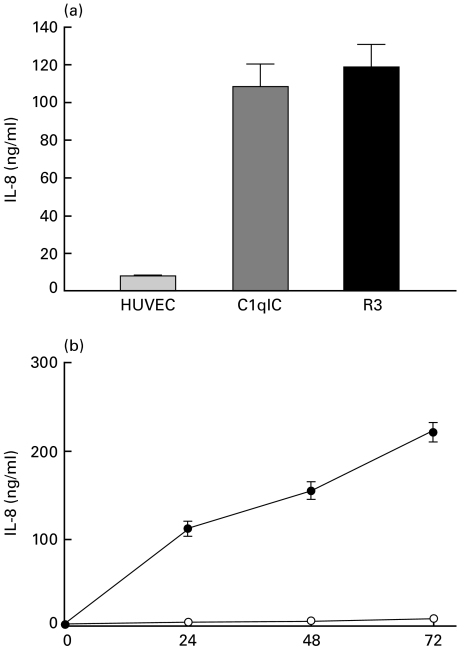
Effects of the anti-C1qRp IgM MoAb R3 on IL-8 production from HUVEC
At least three C1q-binding proteins expressed in HUVEC may mediate cellular activation. However, only the 126 kD C1qRp is unequivocally expressed on the resting cell surface, and only the C1qRp possesses the structure of a signal-transducing transmembrane receptor. Experiments were therefore undertaken to examine the potential role of the C1qRp in inducing IL-8 secretion from HUVEC. HUVEC were incubated with 10 µg/ml mouse IgM R3 MoAb to the 126 kD C1qRp or control mouse IgM for 24 h, after which IL-8 levels were measured in the cell culture supernatants. A significant increase in IL-8 secretion over background was seen in antibody-stimulated cells. (Fig. 4a). The kinetics of IL-8 secretion in response to the R3 were nearly identical to those seen with HUVEC stimulated with C1qIC (Fig. 4b). That is, IL-8 levels above background were detectable within 24 h and continued to increase over the 72 h time period.
Fig. 4.
Effects of anti-C1qR R3 murine IgM MoAb on IL-8 production from HUVEC. (a) Confluent monolayers of HUVEC were treated with 10 µg/ml R3 IgM MoAb to to 127 kD C1qRp or 400 µg/ml C1q-IC for 24-h. (b) Kinetics of IL-8 secretion in HUVEC were treated with 10 µg/ml R3 MoAb or mouse IgM for the indicated time periods. IL-8 was measured in cell cultured supernates by ELISA. Results are means ± s.e.m. of three independent experiments. •, R3 (10 μg/ml); ○, mouse IgM.
In an additional set of experiments, we added R3 and C1q-bearing immune complexes to HUVEC simultaneously to see whether there would be increases in IL-8 secretion through additive or synergistic mechanisms. No increase in IL-8 secretion was seen seen under these conditions (data not shown).
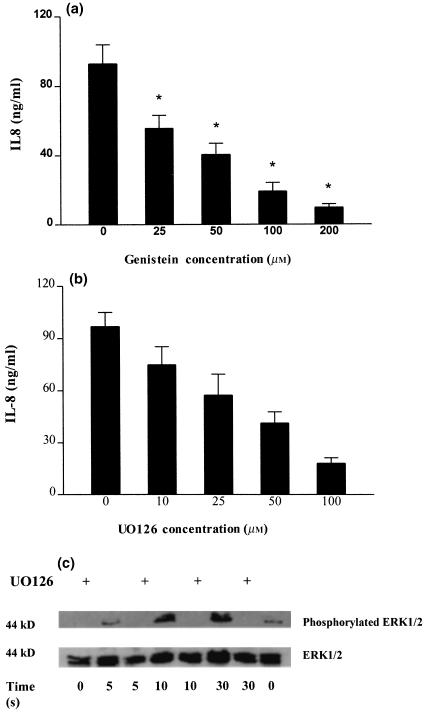
Role of PTK and MAPK in IL-8 secretion from C1qIC-stimulated HUVEC
To assess the role of PTK in the secretion of IL-8 by C1q-IC-stimulated HUVEC, we treated HUVEC with C1q-IC (400 µg/ml) in the presence or absence of the PTK inhibitor, genistein. Genistein reduced IL-8 secretion by 90% at the highest concentration used (200 µm; Fig. 5a). This inhibitory effect was significant (P < 0·05) at all concentrations (25–200 µm).
