Abstract
Studies in humans and murine disease models have clearly shown dietary fish oil to possess anti-inflammatory properties, apparently mediated by the n-3 polyunsaturated fatty acids, eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA). To determine the mechanisms by which dietary EPA and DHA modulate mouse T-cell activation, female C57BL/6 mice were fed diets containing either 2% safflower oil (SAF), 2% fish oil (FO), or a 2% purified EPA/DHA ethyl ester mixture for 14 days. Splenic CD4 T cells (∼90% purity) or CD8 T cells (∼85% purity) were incubated with agonists which act at the plasma membrane receptor level [anti(α)-CD3/anti(α)-CD28], the intracellular level (PMA/Ionomycin), or at both the receptor and intracellular levels (αCD3/PMA). CD4 T cells stimulated with αCD3/αCD28 or PMA/Ionomycin proliferated and produced principally IL-2 (i.e. a Th1 phenotype), whereas the proliferation of CD4 T cells stimulated with αCD3/PMA was apparently driven principally by IL-4 (i.e. a Th2 phenotype). The IL-4 driven proliferation of putative Th2 CD4 cells was enhanced by dietary n-3 fatty acids (P = 0·02). Conversely, IL-2 production by αCD3/α CD28-stimulated CD4 T cells was reduced in FO-fed animals (P < 0·0001). The αCD3/αCD28-stimulated CD8 cells cultured from FO-fed animals exhibited a significant decrease (P < 0·05) in proliferation. There were no dietary effects seen in αCD3/PMA-stimulated CD8 cells, which produced both IL-2 and IL-4, or in PMA/Ionomycin-stimulated CD8 cells, which produced principally IL-2. These data suggest that dietary n-3 fatty acids down-regulated IL-2 driven CD4 and CD8 activation, while up-regulating the activation of the Th2 CD4 T-cell subset. Thus, the anti-inflammatory effects of n-3 fatty acids may result in both the direct suppression of IL-2-induced Th1 cell activation and the indirect suppression of Th1 cells by the enhanced cross-regulatory function of Th2 cells.
Keywords: T cells, cytokines, diet, fatty acids
INTRODUCTION
Epidemiological [1,2], clinical [3–6] and mechanistic studies [7–12] support the conclusion that n-3 polyunsaturated fatty acids (PUFA) found in fish oil (FO) mediate a potent anti-inflammatory role when fed to patients with rheumatoid arthritis. n-3 PUFA have been shown to decrease T-cell proliferation [13,14], cytokine secretion [15–18], intracellular enzyme activity [9,10] and gene transcription [16,19].
Most previous studies have examined the effects of n-3 PUFA on mixed cell populations, e.g. whole splenocytes [13,16,20,21], purified murine T cells [22–24] or immortalized T-cell lines [7,11]. The few studies that have addressed T-cell subsets have produced a wide range of results. Jeffrey et al. [25] demonstrated that FO diets fed to rats did not affect CD4 and CD8 proportions as measured by percent positive cells, whereas Jenski et al. [26] reported that dietary docosahexaenoic acid (DHA) caused a decrease in the surface expression of murine CD8. These results might not be contradictory, as others have observed that dietary fish oil can decrease CD8 expression on rat lymphocytes without altering the number of CD8 T cells [27,28]. We have previously shown that dietary DHA is capable of reducing the proportion of CD8 T cells, but not that of the CD4 T cells, in mouse spleens [21]. Lim et al. [29] reported that calorie restriction combined with FO feeding blunted age-related alterations in CD4 and CD8 T-lymphocyte subset ratios.
Both CD4 and CD8 cells are subdivided, according to the cytokine profiles which they produce [30,31], into Th1 and Th2 cells, and Tc1 and Tc2 cells, respectively. Th1 cells produce interleukin-2 (IL-2) as their principal growth factor [32] and are thought to be involved in the inflammation seen in rheumatoid arthritis and other diseases [33]. Th2 cells produce IL-4 as their primary growth factor [34]. Th1 and Th2 cells cross-regulate each other by inhibiting the production of cytokines of the other cell type [34].
Tc1 and Tc2 subsets of CD8 cells produce cytokine profiles analogous to the Th1 and Th2 CD4 profiles [30,35]. These subsets are also capable of cross-regulation. In a manner analogous to Th subsets, Tc1 cells utilize IL-2 as their principal growth factor, while Tc2 cells rely upon IL-4 [30,35,36].
The development of Th1 and Th2 cells can be determined by the type of stimulation received. Yamashita et al. [37] employed phorbol-12-myristate-13-acetate (PMA) and a calcium ionophore (Ionomycin) to induce a switch to the Th2 pole of the dichotomy. Recently, Noble et al. [38] demonstrated that it is the balance of PKC/calcium signalling that drives the T cells toward the type 1 or type 2 phenotype. PMA induced the Th2/Tc2 phenotype when accompanied by a weak calcium signal, whereas a strong calcium signal and a weak PKC signal produced the Th1/Tc1 phenotype.
We previously reported [22] that purified splenic T cells taken from mice fed diets containing 1% DHA produced less IL-2 when stimulated at the plasma membrane receptor level with antibodies to CD3 and CD28, than T cells from mice fed 1% arachidonic acid (AA). Similar results were seen in Jurkat cells incubated in DHA-enriched culture medium [22]. Thus, in our experimental system, dietary fatty acids appear to modulate T-cell plasma membrane dynamics and alter T-cell receptor (TcR)-dependent or co-stimulatory receptor signal transduction.
Since CD4 cells, especially the Th1 subset, are primarily pro-inflammatory and are implicated in the pathogenesis of human inflammatory and autoimmune diseases [33,39], it is important to determine whether CD4 Th1 cells are being down-regulated by dietary n-3 PUFA. We report here for the first time the effect of dietary PUFA on purified murine CD4 and CD8 T-cell subsets that have been stimulated with agonists which result in type 1 or type 2 cytokine profile polarization. We demonstrate that dietary n-3 PUFA mediate agonist-specific effects on T-cell proliferation, and that the effects are related to the resulting cytokine profiles.
