Abstract
A major problem in the management of SLE patients is to predict a flare or to distinguish between active and quiescent disease. Serological markers are widely used to assess disease activity, but many patients have close to or normal values for these parameters while exhibiting obvious disease-related signs and symptoms. This study aimed to determine which serological parameters, among ESR, ANA and anti-dsDNA antibody titres, CH50 and the HLA-DR expression on circulating T-lymphocyte subsets, best reflected the development of SLE flares. Sixty SLE patients were included, 34 with quiescent disease throughout the entire follow-up period and 26 who experienced an SLE flare defined as having active disease. According to univariate analysis, all parameters were significantly higher for patients with active disease, with the percentage of CD8+DR+ cells being the most significant parameter (P = 10−7). Multivariate logistic regression analysis identified three independent variables enabling the identification of a lupus flare: CH50, the CD8+DR+ and CD4+DR+ cell percentages among total lymphocytes. The CD8+DR+ cell percentage is the biological parameter most significantly associated with a flare (P < 0·001), even more powerful than CH50 (P < 0·01). HLA-DR expression on CD8+ lymphocytes clearly coincided with disease evolution in seven patients enrolled as having quiescent disease, but who experienced one flare during follow-up that subsequently resolved. The percentage of circulating CD8+DR+ lymphocytes appears to be a biological marker which accurately reflects disease activity. A larger prospective study is needed to demonstrate the real efficacy of this marker in predicting an exacerbation in SLE patients.
Keywords: systemic lupus erythematosus, lymphocyte activation, HLA-DR, anti-dsDNA antibodies
INTRODUCTION
Systemic lupus erythematosus (SLE) is a chronic, multi-organ, autoimmune disease characterized by clinical flares and remission, and functional alterations of both B and T lymphocytes. A major problem in the management of SLE patients arises from the inability for the clinician to predict a relapse or to distinguish between active and quiescent disease. The presence of distinct symptoms and/or clinical signs may suggest flares of the SLE, but objective assessment of disease activity remains elusive. Several validated indices based on clinical features, such as systemic lupus activity measure (SLAM) or systemic lupus evaluation disease activity index (SLEDAI) are useful for assessing disease activity [1]. However, these scoring systems are founded on the presumption that symptoms and/or signs are attributable solely to disease activity and are influenced by the user's experience. To escape these drawbacks, investigators tried to identify additional parameters as indicators of SLE activity. Serological markers, such as anti-double-stranded DNA antibodies (dsDNA antibodies), and laboratory indices, such as erythrocyte sedimentation rate (ESR) or C3 levels, are widely used as markers of disease activity, but many patients have normal or almost normal values for these parameters while they present obvious disease-related signs and symptoms. For example, anti-dsDNA antibodies were not detected in more than 40% of SLE patients [2], whereas up to 15% of clinically-asymptomatic patients had high anti-dsDNA antibody titres [3,4]. Finally, some patients, such as those with central nervous system lupus or high SLEDAI but low anti-dsDNA antibody titres, have also been described [5]. Thus, the search continues for markers with the potential to predict and monitor disease activity.
Because of their pivotal role in the immune response, T lymphocytes have been examined with this aim in mind and increased classical signs of T-cell activity, such as the level of soluble interleukin-2 receptor (IL-2R) in serum [6,7], or up-regulation of HLA-DR and IL-2R on circulating T lymphocytes [8,9], have been reported. However, to date, HLA-DR expression on T-cell subsets in SLE patients has never been studied in a large cohort and has never been compared with other classical parameters used in routine practice.
The purpose of this study was to determine which serological parameters, among ESR, antinuclear (ANA) and anti-dsDNA antibody levels, total serum haemolytic complement (CH50) and the HLA-DR expression on subsets of peripheral blood T lymphocytes, best reflected the development of clinically-active exacerbations of the SLE.
PATIENTS AND METHODS
Patients
Sixty SLE patients routinely followed in an Internal Medicine Department were included in the present study between September 1996 and December 1999. Patients met at least four of the American Rheumatism Association (ARA) 1982 revised criteria for SLE [10]. Except for two patients originating from Africa and one from Asia, all of them were Caucasian. For each visit, patients were clinically evaluated for the presence of signs and symptoms of SLE, multiple organ system involvement, and underwent laboratory tests including urinalysis, and blood electrolytes, creatinine, ESR, blood cell count, T-cell subset determinations, ANA titre, anti-dsDNA antibody titre and CH50.
Clinical disease activity was scored using SLEDAI, which includes both clinical and laboratory features of SLE [11,12]. Two groups of patients were defined. The active disease group included patients with a disease flare, which was defined as a minimal three-point increase of the SLEDAI score compared with the last examination [13], or patients at diagnosis. The quiescent group included patients with no variations of their SLEDAI throughout the entire follow-up period. At the time of physical examination and blood tests, some SLE patients were being treated with oral prednisone, azathioprine and/or hydroxychloroquine (see Table 1). After physical and blood examinations, patients diagnosed as having disease exacerbations with major organ involvement were treated with intravenous methylprednisolone and/or intravenous cyclophosphamide.