Fig. 5.
Effects of PTK and PKC inhibitors on IL-8 secretion from C1qIC-treated HUVEC. (a) Dose–response effects observed in confluent monolayers of HUVEC preincubated with 0–200 µm genistein for 30 min and then stimulated with 400 µg/ml C1q-IC for 24 h. DMSO (1%) was used as the solvent control. (b) Dose–response effects observed in HUVEC treated with the MAPK inhibitor, UO126 at 0–100 µm. ELISA assays were used to measure concentrations of IL-8 in cell culture supernates. Results are means ± s.e.m. of three independent experiments. (c) Effects of UO126 on ERK1/2 activation in C1q-IC-stimulated HUVEC. HUVEC were pretreated with 100 µm of UO126 or conditional media for 30 min, followed by 400 µg/ml C1qIC for the indicated time periods. Cells were lysed and proteins separated by SDS-PAGE followed by Western blotting using an anti-ERK1/2 or E10 MoAb that recognizes only the phosphorylated forms of ERK1/ERK2. ERK1 appears as the upper band (44 kD); ERK2 appears as the lower band (42 kD). The results shown are representative of three to five independent experiments.
A common feature of many PTK-dependent receptors is the ‘downstream’ activation of a family of serine–threonine protein phosphokinases known collectively as MAPK. Involvement of MAPK in the activation of HUVEC treated with C1qIC was examined using the specific MAPK inhibitor, UO126. UO126 acts by inhibiting the activation of MEK (‘MAP kinase kinase’), the enzyme that activates the 42/40 kD MAP kinases (Erk-1/Erk-2). Negative controls consisted of cells treated with DMSO, the vehicle used to dissolve the specific inhibitor. As shown in Fig. 5b, UO126 inhibited IL-8 secretion in immune complex-stimulated HUVEC in a dose-dependent fashion. DMSO had no such inhibitory effect (data not shown). Western blotting for activated forms of the ERK-1/2 MAP kinases demonstrated time-dependent phosphorylation of the 44/42 kD doublet that was completely abolished by the addition of UO126 at a concentration of 100 µm.
DISCUSSION
The accumulation of immune complexes in the circulation or tissues is a pathological event [28] and is associated with many human diseases, including systemic lupus erythematosus [29], rheumatoid arthritis [5] and idiopathic glomerulonephritis [30]. A common feature of many diseases associated with immune complex accumulation in the circulation is the presence of vasculitis, inflammation in blood vessel wall and in perivascular tissue. Thus, many of the pathological features of these diseases may be mediated by immune complex binding to and activation of the endothelium, with subsequent leucocyte recruitment to sites of immune complex deposition. Although Fcγ receptors (FcγR) are not constitutively expressed on endothelial cell lines commonly used to model vascular pathogenesis [31] (dermal microvascular endothelial cells are a notable exception [32]), HUVEC are known to bind immune complexes through the first component of complement, C1q [12]. We present data here supporting the hypothesis that the relevant receptor mediating HUVEC activation after immune complex binding is the 126 kD C1qRp, previously recognized for its capacity to enhance phagocytosis in leucocytes [17].
Van den berg and colleagues [20] were the first to demonstrate the capacity of C1q to elicit cytokine secretion from HUVEC. These authors demonstrated that the globular heads of C1q did not elicit cytokine secretion from the cells, consistent with their previous observation that the gC1qR (receptor for the globular heads of C1q) is an intracellular protein in HUVEC [14]. Collagen stalks, however, did elicit IL-8 secretion, and these authors speculated that the previously described cC1qR/calreticulin receptor, may participate in activating HUVEC after C1q binding. However, the C1qRp also binds the collagen-like region of C1q[9,33], and our data strongly support its role in the IL-8 secretion from immune complex-activated HUVEC. Further support for a role for the 126 kD receptor as an activator of endothelial cells was reported recently in abstract form [34]. In that study, Reiss and colleagues demonstrated that the down-regulation of cholesterol 27-hyroxylase on human aortic endothelium that occurs in response to immune complex binding could be inhibited by antibody to the 126 kD C1qRp. In our experiments, we cross-linked the C1qRp using a murine monoclonal IgM antibody to the receptor and observed IL-8 secretion identical in levels and kinetics to those obtained using C1qIC. Our findings differed from those of van den Berg and colleagues in that, in our experiments, uncomplexed C1q did not activate HUVEC and therefore parallel what other authors have described with C1q-activated platelets [35]. The reason for this disparity is not immediately evident, although Van den Berg and colleagues state that there may have been C1q multimers in their C1q preparation [20]. Such multimers may therefore have cross-linked the relevant C1qR sufficiently to cause activation.