MATERIALS AND METHODS
Diet and animals
All experimental procedures using laboratory animals were approved by the University Laboratory Animal Care Committee of Texas A & M University. Female, pathogen-free young (12–14 g) C57BL/6 mice were purchased from Frederick National Cancer Research Facility, Frederick, MD. Animals were housed in groups of six in polycarbonate microisolator cages, had free access to autoclaved water and diet, and were maintained at room temperature (∼25 °C) on a 12 h light:dark cycle. Mice were initially fed standard mouse chow (Teklad 9F Sterilizable Rodent diet, Madison, WI) during a 1 week acclimation period and were subsequently assigned to one of three semi-purified diets: safflower oil ethyl ester (SAF) (control diet containing no n-3 PUFA), FO, or an EPA/DHA ethyl ester combination, for 2 weeks. The purified n-3 ethyl ester combination was mixed at a ratio of 1·4:1·0 to approximate the EPA/DHA ratio found in FO. Diets were analysed by gas chromatography (Table 1) prior to feeding, aliquoted, and stored at − 80 °C; they were changed daily to prevent peroxidation. The analysis confirmed the enrichment of 18:2 (n-6) in the SAF diet (63·2% of total lipid), 20:5 (n-3) and 22:6 (n-3) in the EPA/DHA diet (19·5% and 13·9% of total lipid, respectively), and 20:5 (n-3) and 22:6 (n-3) in the FO diet (5·3% and 3·9% of total lipid, respectively).
Table 1.
Fatty acid composition of the experimental diets
| Fatty acid | SAF | EPA/DHA | FO |
|---|---|---|---|
| 14:0 | 0·00 | 0·27 | 4·23 |
| 16:0 | 9·88 | 7·53 | 14·74 |
| 16:1 (n-7) | 0·00 | 0·00 | 4·82 |
| 18:0 | 2·22 | 1·58 | 2·82 |
| 18:1 (n-7 + n-9) | 21·63 | 18·33 | 21·69 |
| 18:2 (n-6) | 63·21 | 35·18 | 35·44 |
| 18:3 (n-6) | 0·00 | 0·00 | 0·00 |
| 18:3 (n-3) | 0·73 | 0·75 | 1·11 |
| 20:0 | 0·47 | 0·37 | 0·54 |
| 20:1 (n-9) | 0·61 | 0·48 | 0·62 |
| 20:3 (n-6) | 0·00 | 0·00 | 0·00 |
| 20:4 (n-6) | 0·00 | 0·00 | 0·00 |
| 20:5 (n-3) | 0·00 | 19·53 | 5·29 |
| 22:5 (n-3) | 0·00 | 0·00 | 1·18 |
| 22:6 (n-3) | 0·00 | 13·86 | 3·90 |
| Total SFA | 12·57 | 9·75 | 22·33 |
| Total MUFA | 22·24 | 18·81 | 27·13 |
| Total (n-6) PUFA | 63·21 | 35·18 | 35·44 |
| Total (n-3) PUFA | 0·73 | 34·14 | 11·48 |
Only the major fatty acids are listed. SFA, saturated fatty acid; MUFA, monounsaturated fatty acid; PUFA, polyunsaturated fatty acid; SAF, linoleic acid-containing diet; EPA/DHA, purified eicosapentaenoic acid and docosahexaenoic acid-containing diet; FO, fish oil-containing diet. Values are expressed as g/100g of fatty acids in each diet.
There was no significant difference in food intake between dietary groups, and weight gain was similar in all groups (final body weights mean ± s.e.m.: SAF, 19·02 g ± 0·29 g; EPA/DHA, 18·70 g ± 0·29 g; FO 19·25 g ± 0·21 g; n = 12 for the CD4 study and n = 18 for the CD8 study per diet group). The purified diets met National Research Council nutrition requirements and varied only in lipid composition [40]. The basic diet composition, expressed in g/kg of complete diet, was as follows: 200 g casein, 420 g sucrose, 219·8 g starch, 60 g cellulose, 35 g AIN-76 mineral mix, 10 g vitamin mix AIN-76, 3 g dl-methionine, 2 g choline chloride, 0·2 g tertiary butyl hydroquinone, 30 g corn oil (CO), and 20 g PUFA. The three diet groups varied by PUFA lipid source only, containing 20 g/kg SAF, 11·2 g/kg EPA + 8·8 g/kg DHA, or 20 g/kg Menhaden fish oil (FO). The linoleic acid (18:2 n-6) content from CO was 5·6% of total energy and thus met the minimum 1–2% requirement for rodents [40]. The vitamin E levels were approximately equal (mean ± s.e.m. = 169·2 ± 4·4 mg/kg diet) and exceeded the minimum requirement (22 mg Vitamin E/kg diet) [40]. DHA (88·9% as 22:6 n-3), EPA (94·1% as 20:5 n-3) and SAF (70·5% as 18:2 n-6) were obtained in ethyl ester form from the National Institute of Health Test Materials Program (Charleston, SC). Menhaden fish oil (13·1% as 20:5 n-3, 9·7% as 22:6 n-3) was provided by the National Institutes of Health Test Materials Program, and CO (57·3% as 18:2 n-6) was obtained from Traco Labs (Champaign, IL).
Isolation and preparation of splenic lymphocytes
Mice were killed via CO2 asphyxiation. Spleens were placed in 3 ml of RPMI complete medium [(RPMI 1640 with 25 mm HEPES (Irvine Scientific, Santa Ana, CA) supplemented with 10% FBS (Irvine Scientific), 1 × 105 U/l penicillin and 100 mg/l streptomycin (Irvine Scientific), 2 mm l-glutamine, and 10 µm 2-mercaptoethanol] [21]. Spleens were dispersed with glass homogenizers and passed through a 149 micron wire mesh filter to create single-cell suspensions. Splenocytes were washed with RPMI complete medium prior to CD4 or CD8 T-cell enrichment.