Table 1.
Summary of the demographic, clinical and therapeutic characteristics of SLE patients
| At blood-sampling time | |||||
|---|---|---|---|---|---|
| Patient | Sex | Age (years) | Diagnosis according to ARA criteria | SLEDAI | Treatment |
| With quiescent disease | |||||
| 1 | F | 30 | A, Sk,P | 6 | Pred = 20 mg, Hycl |
| 2 | F | 27 | Bl, A | 4 | Pred < 10 mg |
| 3 | F | 25 | Sk, Bl | 2 | Pred = 20 mg |
| 4 | F | 54 | A, Bl | 6 | Pred = 20 mg |
| 5 | F | 21 | A, Sk | 4 | None |
| 6 | F | 59 | Sk, A | 6 | Pred = 25 mg |
| 7 | F | 22 | P, A, NP | 6 | Pred = 10 mg, Hycl |
| 8 | F | 36 | P, A | 2 | Hycl |
| 9 | F | 71 | A, Sk, APS | 4 | Pred = 20 mg |
| 10 | F | 16 | A, NP | 4 | Pred = 15 mg |
| 11 | F | 45 | Bl, A | 3 | Pred < 10 mg |
| 12 | F | 46 | A, Sk, APS | 6 | None |
| 13 | F | 33 | NP, Sk | 2 | None |
| 14 | M | 33 | Sk, A | 6 | Pred > 15 mg |
| 15 | F | 24 | K, Sk, A | 6 | Pred = 10 mg, Aza |
| 16 | F | 50 | A, P | 6 | NSAID |
| 17 | F | 31 | Bl, APS, Sk | 4 | None |
| 18 | F | 22 | A, P | 4 | Pred = 15 mg |
| 19 | F | 26 | K, A | 4 | Pred < 10 mg, Aza |
| 20 | F | 34 | K, A | 4 | Pred = 10 mg |
| 21 | F | 25 | A, Sk | 2 | None |
| 22 | F | 32 | A, K, P | 2 | None |
| 23 | F | 31 | A, Sk | 4 | Hycl |
| 24 | F | 50 | P, A | 2 | Pred < 10 mg |
| 25 | F | 41 | Bl, A, NP | 5 | Pred < 10 mg, Hycl |
| 26 | F | 59 | Sk, A, APS | 3 | None |
| 27 | F | 40 | A, Sk, Bl | 3 | None |
| 28 | M | 44 | P, Bl | 2 | None |
| 29 | F | 41 | APS, A, P | 3 | Pred > 12·5 mg, Aza |
| 30 | F | 74 | A, P, Bl | 2 | Pred = 15 mg |
| 31 | F | 48 | APS, NP | 1 | Pred = 30 mg |
| 32 | F | 30 | Sk, A, APS | 2 | Pred < 10 mg |
| 33 | F | 30 | Sk, Bl, APS | 2 | None |
| 34 | F | 40 | Sk, A, Bl | 3 | None |
| With active disease | |||||
| 101 | M | 79 | K, A | 12 | None |
| 102 | F | 38 | A, P | 12 | None |
| 103 | F | 40 | Sk, A, K | 27 | None |
| 104 | F | 19 | Sk, A | 10 | None |
| 105 | F | 29 | Sk, A | 8 | Pred < 10 mg |
| 106 | M | 23 | A* K* | 25 | NSAID |
| 107 | F | 32 | A*, K*, Sk | 23 | Pred < 10 mg, Aza |
| 108 | F | 26 | A, P | 15 | None |
| 109 | F | 28 | K*, A* | 18 | Pred. = 20 mg |
| 110 | F | 21 | A*, Sk* | 10 | Pred < 10 mg |
| 111 | F | 28 | A*, Sk, P* | 11 | Pred < 10 mg |
| 112 | F | 31 | NP, A | 12 | Pred < 10 mg |
| 113 | F | 38 | A*, Sk* | 9 | Pred < 10 mg, Hycl |
| 114 | F | 65 | Bl, NP*, P | 18 | Pred < 10 mg, Aza |
| 115 | F | 52 | P, A | 8 | Pred < 10 mg |
| 116 | F | 45 | A*, Sk* | 11 | Pred < 10 mg |
| 117 | F | 29 | A*, Sk* | 10 | NSAID |
| 118 | F | 35 | K, P, Bl | 23 | None |
| 119 | F | 16 | Bl, A | 9 | None |
| 120 | F | 36 | A, Sk | 12 | NSAID |
| 121 | F | 30 | NP*, A | 25 | Pred = 15 mg, Hycl |
| 122 | F | 71 | Bl, Sk, A | 12 | None |
| 123 | F | 37 | A, P | 8 | None |
| 124 | F | 55 | Sk*, A*, Bl* | 12 | NSAID |
| 125 | F | 20 | A, APS, Bl | 12 | None |
| 126 | F | 64 | A*, Bl* | 8 | Pred < 10 mg |
Organ affected during the flare (only for the patients having a SLE before the flare; for the others, all mentioned organs in the ARA criteria are involved because it is at diagnosis). A, musculoskeletal system; K, renal disease; Sk, mucocutaneous lesions; Bl, haematological abnormality; NP, neuropsychiatric disorders; P, pericarditis; APS, antiphospholipid syndrome. Pred, prednisone expressed as dose/24 h; Aza, Azathioprine; Hycl, Hydroxychloroquine; NSAID, nonsteroidal anti-inflammatory drug.