The immune complexes we used in this study differed, of course, from complexes that might be found in pathological states. They were intended as a model for those diseases, such as systemic lupus erythematosus (SLE) [36] and rheumatoid arthritis [37], where C1q-bearing complexes are known to be involved in disease pathogenesis or in specific disease manifestations. Since we have shown previously that both size and composition are important regulators of the biological behaviour of immune complexes [22], the model nature of the immune complexes we used here must be considered in interpreting our findings. We have, however, used immune complexes prepared by polyethylene glycol precipitation from synovial fluids of children with juvenile chronic arthritis and obtained results identical to those obtained with the model C1q-bearing immune complexes used here. Indeed, material obtained from patients results in significantly higher levels of IL-8 secretion than we achieve with C1q-BSA-anti-BSA (unpublished data).
It is important to note that what we have modelled here is pathological, not physiological. It is highly unlikely that, in a healthy host, C1q-bearing immune complexes interact in any substantial way with endothelium. Under physiological conditions, immune complexes bind other complement components (C4b, C3b), which facilitates their binding to complement receptors on phagocytic cells and erythrocytes [38]. Once bound, complexes are carried to the liver and spleen, where they are cleared by cells of the monocyte/phagocytic system [39]. Thus, activation of endothelial cells by immune complexes very probably occurs only under pathological situations where normal immune complex buffering and clearance mechanisms are overwhelmed.
Although various studies have recently identified several functional effects of C1q-bearing immune complexes on endothelial cells or other cell types, little is known about postreceptor events that mediate and propagate the C1q-IC-induced signal. Our studies therefore extend those of van den Berg and colleagues by elucidating the mechanisms through which C1q-IC elicit IL-8 secretion from HUVEC. The structure of the C1qRp has recently been deduced from cDNA cloning [40] and its intracytoplasmic domain (47 amino acids) contains a single tyrosine at position 644. This tyrosine resides within a consensus motif (R/KX2–3D/EX2–3Y) recognized by PTK. Of the C1q binding proteins expressed in/on HUVEC, only the C1qRp contains such an activation motif. Thus, the inhibition of IL-8 secretion observed in the presence of genistein, an inhibitor of PTK and angiogenesis [41], further supports a role for the C1qRp in the activation of HUVEC by immune complexes.
A common ‘downstream’ pathway used by PTK-activated receptors is the activation of a family of serime/threonine protein tyrosine kinases known collectively as MAP kinases. Both extracellular signal-regulated kinases (ERK 1/2) and p38 pathways are involved in IL-8 regulation in human monocytes/marophages and neutrophils [42]. Our studies show that the ERK 1/2 pathway is also activated in immune complex-stimulated HUVEC. UO126, a novel and potent inhibitor of MEK, the enzyme that phosphorylates the ERK 1/2 kinases, efficiently blocked IL-8 production in HUVEC in response to C1q-bearing IC. We obtained a similar result using TNFα to stimulate IL-8 secretion from HUVEC (data not shown).