CD4 T-cell purification
Total lymphocytes were initially enriched by density gradient centrifugation using Lympholyte-M (Cedarlane, Toronto, Ontario, Canada) in accordance with the manufacturer's protocol. The resulting cell fractions from two spleens were pooled; approximately 120–180 × 106 lymphoctyes were incubated with an antibody cocktail provided by the manufacturer, loaded onto a negative-selection mouse CD4 T-cell purification column (R & D Systems, Minneapolis, MN) and incubated for 10 min at room temperature. Non-adherent cells were eluted for purity analysis, proliferation or cytokine assays. The purity of the CD4 T-cell population was analysed by flow cytometry (FACScan; Becton-Dickenson, Bedford, MA) as previously described [41] using anti-CD4 antibody conjugated to fluorescein isothiocyanate (PharMingen, San Diego, CA), and determined to be 90·3 ± 1·4% (n = 3).
CD8 T-cell purification
Three spleens were combined at necropsy prior to homogenization. Red blood cells were initially lysed using H-lyse buffer (R & D Systems) in accordance with the manufacturer's protocol. Approximately 180–270 × 106 lymphoctyes were incubated with an antibody cocktail provided by the manufacturer, loaded onto a negative-selection mouse CD8 T-cell purification column (R & D Systems) and incubated for 10 min at room temperature. Non-adherent cells were eluted for purity analysis, proliferation or cytokine assays. The purity of the CD8 T-cell population was analysed by flow cytometry (FACScan) as previously described by Darzynkiewicz and Crissman [41], using anti-CD8 antibody conjugated to phycoerythrin (PharMingen) and determined to be 84·5 ± 1·2% (n = 3).
T-lymphocyte proliferation assay
Purified splenic CD4 and CD8 T cells were cultured at 2 × 105 cells per well (200 µl total volume) in 96-well round-bottomed microtitre plates (Falcon, Becton-Dickenson, Lincoln Park, NJ) with triplicate wells for each stimulus and diet treatment. Cells were cultured in the presence of the following agonists: 1 µg/ml plate-bound purified hamster anti-mouse CD3e (αCD3) monoclonal antibody (PharMingen) with 5 µg/ml soluble purified hamster anti-mouse CD28 (αCD28) monoclonal antibody (PharMingen); 0·5 ng/ml PMA (Sigma, St Louis, MO) with 10 µg/ml bound purified hamster anti-mouse CD3e; and 1 ng/ml PMA with 500 nm Ionomycin (Calbiochem-Novabiochem, San Diego, CA). These concentrations were determined by preliminary proliferation assays using various doses to produce proliferation without compromising viability (> 90% viable cells) (data not shown).
Cells were incubated at 37 °C in an atmosphere of 5% CO2 in air for 72 h. For the final 6 h, 1·0 uCi[3H]-thymidine/well (New England Nuclear, North Bellerica, MA) was added to the cultures. Cells were harvested onto glass fibre filter paper discs (Whatman, Maidstone, UK) using a multiple automated sample harvester unit (MASH II; MA Bioproducts, Walkersville, MD). Cellular uptake of[3H]-thymidine was measured using a liquid scintillation counter (LS 8000, Beckman Instruments, Irvine, CA). Results are expressed as the net disintegrations per minute (DPM) (stimulated minus control) of triplicate cultures [24].
Mouse CD4 and CD8 T-cell interleukin-2 and interleukin-4 quantification
Cells were cultured for 48 h as described above. Supernatant fluids from triplicate wells of splenic CD4 and CD8 T cells for each stimulus were harvested, pooled, and stored at − 80 °C. After thawing, supernatant fluids were assayed in triplicate for IL-2 and IL-4 protein using the Mouse IL-2 Immunoassay (ELISA) Kit and Mouse IL-4 Immunoassay (ELISA) Kit (R & D Systems), respectively. Results are expressed as pg/200 000 cells as we have previously described [21].
Statistical analysis
The effects of stimulus, diet and stimulus-by-diet interaction on proliferation, IL-2 and IL-4 concentrations were evaluated using a split plot design, with diets as main plots, stimuli as subplots and mouse spleens or pools of splenocytes as the sampling units. The analysis was done using PROC GLM in SAS [42]. Box-Cox transformations were used in order to facilitate homoscedasticity and normality in the model. Comparisons across stimuli or diet were done using Student–Newman–Keul's Multiple Range test. When the stimulus-by-diet interaction was significant, the effects of diet were investigated separately for the different stimuli. The number of sampling units was usually six per diet, although variations in the efficiency of CD4 and CD8 T-cell recovery following subset column purification sometimes reduced the ‘n’ to four or five. The actual ‘n’ associated with each mean is indicated in each figure legend.
RESULTS
Effect of diet and agonist on murine CD4 T-cell proliferation
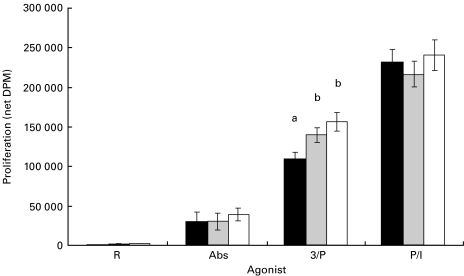
Figure 1 illustrates the proliferative response of CD4 cells taken from mice fed either the SAF, EPA/DHA or FO diets. T cells stimulated with PMA-containing agonists proliferated most vigorously regardless of diet. The net DPM (mean ± s.e.m.) for the three agonists and control summed across diets were: PMA/Ionomycin (P/I) = 230 169 ± 9700; PMA/αCD3 (3/P) = 135 534 ± 7291; αCD3/αCD28 (Abs) = 33 383 ± 5525; RPMI (control) = 852 ± 309; n = 15 per group. Differences in proliferation were significant between all agonist groups (P < 0·0001). CD4 T cells from FO- and EPA/DHA-fed animals stimulated in culture with PMA/αCD3 (3/P) exhibited a significant increase in proliferation relative to cells from SAF-fed animals [FO versus SAF (P = 0·0052); EPA/DHA versus SAF (P = 0·0488)].
Fig. 1.
Effect of dietary fatty acids on CD4 T-cell proliferation. Mice were fed diets enriched in SAF (black bars), EPA/DHA (grey bars) or FO (white bars) for 14 days. Splenic CD4 T lymphocytes were isolated and stimulated with various agonists as described in Materials and methods. Values (n = 5) represent the mean ± s.e.m. of net thymidine uptake (DPM). Different letters denote significant differences within each agonist group (P < 0·05). Abs, αCD3/αCD28 antibodies; 3/P, αCD3/PMA; P/I, PMA/Ionomycin.