For patients who presented a flare (n = 26), the concomitant or the closest biological variables measured within 2 weeks of the clinical episode were considered for statistical analyses. Most of the 26 patients classified as having active disease were enrolled at the date of diagnosis (n = 14), whereas five were seen at our institution for the first time during a flare but had an SLE previously diagnosed, and seven others, who had been enrolled as quiescent disease, developed one SLE flare during the observation period that subsequently resolved during follow-up. For patients with quiescent disease throughout the entire follow-up period (n = 34), the last biological variables were taken for statistical analyses to avoid any potential impact of intercurrent illness not diagnosed by the end of this follow-up.
Antinuclear antibodies
ANA titres (serum dilution) were measured by indirect immunofluorescence microscopy on human epithelioid cells (HEp-2 cells, Gull, Bad Homburg, Germany).
Anti-dsDNA antibodies
Anti-dsDNA antibodies were detected and quantified using a commercially available ELISA (DNA-LISA, Biomedical Diagnostics, Paris, France). Titres are expressed as IU/ml. The positive threshold for this test was 10 IU/ml.
Complement activity
The CH50 in patients' sera was determined using kinetic (1996) or endpoint methods (1997–99) on sheep red blood cells by spectrophotometric (Spectrophotometer, SAFAS, Monaco) measurement of haemoglobin levels after red cell lysis. Results are expressed as percentages of the reference pooled normal sera, with normal values of 100 ± 25%.
Flow cytometric analysis
In a 5 ml polystyrene tube (Becton Dickinson, Mountain View, CA), 0·02 ml of the fluorescent-labelled monoclonal antibody directed against CD3 (fluorecein isothiocyanate (FITC)-conjugated, Becton Dickinson Cat no. 349201) or HLA-DR (phycoerythrin (PE)-conjugated, Becton Dickinson Cat no. 347367) was added to 0·1 ml of whole blood collected the same day on sodium ethylenediaminetetraacetic acid (EDTA) from patients (Vacutainer, Becton Dickinson). Another tube was also set up which included a triple combination of CD4-FITC (Becton Dickinson Cat no. 340133), CD8-peridinin-chlorophyll (PerCP) (Becton Dickinson Cat no. 347314) and DR-PE.
After a 15 min incubation at room temperature in the dark, the red blood cells were lysed and the white blood cells were fixed by adding 2 ml of the Facs lysing solution (Becton Dickinson Cat no. 349202), diluted 1/10 with distilled water, and left at room temperature for another 10 min; white blood cells were then washed once with phosphate-buffered saline prior to analysis in a four-colour FacsCalibur instrument (Becton Dickinson).
Dot plots from 5000 white blood cells gated on forward scatter (FSC) and side scatter (SSC), or SSC/CD3+ cells, were generated with the CellQuest sofware (Becton Dickinson) provided with the apparatus. Total double-positive CD3+ and DR+ cells obtained from the first tube, or total CD4+ and DR+, or total CD8+ and DR+ cells obtained from the second tube, were expressed as percentages of total lymphocytes gated on FSC and SSC in both cases. Positivity thresholds were defined using double-negative cells for CD3−DR−, CD4−DR− or CD8−DR− dot-plots.
Statistical analysis
ANA and anti-dsDNA antibody titres, CH50, ESR, and CD3+DR+, CD4+DR+ and CD8+DR+ T-cell percentages for both patient groups, were compared using the non-parametric Mann–Whitney U-test, with a level of significance of 0·05. To identify the statistically significant independent variables with the aim of characterizing the disease activity, we used a multivariate logistic regression model that took into account only the parameters found by univariate analysis to be associated with active disease. The tests were performed with the statistical software Statistica Inc. (Statsoft, Tucson, AZ).
RESULTS
Patients
All 60 patients fulfilled at least four of the ARA 1982 revised criteria for SLE disease (Table 1). All but four were women and their ages ranged from 16 to 79 years, with a mean of 37·95 years. The duration of the disease ranged from 1 month to 12 years.
Although 34 patients had quiescent disease throughout the entire follow-up period, their prior histories included pericarditis or glomerulonephritis for 10 and 4 patients, respectively, whereas 5 patients had central nervous system involvement. Twenty-eight patients had polyarthritis, 17 had cutaneous manifestations and eight had antiphospholipid syndrome. Biologically, 11 patients were thrombopenic (≤ 110 000/mm3), and anticardiolipin antibodies were detected in eight cases. All patients had ANA titres ≥ 1/250, and anti-dsDNA antibody titres were detected in 13 patients. CH50 was low in six patients.