Another notable finding in our studies was the effect of cycloheximide on IL-8 mRNA expression in C1q-IC-stimulated HUVEC. The IL-8 gene, like most cytokine genes, is under complicated regulatory control mediated through multiple upstream elements, including NFκB, NF-IL-6, AP-1 [43] and C/EBP [44] sites. In monocytes stimulated with either PHA or LPS, IL-8 mRNA expression is inhibited by cycloheximide [45] demonstrating that, in these cells with these stimuli, prior protein synthesis is required for IL-8 mRNA expression. The same finding has been reported in monocytic cell lines stimulated with IFN-γ [46]. These observations are consistent with what we and others have reported in immune complex-stimulated PBMC [26], where IL-1β and TNFα mRNAs appear before IL-8, and support the concept of a ‘cascade’ effect regulating the expression of specific cytokines at the initiation of inflammation [47]. In contrast, we found that cycloheximide enhanced IL-8 mRNA expression in HUVEC, suggesting that IL-8 transcription is a direct effect of C1q receptor ligation and not dependent on prior secretion of other cytokines. This ‘superinduction’ in the presence of cycloheximide has been observed in other models, including IL-8 in TNFα-stimulated synovial fibroblasts [48]. We observed the same effect in TNFα-stimulated HUVEC. These findings may relate to the capacity of cycloheximide to inhibit the regeneration of IκB or related inhibitors after the initial stimulatory events [49].
Taken together, our findings help clarify the mechanisms through which C1q-bearing immune complexes induce IL-8 secretion from HUVEC, as described originally by Van den Berg and colleagues [20]. We propose that the relevant receptor is the C1qRp. Ligation of this receptor leads to a rapid sequence of PTK and MAPK activation and IL-8 gene transcription, which is the direct result of C1qR ligation and not dependent upon the synthesis of intermediary proteins. Furthermore, activation results in prolonged secretion of IL-8 and sustained IL-8 mRNA expression.
These data are informative concerning the role of immune complexes in the pathophysiology of chronic inflammatory disease, particularly those characterized by vascular and perivascular inflammation. The immediate and sustained secretion of IL-8 resulting from immune complex binding to endothelium would provide a potent chemoattractant signal for both neutrophils and lymphocytes. Furthermore, immune complex-stimulated HUVEC also express the cell-surface adhesion molecules required for leucocyte arrest and transmigration [19]. Thus, the presence of circulating immune complexes in human illnesses associated with vasculitis or perivascular inflammation in specific inflamed tissues (e.g. the rheumatoid synovium) may not be coincidental.
Acknowledgments
This work was supported by NIH grant AR-43967 and a grant from the Children Medical Research Institute of Oklahoma City. Dr Jarvis is also the recipient of a grant from the Oklahoma Chapter of the Arthritis Foundation. The authors wish to thank Dr Andrea Tenner for providing the R3 murine IgM antibody to the C1qRp. Thanks also to Dr Rod McEver for review of the manuscript and for many helpful suggestions. Thanks to Dr Terry Stull for his continued support of this work.
REFERENCES
- 1.Vanhoutte PM, Houston DS. Platelets, endothelium, and vasospasm. Circulation. 1985;72:728–34. doi: 10.1161/01.cir.72.4.728. [DOI] [PubMed] [Google Scholar]
- 2.Gimbrone MA., Jr . Vascular endothelium in health and disease. In: Haber E, editor. Molecular cardiovascular medicine. New York: Scientific American Medicine; 1995. p. 49. [Google Scholar]
- 3.Pober JS, Cotran RS. Cytokines and endothelial cell biology. Physiol Rev. 1990;70:427–51. doi: 10.1152/physrev.1990.70.2.427. [DOI] [PubMed] [Google Scholar]