Effect of diet and agonist on murine CD8 T-cell proliferation
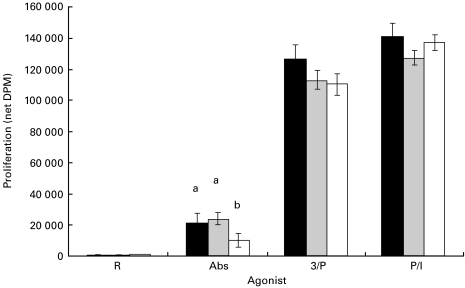
Figure 2 illustrates the proliferative response of CD8 cells taken from mice fed either the SAF, EPA/DHA or FO diets. Purified CD8 T cells were cultured and assessed for proliferation under the same conditions as described for CD4 T cells above. CD8 T cells exhibited a significant diet–stimulus interaction (P = 0·0047), and the two PMA-containing agonists proliferated similarly and most vigorously, regardless of diet. The net DPM (mean ± s.e.m.) for the three agonist groups and control summed across diets were: PMA/Ionomycin (P/I) = 135 432 ± 3530; PMA/αCD3 (3/P) = 116 316 ± 4244; αCD3/αCD28 (Abs) = 18 095 ± 2994; RPMI (control) = 621 ± 68; n = 16 for each group. CD8 T cells from FO-fed animals stimulated in culture with αCD3/αCD28 exhibited a significant decrease in proliferation relative to cells from SAF- and EPA/DHA-fed animals (P = 0·0005 for both comparisons). All other proliferative responses within each agonist group were not significantly affected by diet (P > 0·05).
Fig. 2.
Effect of dietary fatty acids on CD8 T-cell proliferation. Mice were fed diets enriched in SAF (black bars), EPA/DHA (grey bars) or FO (white bars) for 14 days. Splenic CD8 T cells were isolated and stimulated with various agonists as described in Materials and methods. Values (SAF, n = 5; EPA/DHA, n = 5; FO, n = 6) represent the mean ± s.e.m. of net thymidine uptake (DPM). Different letters denote significant differences within each agonist group (P < 0·05). Abs, αCD3/αCD28 antibodies; 3/P, αCD3/PMA; P/I, PMA/Ionomycin.
Effect of diet and agonist on interleukin-2 (IL-2) and interleukin-4 (IL-4) production by CD4 T cells
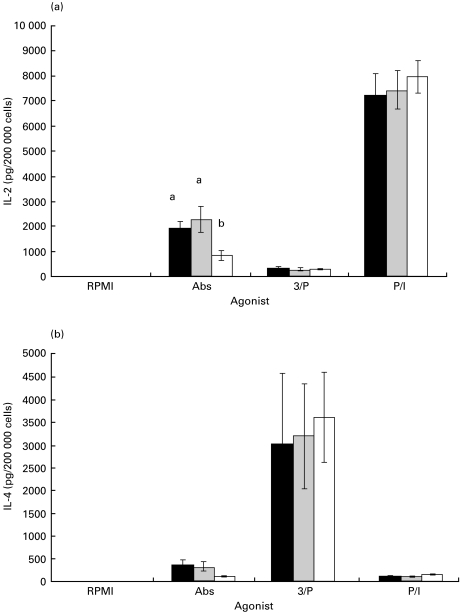
Figure 3(a) illustrates the amount (pg/200 000 cells) of immunoreactive IL-2 protein that was secreted from purified CD4 cells cultured for 48 h in the presence of the same agonists listed under Fig. 1, and assayed as described in Materials and methods. The two PMA-stimulated groups that exhibited the greatest proliferation in Fig. 1 secreted highly different total amounts of IL-2. The means (± s.e.m.) for these two groups summed across diets were: PMA/Ionomycin (P/I) = 7563 ± 412 versus PMA/αCD3 (3/P) = 296 ± 28. The amount of IL-2 secreted by the αCD3/αCD28 (Abs)-stimulated cells was 1710 ± 248, whereas levels in the RPMI (control) group were below those minimally detectable using this assay (3 pg/200 000 cells). CD4 T cells from FO-fed animals secreted significantly less IL-2 compared with SAF- and EPA/DHA-fed animals when the cells were activated via the TcR and CD28 receptors, i.e. αCD3/αCD28 (Abs) (P < 0·0001). Conversely, αCD3/PMA (3/P)- and PMA/Ionomycin (P/I)-stimulated CD4 T cells secreted levels of IL-2 which were not different between diets.
Fig. 3.
Effect of dietary fatty acids on IL-2 (a) and IL-4 (b) production by purified murine splenic CD4 T cells. Mice were fed diets enriched in SAF (black bars), EPA/DHA (grey bars) or FO (white bars) for 14 days, and purified splenic CD4 T cells were cultured with various agonists for 48 h. IL-2 and IL-4 in culture supernatant fluids were quantified by ELISA as described in Materials and methods. Values (n = 4) represent the mean ± s.e.m. in pg/200 000 cells. Different letters denote significant differences within each agonist group (P < 0·05). R, RPMI; Abs, αCD3/αCD28; 3/P, αCD3/PMA; P/I, PMA/Ionomycin.
CD4 T cells treated with αCD3/PMA (3/P) proliferated robustly (Fig. 1), but did not secrete IL-2 as their primary growth factor (Fig. 3a). Since it has been shown that PKC up-regulation via PMA can bias cells in culture towards a Th2 response [38], we assayed culture supernatant fluids for concentrations of immunoreactive IL-4 protein (Fig. 3b) using the same set of culture conditions employed for the IL-2 assays. The means (± s.e.m.) in pg/200 000 cells of secreted IL-4 clearly illustrate that the αCD3/PMA (3/P)-stimulated CD4 cells employed IL-4 as their primary growth factor (3313 ± 648) whereas CD4 cells stimulated with PMA/Ionomycin (P/I) (128 ± 9) or αCD3/αCD28 (Abs) (278 ± 58) did not. Control cells secreted only 3 ± 2 pg/200 000 cells of IL-4. There were no significant differences in IL-4 secretion between dietary groups when analysed separately for each stimulus.