Among the 26 patients with active disease, 14 were considered to be active at diagnosis, five exhibited a flare but had a SLE previously diagnosed in another institution, and seven experienced a flare during follow-up. The past clinical histories of these patients included six renal biopsies which revealed SLE-related nephropathies. In addition, 24 patients had arthritis, 12 had cutaneous involvement, three had neurological disease and seven had pericarditis. Seven were thrombopenic. Anticardiolipin antibodies were detected in two, but only patient 125 had antiphospholipid syndrome. ANA titres were ≥ 1/500 in all patients except patient 113, and CH50 was low in 14 patients. Anti-dsDNA antibody titres were detected in 21 cases.
Univariate analysis
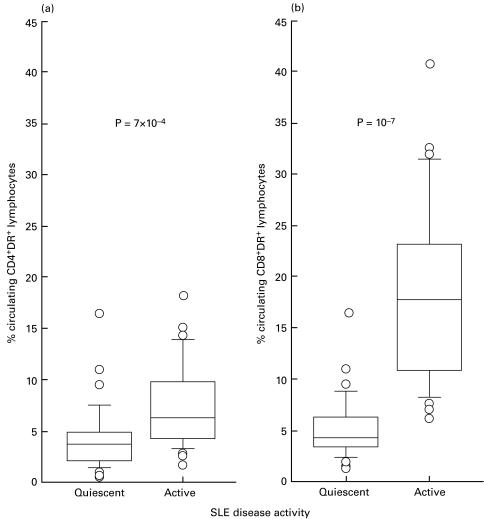
ESR, ANA and anti-dsDNA antibody titres, CH50 percentages and percentages of DR+-lymphocyte subpopulations were compared between patients with quiescent disease and those with active disease using the non-parametric Mann–Whitney U-test (Table 2). According to this univariate analysis, all parameters were significantly higher for patients with active disease. As shown in Table 2, the percentage of CD3+DR+ cells was significantly higher in patients with active disease in comparison with those with quiescent disease, and the contribution of the CD8+ subpopulation (Fig. 1b) was even more significant than that of the CD4+ subset (Fig. 1a).
Table 2.
Comparison of ESR, ANA, anti-dsDNA Ab, CH50 and the percentages of lymphocyte subsets between active and quiescent SLE
| Parameters | Quiescent disease (n = 34) | Active disease (n = 26) | P* |
|---|---|---|---|
| ESR (mm/1st h) | |||
| Median | 14 | 42 | 0·00012 |
| [range] | [2–120] | [3–120] | |
| (25th, 75th percentiles) | (8, 22) | (20, 62) | |
| ANA (serum dilution) | |||
| Median | 500 | 8000 | 0·000003 |
| [range] | [250–16000] | [250–64000] | |
| (25th, 75th percentiles) | (500, 2000) | (2000, 32 000) | |
| Anti-dsDNA antibody (IU/ml) | |||
| Median | 0 | 22 | 0·001 |
| [range] | [0–618] | [0–459] | |
| (25th, 75th percentiles) | (0, 20) | (13, 63) | |
| CH50 | |||
| (% of normal pooled sera) | |||
| Median | 90 | 70 | 0·009 |
| [range] | [46–143] | [12–136] | |
| (25th, 75th percentiles) | (78, 106) | (60, 90) | |
| % CD3+DR+ lymphocytes† | |||
| Median | 9·2 | 22·7 | 0·000001 |
| [range] | [3·58–36·2] | [10·66–55·78] | |
| (25th, 75th percentiles) | (7·16, 12·44) | (20·06, 30·00) | |
| % CD3+CD4+DR+ lymphocytes | |||
| Median | 4·63 | 7·34 | 0·0007 |
| [range] | [1·48–17·3] | [2·66–19·06] | |
| (25th, 75th percentiles) | (3·04, 5·78) | (5·16, 10·59) | |
| % CD3+CD8+DR+ lymphocytes | |||
| Median | 4·33 | 17·52 | 0·0000001 |
| [range] | [1·3–16·08] | [6·56–40·86] | |
| (25th, 75th percentiles) | (3·46, 6·34) | (9·44, 19·90) | |
Comparisons were made with the nonparametric Mann–Whitney U-test.
Among total lymphocytes.
Fig. 1.
Comparisons of the percentages of circulating CD4+DR+ (a) and CD8+DR+ (b) T-cell subsets among total lymphocytes between patients with active (n = 24) and quiescent (n = 36) SLE (non-parametric Mann–Whitney U-test). The medium bar within the box represents the median value. The outlines of the boxes show the 25% and the 75% percentiles, while the bars outside the boxes represent the 10% and 90% percentiles. (○), Values outside this range. In (a), P = 7 × 10−4; in (b), P = 10−7.