- 4.Moore TL, Dorner RW, Zuckner J. 19S IgM rheumatoid factor-7S IgG rheumatoid factor immune complexes isolated in patients with rheumatoid arthritis. J Lab Clin Med. 1986;107:465–70. [PubMed] [Google Scholar]
- 5.Reynolds WJ, Yoon SJ, Emin M, Chapman KR, Klein MH. Circulating immune complexes in rheumatoid arthritis. A prospective study using five immunoassays. J Rheumatol. 1986;13:700–6. [PubMed] [Google Scholar]
- 6.Jarvis JN, Diebold MM, Chadwell MK, Iobidze M, Moore HT. Composition and biological behaviour of immune complexes isolated from synovial fluid of patients with juvenile rheumatoid arthritis (JRA) Clin Exp Immunol. 1995;100:514–8. doi: 10.1111/j.1365-2249.1995.tb03731.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jarvis JN, Taylor H, Iobidze M, Krenz M. Complement activation and immune complexes in children with polyarticular juvenile rheumatoid arthritis: a longitudinal study. J Rheumatol. 1994;21:1124–7. [PubMed] [Google Scholar]
- 8.Tung USK, DeHoratius RJ, Williams RC. Study of circulating immune complex size in systemic lupus erythematosus. Clin Exp Immunol. 1981;43:615–25. [PMC free article] [PubMed] [Google Scholar]
- 9.Schifferli JA, Taylor RP. Physiological and pathological aspects of circulating immune complexes. Kidney Int. 1989;35:993–1003. doi: 10.1038/ki.1989.83. [DOI] [PubMed] [Google Scholar]
- 10.Foreman KE, Vaporclyan AA, Bonish BK, Jones ML, Johnson KJ, Glovsky MM. C5a-induced expression of P-selectin in endothelial cells. J Clin Invest. 1994;94:1147–55. doi: 10.1172/JCI117430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hattori R, Hamilton KK, McEver RP, Sims PJ. Complement protein C5b-9 induces secretion of high molecular weight multimers of endothelial von Willebrand factor and translocation of granule membrane protein GMP-140 to the cell surface. J Biol Chem. 1989;264:9053–60. [PubMed] [Google Scholar]
- 12.Daha MR, Mittenburg AMM, Hiemstra PS, Klar-Mohamad N, Van Es LA, Van Hinsbergh VWM. The complement subcomponent C1q mediates binding of immune complexes and aggregates to endothelial cells in vitro. Eur J Immunol. 1988;18:783–7. doi: 10.1002/eji.1830180519. [DOI] [PubMed] [Google Scholar]
- 13.Ghebrehiwet B, Lim B-L, Peerschke EIB, Willis AC, Reid KM. Isolation, cDNA cloning, overexpression of a 33 kD cell surface glycoprotein that that binds the globular ‘heads’ of C1q. J Exp Med. 1994;179:1809–21. doi: 10.1084/jem.179.6.1809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Van den Berg RH, Prins F, Faber-Krol MC, et al. Intracellular localization of the human receptor for the globular domains of C1q. J Immunol. 1997;158:3909–16. [PubMed] [Google Scholar]
- 15.Reid KB, Colomb MMG, Loos M. Complement component C1 and the collectins: parallels between routes of acquired of acquired and innate immunity. Immunol Today. 1998;19:56–9. doi: 10.1016/s0167-5699(97)01207-3. [DOI] [PubMed] [Google Scholar]
- 16.Korb LC, Ahearn JM. C1q binds directly and specifically to surface blebs of apoptotic human keratinocytes. Complement deficiency and systemic lupus erythematosus reconsidered. J Immunol. 1997;158:4525–8. [PubMed] [Google Scholar]
- 17.Guan E, Robinson SL, Goodman EB, Tenner AJ. Cell-surface protein identified on phagocytic cells modulates the C1q-mediated enhancement of phagocytosis. J Immunol. 1994;154:4005–16. [PubMed] [Google Scholar]
- 18.Nepomuceno RR, Tenner AJ. C1qRp, the C1q receptor that enhances phagocytosis, is detected specifically in human cells of myeloid lineage endothelial cells, and platelets. J Immunol. 1998;160:1929–235. [PubMed] [Google Scholar]