Effect of diet and agonist on interleukin-2 (IL-2) and interleukin-4 (IL-4) production by CD8 T cells
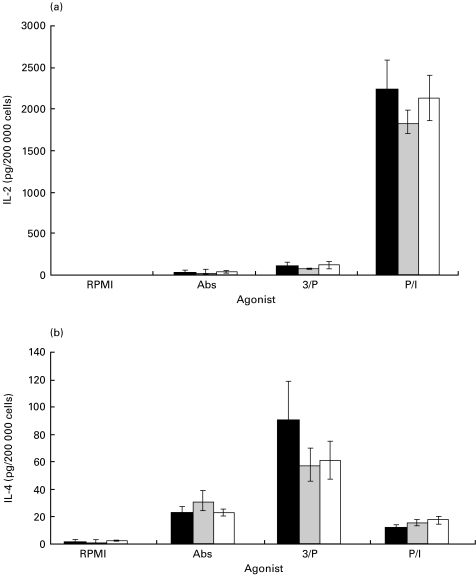
Figure 4(a) illustrates the amount of immunoreactive IL-2 protein secreted from purified CD8 cells cultured for 48 h in the presence of the same agonists listed under Fig. 2. Mean IL-2 concentrations (± s.e.m.) in pg/200 000 cells summed across diets were significantly different (P < 0·0001): PMA/Ionomycin (P/I) = 2078 ± 147; PMA/αCD3 (3/P) = 101 ± 19; αCD3/αCD28 (Abs) = 42 ± 10. Levels of IL-2 secreted from the unstimulated (RPMI) control cells were below the minimal detectable level for the assay used (3 pg/200 000 cells). Once again, as in the case of CD4 T cells, the PMA/Ionomycin (P/I)-stimulated CD8 T cells secreted IL-2 as their primary growth factor, whereas PMA/αCD3-cultured cells did not (Fig. 4a). No dietary effects were observed.
Fig. 4.
Effect of dietary fatty acids on IL-2 (a) and IL-4 (b) production by purified murine splenic CD8 T cells. Mice were fed diets enriched in SAF (black bars), EPA/DHA (grey bars) or FO (white bars) for 14 days, and purified splenic CD8 T cells were cultured with various agonists for 48 h. IL-2 and IL-4 in the culture supernatant fluids were quantified by ELISA as described in Materials and methods. Values (n = 5 or 6) represent the mean ± s.e.m. in pg/200 000 cells. Different letters denote significant differences within each agonist group (P < 0·05). R, RPMI; Abs, αCD3/αCD28; 3/P, αCD3/PMA; P/I, PMA/Ionomycin.
Figure 4(b) illustrates the amount of IL-4 protein secreted by CD8 T cells cultured in the same manner as stated above. There was an overall stimulus effect (P < 0·0001), with mean IL-4 concentrations (± s.e.m.) in pg/200 000 cells summed across diets of: PMA/Ionomycin (P/I) = 15 ± 1; PMA/αCD3 (3/P) = 69 ± 11; αCD3/αCD28 (Abs) = 25 ± 3; the RPMI group secreted IL-4 below the minimal detectable level for this assay (2 pg/200 000 cells). It is apparent that PMA/αCD3 (3/P)-stimulated CD8 T cells employed IL-4 as their principal growth factor (Fig. 4b).
DISCUSSION
Murine CD4 T cells stimulated in culture with PMA/αCD3 apparently produced a Th2 cytokine, IL-4, as their principal growth factor (Fig. 3b), while CD4 T cells stimulated in culture with either αCD3/αCD28 or PMA/Ionomycin displayed a Th1 cytokine profile and secreted IL-2 as their primary growth factor (Fig. 3a). This agonist-dependent polarizing effect allowed us to examine the differential effect of diet on Th1 versus Th2 CD4 subsets. These observations are consistent with recent studies which indicate that PMA-containing agonists have the ability to polarize T cells toward a Th2/Tc2 subset. Th2 cells apparently do not require the mitogen-activated protein (MAP) kinase Jun kinase (JNK) pathway [43]. PMA induces PKC activation and therefore is not a direct activator of the MAP kinase/JNK pathway. This provides one possible mechanistic basis for selective PMA activation of Th2 cells. Yamashita et al. [37] and Funauchi et al. [44] have shown that PMA/Ionomycin polarizes CD4 T cells towards the Th2 phenotype. However, Noble et al. [38] demonstrated that it is the balance of PKC/calcium signalling induced via TcR stimulation which redirects T cells towards the type 1 or type 2 phenotype in primary culture. They showed that PMA combined with a weak calcium signal produced a Th2/Tc2 phenotype, whereas PMA in combination with a strong calcium signal produced the Th1/Tc1 phenotype. Since stimulating the TcR results in a weak calcium signal, in combination with a direct PKC signal from PMA, culturing CD4 T cells with PMA/αCD3 could provide the weak calcium/strong PKC signal reported by Noble et al. [38], and explain the Th2 phenotype which we observed in this study. Conversely, the PMA/Ionomycin agonist was composed of a relatively low PMA concentration relative to Ionomycin (1 ng:500 nm), which would provide a weak PKC/strong calcium signal and possibly explain the Th1 phenotype which we observed in this study. Based on these findings, we hypothesize that the cytokine profiles we observed are a result of agonist polarization during the period of stimulation in vitro, rather than selective activation of a pre-formed population within the culture.
In contrast, in purified CD8 T cells, αCD3/αCD28 and αCD3/PMA (Fig. 4b) stimulated a mixed Tc1/Tc2 cytokine profile, with significant production of both IL-2 and IL-4, whereas PMA/Ionomycin (Fig. 4a) induced primarily a Tc1 profile. This latter effect is likely the result of the same polarizing effect just described for CD4 T cells. Since the nature or intensity of TcR engagement can influence T-cell development [38], we hypothesize that the strength of TcR stimulus received following incubation with both of the αCD3-containing agonists was capable of activating both Tc1 and Tc2 phenotypic differentiation.