As CD3+CD4+ and CD3+CD8+ T cells are the major contributors to the CD4+ and CD8+ subsets, and represent the parent populations from which we subclassified the DR+ cells, we also compared their percentages and absolute counts between quiescent and active disease. The respective median absolute counts of total CD3+ cells (1289/mm3 versus 1024/mm3; P = 0·07) and CD3+CD8+ cells (388/mm3 versus 392/mm3; P = 0·81) did not differ significantly between groups, whereas patients with active disease had significantly lower median CD3+CD4+ counts (766/mm3 versus 554/mm3; P = 0·004). This latter difference accounted for the slight difference observed between the total CD3+ counts and thus excluded the participation of any other CD3+ subpopulation. The profile was similar when subset percentages for patients with quiescent versus active SLE were considered. The respective median percentages of CD3+ T cells (77·95% versus 75·95%; P = 0·34) did not differ between groups, whereas the median percentages of CD3+CD4+ T cells (49·30% versus 40·88%; P = 0·0005) were highly significantly different. Concerning median CD3+CD8+ T-cell percentages (21·68% for quiescent versus 29·70% for active disease; P = 0·002), the difference was statistically significant, but the overall variations of the CD3+CD4+ and CD3+CD8+ subsets within the total CD3+ population did not allow a distinction to be made between a rise of CD8+ or a fall of CD4+ T cells during a disease flare.
In the active SLE group using the Mann–Whitney test, we did not find any statistically significant difference in the percentage of DR+ CD8+ lymphocytes between the subgroup of patients receiving treatment (n = 16) and the subgroup of non-treated patients (n = 10) (P = 0·67).
Multivariate analysis
Multivariate logistic regression analysis was conducted to characterize the independent variables selected by univariate analysis that would identify a lupus flare. Only three were found to be independent: CH50, CD8+DR+ and CD4+DR+ cell percentages (Table 3). However, the CD8+DR+ cell percentage appeared to be the best biological parameter associated with a lupus flare, even more powerful than the CH50%.
Table 3.
Results of the multivariate analysis
| Variables | β coefficient | P |
|---|---|---|
| CH50 | − 0·024 | < 0·01 |
| CD8+DR+ | 0·149 | < 0·001 |
| CD4+DR+ | 0·063 | < 0·01 |
βcoefficient: coefficient automatically attributed to the independent variables selected by the multivariate analysis.
Variations in the size of the CD8+DR+ lymphocyte subset in relation to the SLE flare
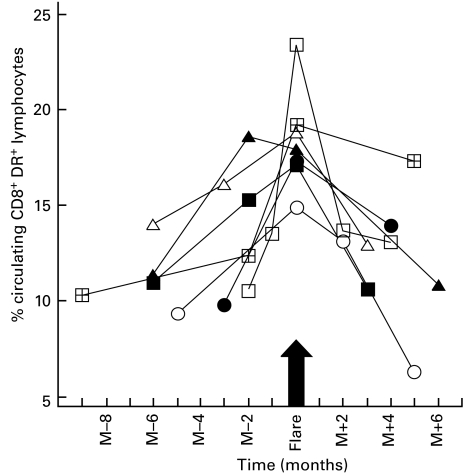
Seven active-disease patients who developed one exacerbation during the follow-up period gave us the opportunity to analyse serially the blood samples drawn before, during and after these episodes. To assess the kinetics of lymphocyte-subset activation, we analysed individual changes in HLA-DR expression on lymphocyte subsets (Fig. 2) in parallel with variations of ANA, anti-dsDNA antibody or CH50 levels (Table 4). HLA-DR expression on CD8+ lymphocytes clearly increased during the 1–3 months preceding the flare in these patients and peaked at the time that they were consulting for disease-related symptoms.
Fig. 2.
Longitudinal follow-up of the percentages of circulating CD8+DR+ T cells among total lymphocytes in patients with active SLE (n = 7). □ Patient 106; ▵ patient 110; ○ patient 111; ▪ patient 113; ▴ patient 114; • patient 117; ⊞ patient 121.
Table 4.
Longitudinal survey of seven patients with active SLE
| Patient | Time* | ANA (dilution) | Anti-dsDNA antibody (IU/ml) | CH50 (%) | CD8+DR+ cells (%) |
|---|---|---|---|---|---|
| 106 | − 2 | 4000 | 359 | 45 | 10·60 |
| − 1 | 4000 | 380 | 35 | 13·50 | |
| Flare | 4000 | 459 | 27 | 23·36 | |
| + 2 | 1000 | 35 | 80 | 13·66 | |
| + 4 | 500 | 21 | 120 | 13·06 | |
| 110 | − 6 | 8000 | 10 | 66 | 14·02 |
| − 3 | 16000 | 45 | ND† | 16·06 | |
| Flare | 32000 | 121 | 67 | 18·78 | |
| + 3 | 8000 | 15 | 70 | 12·90 | |
| 111 | − 5 | 32000 | 75 | 65 | 9·36 |
| Flare | 32000 | 60 | 90 | 14·86 | |
| + 2 | 16000 | 45 | 80 | 13·12 | |
| + 5 | 32000 | 166 | 80 | 6·30 | |
| 113 | − 6 | 250 | 0 | 96 | 10·98 |
| − 2 | 250 | 0 | 82 | 15·28 | |
| Flare | 250 | 0 | 80 | 17·12 | |
| + 3 | 250 | 0 | 92 | 10·62 | |
| 114 | − 6 | 500 | 0 | 124 | 11·39 |
| − 2 | 500 | 0 | 80 | 18·56 | |
| Flare | 500 | 0 | 70 | 17·92 | |
| + 6 | 100 | 0 | 88 | 10·84 | |
| 117 | − 3 | 2000 | 60 | 100 | 9·84 |
| Flare | 2000 | 37 | 75 | 17·28 | |
| + 4 | 1000 | 58 | 75 | 13·96 | |
| 121 | − 9 | 16000 | 78 | 65 | 10·27 |
| − 2 | 8000 | 85 | 78 | 12·36 | |
| Flare | 8000 | 32 | 65 | 19·20 | |
| + 5 | 16000 | 37 | 86 | 17·30 |
Time points are expressed in month(s) before flare (− number) and after flare (+ number).