- 19.Lozada C, Levin RI, Huie M, et al. Identification of C1q as the heat-labile serum cofactor required for immune complexes to stimulate endothelial expression of adhesion molecules E-selectin and intercellular and vascular adhesion molecules 1. Proc Natl Acad Sci USA. 1995;92:8378–82. doi: 10.1073/pnas.92.18.8378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Van de Berg RH, Faber-Krol MC, Sim RB, Daha MR. The first subcomponent of complement, C1q triggers the production of IL-8, IL-6 and monocyte chemoattractant peptide-1 by human umbilical vein endothelial cells. J Immunol. 1998;161:6924–30. [PubMed] [Google Scholar]
- 21.Jaffe EA, Nachman RL, Becker CG, Minick CR. Culture of human endothelial cells derived from umbilical veins. J Clin Invest. 1973;52:2745–56. doi: 10.1172/JCI107470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jarvis JN, Xu C-S, Wang W, et al. Immune complex size and complement regulate cytokine production by peripheral blood mononuclear cells. Clin Immunol. 1999;93:274–82. doi: 10.1006/clim.1999.4792. [DOI] [PubMed] [Google Scholar]
- 23.Amit A, Kindzelskii AL, Zanoni J, Jarvis JN, Petty HR. Complement deposition on immune complexes reduces the frequencies of metabolic, proteolytic, and superoxide oscillations of migrating neutrophils. Cell Immunol. 1999;194:47–53. doi: 10.1006/cimm.1999.1481. [DOI] [PubMed] [Google Scholar]
- 24.Huber C, Rüger A, Hermann M, Krapf F, Kalden JR. C3-containing serum immune complexes in patients with systemic lupus erythematosus: correlation to disease activity and comparison with other rheumatic diseases. Rheumatol Int. 1989;9:59–64. doi: 10.1007/BF00270246. [DOI] [PubMed] [Google Scholar]
- 25.Yao Y, Pan. J, Sediati H, Patel KD, McEver RP. Interleukin 4 or oncostatin M induces a prolonged increase in p-selectin mRNA and protein in human endothelial cells. J Exp Med. 1996;184:81–92. doi: 10.1084/jem.184.1.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jarvis JN, Wang W, Moore HT, Zhao L, Xu C-S. In vitro induction of proinflammatory cytokine secretion by juvenile rheumatoid arthritis synovial fluid immune complexes. Arthritis Rheum. 1997;40:2039–46. doi: 10.1002/art.1780401117. [DOI] [PubMed] [Google Scholar]
- 27.Jarvis JN, Xu C-S, Zhao L, Iobidze M, Moore HT. Human choriocarcinoma JAR cells constitutively express pro-interleukin-1β that can be released with Fcγ receptor engagement. Pediatr Res. 1998;43:509–13. doi: 10.1203/00006450-199804000-00012. [DOI] [PubMed] [Google Scholar]
- 28.Jarvis JN. Pathogenesis, mechanisms of inflammation in childhood rheumatic diseases. Curr Opin Rheum. 1998;10:459–67. doi: 10.1097/00002281-199809000-00011. [DOI] [PubMed] [Google Scholar]
- 29.Greisman SG, Redechan PB, Kimberley RP, Christian CI. Differences among immune complexes: association of C1q in SLE immune complexes with renal disease. J Immunol. 1987;138:739–45. [PubMed] [Google Scholar]
- 30.Siegert CEH, Daha MR, van der Voort EAM, Breedveld FC. IgG and IgA antibodies to the collagen-like region of C1q in rheumatoid vasculitis. Arthritis Rheum. 1990;33:1646–54. doi: 10.1002/art.1780331107. [DOI] [PubMed] [Google Scholar]
- 31.Pan L-F, Kreisel RA, Shi Y-D. Detection of Fcγ receptors on human endothelial cells stimulated with cytokines tumor necrosis factor alpha (TNFα) and interferon-gamma (IFN-γ) Clin Exp Immunol. 1998;112:533–8. doi: 10.1046/j.1365-2249.1998.00597.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gröger M, Sarmay G, Fiebiger G, Wolff K, Petzelbauer P. Dermal microvascular endothelial cells express CD32 receptors in vivo and in vitro. J Immunol. 1996;156:1549–56. [PubMed] [Google Scholar]
- 33.Guan E, Burgess WH, Robinson SL, Goodman EB, McTigue KJ, Tenner AJ. Phagocytic molecules that bind the collagen-like region of C1q. Involvement in the C1q-mediated enhancement of phagocytosis. J Biol Chem. 1991;266:20345–55. [PubMed] [Google Scholar]
- 34.Reiss AB, Awadallah NW, Chan ESL, Montesinos MC, Cronstein BN. Immune complex mediated downregulation of cholesterol 27-hydroxylase in human arterial endothelial cells (HAEC) and THP-1 monocytoid cells is mediated by the 126 kD C1q receptor. Arthritis Rheum. 2000;43:S356. [Google Scholar]