We and others have previously shown that dietary n-3 PUFA mediate an anti-inflammatory response, including reduced mitogen-stimulated proliferation and IL-2 production in whole splenocyte or human peripheral blood lymphocyte populations [17,21,45,46]. Somewhat surprisingly, in purified T cells containing a mixture of CD4 and CD8 cells, we did not see a dietary effect on proliferation induced by PMA/αCD3 [22]. However, our most recent results demonstrate that when purified CD4 T cells are stimulated with an agonist (PMA/αCD3) which drives the cells toward the Th2 phenotype (Fig. 3b), we see an increase in proliferation following n-3 PUFA feeding (Fig. 1). This is consistent with earlier observations that point to a down-regulation of pro-inflammatory T cells (Th1) due to the ability of Th2 cells to suppress Th1 responses. Thus, a Th2 population would secrete IL-4 that would inhibit Th1 proliferation.
Another major novel observation in this study is that CD4 T cell IL-2 production is blunted when cells from FO-fed animals are driven toward the Th1 pro-inflammatory phenotype by stimulation with αCD3/αCD28 (Fig. 3a). Thus, under culture conditions which favour an inflammatory Th1 phenotype, dietary FO is capable of mediating a decrease in IL-2 secretion, whereas when the agonist (PMA/αCD3) preferentially induces an anti-inflammatory Th2 phenotype, we see that an FO diet is accompanied by an increase in proliferation of the Th2 subpopulation (Figs 1 and 3b). Thus, the anti-inflammatory effects of dietary n-3 PUFA may be the combined result of two separate mechanisms, i.e. the direct suppression of IL-2-induced Th1 cell activation combined with the indirect suppression of Th1 cells by the enhanced cross-regulatory function of Th2 cells.
Interestingly, IL-2 levels were low while proliferation was high in αCD3/αCD28-stimulated CD4 T cells from FO-fed mice (Figs 1 and Fig 3a). Although we have measured the known principal T-cell growth factors, IL-2 and IL-4, our data indicate that cells from FO-fed animals may take up soluble IL-2 more efficiently, leaving less available in the supernatant fluid for ELISA quantification. However, this hypothesis is not consistent with our earlier findings that levels of IL-2 receptor alpha (IL-2Rα) chain mRNA were reduced in whole splenoctyes from DHA-fed mice, which might cause the cells to be less efficient in IL-2 uptake [21]. To clarify this issue, future experiments need to include a more detailed assessment of the expression of high affinity IL-2R and total T-cell IL-2 protein production by intracellular cytokine staining in our purified T-cell cultures.
Figure 2 demonstrates that αCD3/αCD28 antibody-stimulated CD8 T cells from FO-fed animals exhibited decreased proliferation compared with similar cultures of CD8 T cells taken from SAF- and EPA/DHA-fed animals, and that a mixed Tc1 and Tc2 cytokine-secreting phenotype was present in the cultures (Fig. 4). Thus, dietary FO does not appear to influence a specific CD8 subset, but may mediate an overall decrease in proliferation by CD8 T cells. Significantly, these data are the first contribution to an understanding of the effects of dietary PUFA on CD8 T-cell function since, to our knowledge, no other published studies have examined this effect in purified CD8 T cells.
In murine T cells and the human Jurkat T-cell line, we have previously shown that agonists which engage T-cell membrane receptors reveal a PUFA effect whereas agonists which bypass the membrane demonstrate no such effect [22]. The data presented here support our previous findings, in that agonists which ligated the CD3 or CD28 receptor (either αCD3/αCD28 or PMA/αCD3) showed a dietary effect (Fig,Fig 2,Fig 3), whereas the agonist which bypassed the membrane entirely (PMA/Ionomycin) did not. Thus, it appears likely that n-3 PUFA mediate their effect on T cells, in part, via alterations in membrane lipid composition and receptor signalling functions [11].
The principal dietary effects on CD4 or CD8 T-cell activation observed in this study were seen in mice fed whole FO as opposed to the purified EPA/DHA ethyl ester blend. When a comparison was made of total n-3 PUFA between the two biologically-effective diets in the present study (FO) and the prior T-cell study (DHA) [22], it becomes apparent that the amount of n-3 PUFA in these two diets was similar (∼0·5 g n-3 PUFA/100 g diet). This observation points to the fact that n-3 PUFA are capable of mediating biological effects whether they are part of a purified ethyl ester or a whole oil. Interestingly, the n-3 PUFA content of the biologically-ineffective diets were either lower (0·2 g n-3 PUFA/100 g in the FO diet) or higher (1·7 g n-3 PUFA/100 g in the DHA/EPA diet). Thus, in our model system, there seems to be an optimal dose requirement for total n-3 PUFAs similar to that which was alluded to in a recent review of therapeutic interventions in clinical trials [47].
Taken together, these data support our earlier conclusions that dietary modulation of T-cell function occurs, at least in part, at the level of the T-cell plasma membrane. Furthermore, a comparison of ethyl esters versus whole FO suggests that the amount of total n-3 PUFA contained in the diet is more critical than the vehicle of delivery. More significantly though, we have shown for the first time the differential ability of dietary FO to selectively modulate the T-cell response according to the T-cell subset and cytokine profile. Our results suggest that the well established anti-inflammatory effects of dietary n-3 PUFA may be the net result of two interrelated mechanisms, i.e. the loss of Th1 proliferative capacity due to a reduction in IL-2 production and/or function, and the down-regulation of Th1 proliferation due to enhancement of counter-regulatory, IL-4-driven Th2 cells.
Acknowledgments
This research was supported, in part, by NIH grants R01 DK53055, P30 ES0106, and USDA Hatch Funds (H-6983).