Not determined. For other abbreviations, see text.
During this period, ANA titres rose before the SLE flare only in patient 110, who was also the only patient to have had a substantial concomitant increase of her anti-dsDNA antibody titre (positive for five patients), while CH50 values declined in three patients (106, 113 and 114), excluding patient 110.
DISCUSSION
SLE is an autoimmune disorder of unknown aetiology which is characterized by a variety of autoantibodies to nuclear components (ANA) and, among them, anti-dsDNA antibodies are specific to the disease [14]. A major clinical problem faced by physicians treating SLE patients is to identify those who will experience severe life-threatening disease or develop with certitude an exacerbation of the disease. To address these issues, several potential markers of disease activity have been proposed, such as routine immunology laboratory parameters (ANA and anti-dsDNA antibodies, complement components) [15,16], cytokine levels and soluble receptors in the serum [6,17–19], or induced protein expression on the surface of activated lymphocytes [9,20,21]. Among these markers, several are either not routinely available or have turned out to be unreliable. In routine practice, ESR, serum complement levels and anti-dsDNA antibodies are widely accepted and used as indicators of SLE activity. However, while some patients may present abnormal values for these markers for considerable periods of time, they might not yet have developed clinical symptoms or functional deterioration of a major organ. Conversely, others are markedly symptomatic with only minor abnormalities in these test results. Moreover, it is commonly accepted that the appearance of anti-dsDNA antibodies does not necessarily correlate with disease activity [22]. Therefore, a reliable marker of SLE activity is still needed. In this regard, many of the clinical features of SLE are considered to be the result of B-cell hyperactivity, but T-cell activation also plays a pivotal role in this phenomenon and is held responsible for the subsequent B-cell activation, cytokine secretion and antibody production [23]. Among the ‘T-cell activation antigens’, the HLA-DR antigen, expressed primarily on B lymphocytes and monocytes, can be found at the cell surface of a significant percentage of T cells upon activation. These DR antigens, which are cell-surface glycoproteins encoded for by genes of the HLA-DR region of the major histocompatibility complex, are normally absent on resting T cells [24], and may represent a potential marker of the immune system activation, as has been proposed for post-transplant monitoring [25]. This hypothesis was partially supported by earlier reports [8,26,27].
In this present study, we re-examined DR expression on T-lymphocyte subsets as a potential marker of SLE activity on a large number of SLE patients, and compared it with the biological parameters that were usually found to coincide with the development of clinical exacerbations. The most salient result came from the observation that a significantly higher percentage of CD8+ cells bore the DR+ molecule in patients with active SLE than in patients with quiescent disease. Pertinently, according to a multivariate analysis, DR expression on CD8+ T lymphocytes was also the laboratory parameter most closely associated with disease exacerbation. CD8+ T-cell activation could not be explained by an on-going infectious illness capable of stimulating the immune system which, if present, would have blurred the interpretation of this enhanced DR expression and restricted the applicability of this variable. On the same ground, we did not observe any influence of the treatment on the DR+ lymphocyte percentage magnitude during flares.
In a normal population of 101 individuals of 18 to 70 years old, HLA-DR expression was detected on total CD3+ T lymphocytes with a median value of 10%, and the 25th and 75th percentiles at 8% and 15%, respectively [28], thus demonstrating that this value varies widely among individuals, reflecting their own immune status at the time of the determination. This variation was also found in our study and was further illustrated by the seven patients we were able to monitor before, at the time of, and after a flare. Although they all showed a clear elevation in the percentage of circulating CD8+DR+ T cells among total lymphocytes concomitantly with the flare, they did not have similar percentages before it, thereby suggesting that, for a given patient, the CD8+DR+ percentage will only be useful in the context of continuous monitoring, with the patient serving as his own control. A few groups have reported on HLA-DR expression on lymphocytes from SLE patients and either compared it with normal controls, or compared active versus quiescent diseases [9,26,29–31]. Discrepancies in the increase of CD3+DR+ cells exist and may be related to the small numbers of patients studied [27,30]. The differential expression of HLA-DR on CD4+ versus CD8+ cells led to the conclusion that the DR antigen is preferentially expressed on the CD4+ T cells of patients with active disease [8,27,30]; however, this conclusion should be viewed cautiously due to the very small size of the sample in two instances out of three [8,27]. These preliminary results call for a further evaluation of this parameter on a larger number of SLE patients, examining both the CD4+ and the CD8+ subpopulations.