- 35.Peerschke EIB, Reid KBM, Ghebrehiwet B. Platelet activation by C1q results in the induction of alpahllb/beta3 integrins (GPllb/llla) and the expression of P-selectin and procoagulant activity. J Exp Med. 1993;178:579–87. doi: 10.1084/jem.178.2.579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bengtsson A, Nezlin R, Shoenfeld Y, Sturfelt G. DNA levels in circulating immune complexes decrease at severe SLE flares — correlation with complement component C1q. J Autoimmunity. 1999;13:111–9. doi: 10.1006/jaut.1999.0300. [DOI] [PubMed] [Google Scholar]
- 37.Melson RD, Horsfall AC, Schrieber L, Charles P, Maini RN. AntiC1q affinity isolated circulating immune complexes correlate with extra-articular rheumatoid disease. Rheumatol Int. 1986;6:227–31. doi: 10.1007/BF00541372. [DOI] [PubMed] [Google Scholar]
- 38.Cornacoff JB, Hebert LA, Smead WL, VanAman ME, Birmingham DJ, Waxman FJ. Primate erythrocyte-immune complex-clearing mechanism. J Clin Invest. 1983;71:236–47. doi: 10.1172/JCI110764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schifferli J, Ng Y, Peters K. The role of complement and its receptors in the elimination of immune complexes. N Engl J Med. 1986;315:488–95. doi: 10.1056/NEJM198608213150805. [DOI] [PubMed] [Google Scholar]
- 40.Nepomuceno RR, Henschen-Edman AH, Burgess WH, Tenner AJ. cDNA cloning and primary structure analysis of C1qRp, the human C1q/MBL/SPA receptor that mediates enhances phagocytosis in vitro. Immunity. 1997;6:119–29. doi: 10.1016/s1074-7613(00)80419-7. [DOI] [PubMed] [Google Scholar]
- 41.Fotsis T, Pepper M, Adlercreutz H, et al. Genistein, a dietary-derived inhibitor of in vitro angiogenesis. Proc Natl Acad Sci USA. 1993;90:2690–4. doi: 10.1073/pnas.90.7.2690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Marie C, Roman-Roman S, Rawadi G. Involvement of mitogen-activated protein kinase pathways in interleukin-8 production by human monocytes and polymorphonuclear cells stimulated with lipopolysaccharide or mycoplasma fermentans membrane lipoproteins. Infect Immun. 1999;67:688–93. doi: 10.1128/iai.67.2.688-693.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mukaida N, Okamoto S, Ishakawa Y, Matsushima K. Molecular mechanism of interleukin-8 gene expression. J Leukoc Biol. 1994;56:554–8. [PubMed] [Google Scholar]
- 44.Stein B, Sullivan AS. Distinct mechanisms for regulation of the IL- 8 gene involve synergism and cooperativity between C/EBP and ΝFκΒ. Mol Cell Biol. 1993;13:7191–8. doi: 10.1128/mcb.13.11.7191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Liebler JM, Kunkel SL, Burdick MD, Standiford TJ, Rolfe TW, Strieter RM. Production of IL-8 and monocyte chemotactic peptide-1 by peripheral blood monocytes. Disparate responses to phytohemagglutinin and lipopolysaccharide. J Immunol. 1994;152:241–9. [PubMed] [Google Scholar]
- 46.Bosco MC, Gusella GL, Esponoza-Delgado I, Longo DL, Varesio L. Interferon-γ upregulates interleukin-8 gene expression in human monocytic cells by a posttranscriptional mechanism. Blood. 1994;83:537–42. [PubMed] [Google Scholar]
- 47.Feldmann M, Brennan FM, Maini RN. Role of cytokines in rheumatoid arthritis. Ann Rev Immunol. 1996;14:397–440. doi: 10.1146/annurev.immunol.14.1.397. [DOI] [PubMed] [Google Scholar]
- 48.Paliniswami R, Hachicha M, Sadick M, Schall TJ, McColl SR. Expression of the cytokine RANTES in human rheumatoid synovial fibroblasts. Differential regulation of RANTES and interleukin-8 genes by inflammatory cytokines. J Biol Chem. 1993;268:5834–9. [PubMed] [Google Scholar]
- 49.Osipovich OE, Fegeding KV, Misuno NI, et al. Differential action of cycloheximide and activation stimuli on transcription of tumor necrosis factor-α, IL-1β, IL-8, and P53 genes in human monocytes. J Immunol. 1993;150:4958–65. [PubMed] [Google Scholar]