REFERENCES
- 1.Simopoulos AP. Omega-3 fatty acids in health and disease and in growth and development. Am J Clin Nutr. 1991;54:438. doi: 10.1093/ajcn/54.3.438. [DOI] [PubMed] [Google Scholar]
- 2.Kromann N, Green A. Epidemiological studies in the Upernavik District. Greenland Acta Med Scand. 1980;208:401–6. [PubMed] [Google Scholar]
- 3.Cleland LG, French JK, Betts WH, et al. Clinical and biochemical effects of dietary fish oil supplements in rheumatoid arthritis. J Rheumatol. 1988;15:1471–5. [PubMed] [Google Scholar]
- 4.Esperson GT, Grunnet N, Lerang HH, et al. Decreased interleukin-1 beta levels in plasma from rheumatoid arthritis patients after dietary supplementation with n-3 polyunsaturated fatty acids. Clin Rheum. 1992;11:393–5. doi: 10.1007/BF02207200. [DOI] [PubMed] [Google Scholar]
- 5.Kremer JM, Bigauoetta J, Michalek AU, et al. Effects of manipulating dietary fatty acids on clinical manifestations of rheumatoid arthritis. Lancet. 1985;1:184–7. doi: 10.1016/s0140-6736(85)92024-0. [DOI] [PubMed] [Google Scholar]
- 6.van der Tempel H, Tulleken JE, Limburg PC, et al. Effects of fish oil supplementation in rheumatoid arthritis. Ann Rheum Dis. 1990;49:76–80. doi: 10.1136/ard.49.2.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chow SC, Ansotegui IJ, Jondal M. Inhibition of receptor-mediated calcium influx in T cells by unsaturated non-esterified fatty acids. Biochem J. 1990;267:727–32. doi: 10.1042/bj2670727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hughes DA, Southon S, Pinder AC. (n-3) Polyunsaturated fatty acids modulate the expression of functionally associated molecules on human monocytes in vitro. J Nutr. 1996;126:603–10. doi: 10.1093/jn/126.3.603. [DOI] [PubMed] [Google Scholar]
- 9.May CL, Southworth AJ, Calder PC. Inhibition of lymphocyte protein kinase C by unsaturated fatty acids. Biochem Biophys Res Comm. 1993;195:823–8. doi: 10.1006/bbrc.1993.2119. [DOI] [PubMed] [Google Scholar]
- 10.Speizer LA, Watson MJ, Brunton LL. Differential effects of omega-3 fish oils on protein kinase activities in vitro. Am J Physiol. 1991;261:E109–14. doi: 10.1152/ajpendo.1991.261.1.E109. [DOI] [PubMed] [Google Scholar]
- 11.Stulnig TM, Berger M, Sigmund T, et al. Polyunsaturated fatty acids inhibit T cell signal transduction by modification of detergent-insoluble membrane domains. J Cell Biol. 1998;143:637–44. doi: 10.1083/jcb.143.3.637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Webb Y, Hermida-Matsumoto L, Resh MD. Inhibition of protein palmitoylation, raft localization, and T cell signaling by 2-bromopalmitate and polyunsaturated fatty acids. J Biol Chem. 2000;275:261–70. doi: 10.1074/jbc.275.1.261. [DOI] [PubMed] [Google Scholar]
- 13.Jolly CA, Laurenz JC, McMurray DN, et al. Diacylglycerol and ceramide kinetics in primary cultures of activated T-lymphocytes. Immunol Lett. 1996;49:43–8. doi: 10.1016/0165-2478(95)02486-7. [DOI] [PubMed] [Google Scholar]
- 14.Peterson LD, Jeffery NM, Thies F, et al. Eicosapentaenoic and docosahexaenoic acids alter rat spleen leukocyte fatty acid composition and prostaglandin E2 production but have different effects on lymphocyte functions and cell-mediated immunity. Lipids. 1998;33:171–80. doi: 10.1007/s11745-998-0193-y. [DOI] [PubMed] [Google Scholar]
- 15.Calder PC. n-3 polyunsaturated fatty acids and cytokine production in health and disease. Ann Nutr Metab. 1997;41:203–34. doi: 10.1159/000177997. [DOI] [PubMed] [Google Scholar]
- 16.Fritsche KL, Byrge M, Feng C. Dietary omega-3 polyunsaturated fatty acids from fish oil reduce interleukin-12 and interferon-gamma production in mice. Immunol Lett. 1999;65:167–73. doi: 10.1016/s0165-2478(98)00109-6. [DOI] [PubMed] [Google Scholar]
- 17.Meydani SN, Endres S, Woods MM, et al. Oral (n-3) fatty acid supplementation suppresses cytokine production and lymphocyte proliferation: comparison between young and older women. J Nutr. 1991;121:547–55. doi: 10.1093/jn/121.4.547. [DOI] [PubMed] [Google Scholar]
- 18.Virella G, Fourspring K, Hyman B, et al. Immunosuppressive effects of fish oil in normal human volunteers: correlation with the in vitro effects of eicosapentaenoic acid on human lymphocytes. Clin Immunol Immunopathol. 1991;61:161–76. doi: 10.1016/s0090-1229(05)80021-2. [DOI] [PubMed] [Google Scholar]
- 19.Sellmayer A, Danesch U, Weber PC. Effects of different polyunsaturated fatty acids on growth-related early gene expression and cell growth. Lipids. 1996;31:S37–40. doi: 10.1007/BF02637048. [DOI] [PubMed] [Google Scholar]
- 20.Jenski LJ, Scherer JM, Caldwell LD, et al. The triggering signal dictates the effect of docosahexaenoic acid on lymphocyte function in vitro. Lipids. 1998;33:869–78. doi: 10.1007/s11745-998-0283-x. [DOI] [PubMed] [Google Scholar]
- 21.Jolly CA, Jiang YH, Chapkin RS, et al. Dietary (n-3) polyunsaturated fatty acids suppress murine lymphoproliferation, interleukin-2 secretion, and the formation of diacylglycerol and ceramide. J Nutr. 1997;127:37–43. doi: 10.1093/jn/127.1.37. [DOI] [PubMed] [Google Scholar]
- 22.Arrington JL, Switzer KC, Fan YY, et al. Docosahexaenoic acid suppresses function of the CD28 costimulatory membrane receptor in primary murine and Jurkat T cells. J Nutr. 2001;131:1147–53. doi: 10.1093/jn/131.4.1147. [DOI] [PubMed] [Google Scholar]