The results of the present analysis lend support to the idea of an elevation in the number of HLA-DR+ lymphocytes in patients with SLE flares, while it is the CD8+ subset which is concerned. The only, but nonetheless serious, limitation of the percentage of CD8+DR+ T cells among total lymphocytes to predict SLE flares comes from the various conditions able to switch on the immune system and therefore diminish its specificity.
Acknowledgments
We are indebted to N. Berrié, J.-C. Carron, M. Garcie and F. Saussais, and to all the members of the clinical Immunology Laboratory of the ‘Centre Hospitalier Régional de Bordeaux’, who skillfully contributed to this study.
REFERENCES
- 1.Gladman DD, Goldsmith CH, Urowitz MB, et al. Sensitivity to change of three systemic lupus erythematosus disease activity indices: international validation. J Rheumatol. 1994;21:1468–71. [PubMed] [Google Scholar]
- 2.Worrall JG, Snaith ML, Batchelor JR, Isenberg DA. SLE: a rheumatological view. Analysis of the clinical features, serology and immunogenetics of 100 SLE patients during long-term follow-up. Q J Med. 1990;74:319–30. [PubMed] [Google Scholar]
- 3.Schur PH, Sandson J. Immunologic factors and clinical activity in systemic lupus erythematosus. N Engl J Med. 1968;278:533–8. doi: 10.1056/NEJM196803072781004. [DOI] [PubMed] [Google Scholar]
- 4.Gladman DD, Urowitz MB, Keystone EC. Serologically active clinically quiescent systemic lupus erythematosus: a discordance between clinical and serologic features. Am J Med. 1979;66:210–5. doi: 10.1016/0002-9343(79)90529-1. [DOI] [PubMed] [Google Scholar]
- 5.Bluestein HG. Neuropsychiatric manifestations of systemic lupus erythematosus. N Engl J Med. 1987;317:309–11. doi: 10.1056/NEJM198707303170509. [DOI] [PubMed] [Google Scholar]
- 6.Ter Borg EJ, Horst G, Limburg PC, Kallenberg CG. Changes in plasma levels of interleukin-2 receptor in relation to disease exacerbations and levels of anti-dsDNA and complement in systemic lupus erythematosus. Clin Exp Immunol. 1990;82:21–6. doi: 10.1111/j.1365-2249.1990.tb05398.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Danieli MG, Paoletti P, Recchioni A, Gabrielli A, Danieli G. Serum levels of soluble interleukin-2 receptor in patients with systemic lupus erythematosus and systemic idiopathic vasculitis. Scand J Rheumatol. 1993;22:215–9. doi: 10.3109/03009749309095125. [DOI] [PubMed] [Google Scholar]
- 8.Raziuddin S, Nur MA, Al-Wabel AA. Increased circulating HLA-DR+ CD4+ T cells in systemic lupus erythematosus: alterations associated with prednisolone therapy. Scand J Immunol. 1990;31:139–45. doi: 10.1111/j.1365-3083.1990.tb02753.x. [DOI] [PubMed] [Google Scholar]
- 9.Spronk PE, van der Gun BT, Limburg PC, Kallenberg CG. B cell activation in clinically quiescent systemic lupus erythematosus (SLE) is related to immunoglobulin levels, but not to levels of anti-dsDNA, nor to concurrent T cell activation. Clin Exp Immunol. 1993;93:39–44. doi: 10.1111/j.1365-2249.1993.tb06494.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tan EM, Cohen AS, Fries JF, et al. The 1982 revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1982;25:1271–7. doi: 10.1002/art.1780251101. [DOI] [PubMed] [Google Scholar]
- 11.Bombardier C, Gladman DD, Urowitz MB, Caron D, Chang CH. Derivation of the SLEDAI: a disease activity index for lupus patients. Arthritis Rheum. 1992;35:630–40. doi: 10.1002/art.1780350606. [DOI] [PubMed] [Google Scholar]
- 12.Gladman DD, Goldsmith CH, Urowitz MB, et al. Crosscultural validation and reliability of three disease activity indices in systemic lupus erythematosus. J Rheumatol. 1992;19:608–11. [PubMed] [Google Scholar]
- 13.Petri M, Genovese M, Engle E, Hochberg M. Definition, incidence, and clinical description of flare in systemic lupus erythematosus. A prospective cohort study. Arthritis Rheum. 1991;34:937–44. doi: 10.1002/art.1780340802. [DOI] [PubMed] [Google Scholar]
- 14.Hohler T, Buschenfelde KH. Systemic lupus erythematosus. N Engl J Med. 1994;331:1235. doi: 10.1056/nejm199411033311816. [DOI] [PubMed] [Google Scholar]
- 15.Lloyd W, Schur PH. Immune complexes, complement, and anti-DNA in exacerbations of systemic lupus erythematosus (SLE) Medicine (Baltimore) 1981;60:208–17. doi: 10.1097/00005792-198105000-00004. [DOI] [PubMed] [Google Scholar]
- 16.Ter Borg EJ, Horst G, Hummel EJ, Limburg PC, Kallenberg CG. Measurement of increases in anti-double-stranded DNA antibody levels as a predictor of disease exacerbation in systemic lupus erythematosus. A long-term, prospective study. Arthritis Rheum. 1990;33:634–43. doi: 10.1002/art.1780330505. [DOI] [PubMed] [Google Scholar]
- 17.Spronk PE, Ter Borg EJ, Limburg PC, Kallenberg CG. Plasma concentration of IL-6 in systemic lupus erythematosus; an indicator of disease activity? Clin Exp Immunol. 1992;90:106–10. doi: 10.1111/j.1365-2249.1992.tb05840.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Viallard JF, Taupin JL, Miossec V, Pellegrin JL, Moreau JF. Analysis of interleukin-6, interleukin-10 and leukemia inhibitory factor (LIF) production by peripheral blood cells from patients with systemic lupus erythematosus identifies LIF as a potential marker of disease activity. Eur Cytokine Netw. 1999;10:17–24. [PubMed] [Google Scholar]
- 19.Davas EM, Tsirogianni A, Kappou I, et al. Serum IL-6, TNFalpha, p55srTNFalpha, p75srTNFalpha, srIL-2alpha levels and disease activity in systemic lupus erythematosus. Clin Rheumatol. 1999;18:17–22. doi: 10.1007/s100670050045. [DOI] [PubMed] [Google Scholar]
- 20.Su CC, Shau WY, Wang CR, Chuang CY, Chen CY. CD69 to CD3 ratio of peripheral blood mononuclear cells as a marker to monitor systemic lupus erythematosus disease activity. Lupus. 1997;6:449–54. doi: 10.1177/096120339700600507. [DOI] [PubMed] [Google Scholar]
- 21.Crispin JC, Martinez A, de Pablo P, Velasquillo C, Alcocer-Varela J. Participation of the CD69 antigen in the T-cell activation process of patients with systemic lupus erythematosus. Scand J Immunol. 1998;48:196–200. doi: 10.1046/j.1365-3083.1998.00366.x. [DOI] [PubMed] [Google Scholar]
- 22.Walz LeBlanc BA, Gladman DD, Urowitz MB. Serologically active clinically quiescent systemic lupus erythematosus-predictors of clinical flares. J Rheumatol. 1994;21:2239–41. [PubMed] [Google Scholar]
- 23.Lorenz HM, Grunke M, Hieronymus T, et al. In vitro apoptosis and expression of apoptosis-related molecules in lymphocytes from patients with systemic lupus erythematosus and other autoimmune diseases. Arthritis Rheum. 1997;40:306–17. doi: 10.1002/art.1780400216. [DOI] [PubMed] [Google Scholar]
- 24.Winchester RJ, Kunkel HG. The human Ia system. Adv Immunol. 1979;28:221–92. [PubMed] [Google Scholar]
- 25.Oliveira JGG, Ramos JP, Xavier P, et al. Analysis of fine-needle aspiration biopsies by flow cytometry in kidney transplant patients. Transplantation. 1997;64:97–102. doi: 10.1097/00007890-199707150-00018. [DOI] [PubMed] [Google Scholar]
- 26.Kitani A, Hara M, Hirose T, et al. Kinetic analysis of Ia expression on T cells in patients with systemic lupus erythematosus. J Clin Lab Immunol. 1986;19:59–63. [PubMed] [Google Scholar]
- 27.Raziuddin S, Danial HB, Kelley M. OKT4+ T cell abnormality in patients with active systemic lupus erythematosus: HLA-DR antigen expressions. Clin Immunol Immunopathol. 1988;48:42–9. doi: 10.1016/0090-1229(88)90155-9. [DOI] [PubMed] [Google Scholar]
- 28.Erkeller-Yüksel FM, Deneys V, Yüksel B, et al. Age-related changes in human blood lymphocyte subpopulations. J Pediatr. 1992;120:216–22. doi: 10.1016/s0022-3476(05)80430-5. [DOI] [PubMed] [Google Scholar]
- 29.Erkeller-Yüksel F, Hulstaart F, Hannet I, Isenberg D, Lydyard P. Lymphocyte subsets in a large cohort of patients with systemic lupus erythematosus. Lupus. 1993;2:227–31. doi: 10.1177/096120339300200404. [DOI] [PubMed] [Google Scholar]
- 30.Spronk PE, Horst G, Van der Gun BT, Limburg PC, Kallenberg CG. Anti-dsDNA production coincides with concurrent B and T cell activation during development of active disease in systemic lupus erythematosus (SLE) Clin Exp Immunol. 1996;104:446–53. doi: 10.1046/j.1365-2249.1996.44754.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Al-Janadi M, Raziuddin S. B cell hyperactivity is a function of T cell derived cytokines in systemic lupus erythematosus. J Rheumatol. 1993;20:1885–91. [PubMed] [Google Scholar]