- 23.DeMarco DM, Santoli D, Zurier RB. Effects of fatty acids on proliferation and activation of human synovial compartment lymphocytes. J Leuk Biol. 1994;56:612–5. doi: 10.1002/jlb.56.5.612. [DOI] [PubMed] [Google Scholar]
- 24.Hosack-Fowler K, Chapkin RS, McMurray DN. Effects of purified dietary n-3 ethyl esters on murine T lymphocyte function. J Immunol. 1993;151:5186–97. [PubMed] [Google Scholar]
- 25.Jeffery NM, Sanderson P, Newsholme EA, et al. Characteristics of lipid and lymphocytes collected from the lymph of rats fed a low fat diet or high fat diets rich in n-6 or n-3 polyunsaturated fatty acids. Nutr Res. 1998;18:299–308. [Google Scholar]
- 26.Jenski LJ, Bowker GM, Johnson MA, et al. Docosahexaenoic acid-induced alteration of Thy-1 and CD8 expression on murine splenocytes. Biochim Biophys Acta. 1995;1236:39–50. doi: 10.1016/0005-2736(95)00034-z. [DOI] [PubMed] [Google Scholar]
- 27.Sanderson P, Yaqoob P, Calder PC. Effect of dietary lipid manipulation upon graft vs host and host vs graft responses in the rat. Cell Immunol. 1995;164:240–7. doi: 10.1006/cimm.1995.1167. [DOI] [PubMed] [Google Scholar]
- 28.Sanderson P, Yaqoob P, Calder PC. The effect of dietary lipid manipulation upon rat spleen lymphocyte functions and expression of lymphocyte surface markers. J Nutr Environ Med. 1994;5:199–32. [Google Scholar]
- 29.Lim BO, Jolly CA, Zaman K, et al. Dietary (n-6) and (n-3) fatty acids and energy restriction modulate mesenteric lymph node lymphocyte function in autoimmune-prone (NZB x NZW) F1 mice. J Nutr. 2000;130:1657–64. doi: 10.1093/jn/130.7.1657. [DOI] [PubMed] [Google Scholar]
- 30.Cerwenka A, Carter LL, Reome JB, et al. In vivo persistence of CD8 polarized T cell subsets producing Type 1 or Type 2 cytokines. J Immunol. 1998;161:97–105. [PubMed] [Google Scholar]
- 31.Mosmann TR, Cherwinski H, Bond MW, et al. Two types of murine helper T cell clone. I. Definition according to profiles of lymphokine activities and secreted proteins. J Immunol. 1986;136:2348–57. [PubMed] [Google Scholar]
- 32.Manickasingham SP, Anderton SM, Burkhart C, et al. Qualitative and quantitative effects of CD28/B7-mediated costimulation on naïve T cells in vitro. J Immunol. 1998;161:3827–35. [PubMed] [Google Scholar]
- 33.Moreland LW, Heck LW, Koopman WJ. Biologic agents for treating rheumatoid arthritis. Arthr Rheum. 1997;40:397–409. doi: 10.1002/art.1780400302. [DOI] [PubMed] [Google Scholar]
- 34.Salgame P, Abrams JS, Clayberger C, et al. Differing lymphokine profiles of functional subsets of human CD4 and CD8 T cell clones. Science. 1991;254:279–82. doi: 10.1126/science.254.5029.279. [DOI] [PubMed] [Google Scholar]
- 35.Erard F, Garcia-Sanz JA, Moriggl R, et al. Presence or absence of TGF-β determines IL-4-induced generation of type 1 or type 2 CD8 T cell subsets. J Immunol. 1999;162:209–14. [PubMed] [Google Scholar]
- 36.Sad S, Krishnan L. Cytokine deprivation of naïve CD8+ T cells promotes minimal cell cycling but maximal cytokine synthesis and autonomous proliferation subsequently: a mechanism of self-regulation. J Immunol. 1999;163:2443–51. [PubMed] [Google Scholar]
- 37.Yamashita M, Katsumata M, Iwashima M, et al. T cell receptor-induced calcineurin activation regulates T helper type 2 cell development by modifying the interleukin 4 receptor signaling complex. J Exp Med. 2000;191:1869–79. doi: 10.1084/jem.191.11.1869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Noble A, Truman JP, Vyas B, et al. The balance of protein kinase C and calcium signaling directs T cell subset development. J Immunol. 2000;164:1807–13. doi: 10.4049/jimmunol.164.4.1807. [DOI] [PubMed] [Google Scholar]
- 39.Schmidt D, Goronzy JJ, Weyand CM. CD4+ CD7–CD28- T cells are expanded in rheumatoid arthritis and are characterized by autoreactivity. J Clin Invest. 1996;97:2027–37. doi: 10.1172/JCI118638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.National Research Council. Nutrient requirements of laboratory animals. 4. Washington D.C: National Academy Press; 1995. Nutrient requirements of the mouse; pp. 80–102. [Google Scholar]
- 41.Coligan JE, Kruisbeck AM, Margueles DH, et al. Current Protocols in Immunology. I. John Wiley & Sons Inc.; 1992. Immunofluorescence and cell sorting; pp. 5.4.1–5.4.12. [Google Scholar]
- 42.Harry NC, Helwig JT, Council KA. Statistical system user's guide. Cary, NC: SAS Institute; 1990. [Google Scholar]
- 43.Yang DD, Conze D, Whitmarsh AJ, et al. Differentiation of CD4+ T cells to Th1 cells requires MAP kinase JNK2. Immunity. 1998;9:575–85. doi: 10.1016/s1074-7613(00)80640-8. [DOI] [PubMed] [Google Scholar]
- 44.Funauchi M, Ikoma S, Enomoto H, et al. Decreased Th1-like and increased Th2-like cells in systemic lupus erythematosus. Scand J Rheum. 1998;27:219–24. doi: 10.1080/030097498440859. [DOI] [PubMed] [Google Scholar]
- 45.Kumar GS, Das UN, Kumar KV, et al. Effect of n-6 and n-3 fatty acids on the proliferation of human lymphoctyes and their secretion of TNF-α and IL-2 in vitro. Nutr Res. 1992;12:815–23. [Google Scholar]
- 46.Lau CS, Morley KD, Belch JJF. Effects of fish oil supplementation on non-steroidal anti-inflammatory drug requirement in patients with mild rheumatoid arthritis—a double-blind placebo controlled study. Br J Rheum. 1993;32:982–9. doi: 10.1093/rheumatology/32.11.982. [DOI] [PubMed] [Google Scholar]
- 47.Kremer JM. n-3 Fatty acid supplements in rheumatoid arthritis. Am J Clin Nutr. 2000;71:S349–51. doi: 10.1093/ajcn/71.1.349s. [DOI] [PubMed] [Google Scholar]