Abstract
Transfer of contact sensitivity (CS) responses by immune lymphoid cells was the first finding that distinguished cellular from humoral immunity. CS has remained the most studied T cell reaction in vivo, and is the prototype for a variety of delayed-type hypersensitivity (DTH) responses. DTH in essence is the recruitment of effector αβ-T cells out of vessels into peripheral tissues. The T cells then are activated by antigen presenting cells to produce pro-inflammatory cytokines. It has been assumed that the αβ-T cells alone are responsible, but recent studies show that three other lymphocyte subsets are involved: CS-inducing NK T cells, CS-initiating B-1 cells, and CS-assisting γδ-T cells. Therefore, the effector αβ-T cells are essential, but cannot be recruited into the tissues without the local action of IgM antibodies produced by B-1 cells rapidly (1 day) post-immunization. The IgM complexes with the challenge antigen to locally activate complement to lead to vascular activation required for T cell recruitment. This process occurs early (1-2 hours) in the elicitation phase, and is called CS-initiation. The essential CS-inducing NK T cells activate the B-1 cells by producing IL-4 rapidly (1 hour) after immunization, and γδ-T cells assist the local inflammatory function of the recruited CS-effector αβ-T cells. Thus, four lymphocyte subsets are required for elicitation of responses: CS-inducing NK T cells, CS-initiating B-1 cells, CS-assisting γδ-T cells, and finally the CS-effector αβ-T cells. Three of these four cell types are present in the immune lymphoid cell population that adoptively transfers CS: B-1 cells, γδ-T cells, and the αβ-T cells.
Keywords: contact hypersensitivity, γδ T cells, αβ T cells, NK T cells
INTRODUCTION
Contact sensitivity (CS) is the oldest and most frequently studied form of in vivo T cell mediated immunity. Experiments in the 1940s first established the concept of cellular immunity by transfer of CS responses with immune cells, and not by serum antibodies [1]. Until recently, the paradigm for these prototypic in vivo cell mediated immune responses was that they were mediated by sensitized effector αβ-T cells recruited into the tissues to bind antigen peptides complexed with MHC molecules on the surface of local antigen-presenting cells. This cognate recognition caused T cell activation for production of cytokines like IFN-γ, to orchestrate local inflammation featuring perivascular infiltrates of mononuclear cells [1]. Lately, there has been an explosion of new knowledge about CS that overturns this simple conception. These findings indicate that yes, T cells are essential, but in fact three different kinds of T cells; namely the CS-effector αβ-T cells [1], but also CS-assisting γδ-T cells [2], and interesting and very recently, CS-inducing NK (natural killer) T cells are required [3]. Finally, and perhaps most surprising for responses defined as free of B cells and antibodies, it has been found that CS-initiating B-1 cells producing IgM antibody also are required (unpublished observation). A paper in the current issue by Yokozeli et al. [4] confirms the involvement of CS-assisting γδ T cells. This review will place these data in the context of the four different specific cells that are all necessary for the induction and elicitation of CS responses.
CS-effector αβ-T cells and Langerhans cells
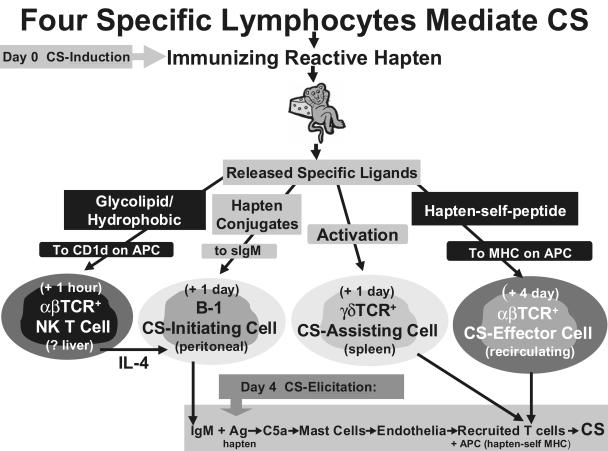
Induction of CS begins with skin painting contact immunization employing simple reactive chemicals, such as picryl chloride (PCl), oxazolone (OX), or DNFB in the laboratory, or poison ivy, nickel, chromium and many other substances in the clinic. These chemicals bind, often covalently, to the terminal amino group on lysines in host skin proteins to constitute neo-antigens that are processed in skin Langerhans presenting cells (APC) into hapten-self peptides that complex with surface MHC and are borne to draining lymph nodes by the APC. Then the Langerhans cells differentiate into mature dendritic cells that activate recirculating T cells that have specific αβ-TCR appropriate for binding the hapten-peptide-MHC complex. By day 4, this results in activation to become sensitized CS-effector T cells, which again recirculate to be able to mediate the late classical 24 h component of CS if recruited into the tissues [1], but actually are the fourth-acting lymphocyte subset in CS (Fig. 1).
Fig. 1.
The lymphocytes involved in CS. At priming for induction of CS (i.e. immunization), released glycolipid ligands activate the first-acting T cells via their unusual semi-invariant αβ-TCR. These are CS-inducing NK T cells that provide crucial IL-4 to allow coactivation of the second-acting CS-initiating B-1 cells in the peritoneal cavity, along with systemically dispersed hapten-self protein conjugate Ag. This stimulates the B-1 cells to produce specific IgM antibodies that enter the circulation. This priming sensitization also activates and mobilizes the third acting CS-assisting γδ-T cells from the spleen. Finally, CS-effector αβ-T cells are activated in the lymph nodes by Ag-MHC-APC on dendritic APC, as the fourth and final effector cells of CS.Then, at secondary hapten Ag challenge for elicitation of CS, the locally available B-1 cell-derived IgM binds Ag to lead to an early Ag-dependent local CS-initiating processes that generates C5a, to locally recruit the CS-effector αβ-T cells, that are aided by corecruited CS-assisting γδ-T cells for optimal activation by the APCs to produce cytokines that mediate CS.
CS-inducing NK T cells
Recent work indicates that αβ-T cells are necessary but not sufficient for the subsequent elicitation of CS responses. Three other classes of lymphocytes with specific receptors also are required. During the first day following sensitization important separate processes are triggered by contact skin painting that activate the other lymphocytes needed to bring about the final elicitation of CS. Amazingly, NK T cells are activated within the first hour following sensitization to become the first-acting required lymphocyte subset in CS. NK T cells were discovered only 14 years ago and have been shown to mediate several regulatory functions in other systems [5]. They have NK cell markers and receptors, but also express αβ-TCR that however, are semi-invariant. In mice, most NK T cells express a single TCR-α chain of Vα14, Jα281 that is paired with either of 3 Vβ chains. This canonical αβ-TCR of the dominant NK T cells preferentially recognizes glycolipids, like α-Galactosyl-Ceramide (α-Gal Cer), derived from marine sponges, and also binds some hydrophobic peptides [6]. Lipid binding usually is in the context of the MHC class I-like molecule CD1d on APC that has a deep lipid-binding hydrophobic groove [6]. The complex of glycolipid and CD1d activates NK T cell Vα14+ TCR causing rapid and strong production of several cytokines, mostly IL-4 and/or IFN-γ [5,6].
The location of NK T cells activated very early in CS is not known. The major population residing in the liver or bone marrow [5,6], or minor numbers in the peritoneal cavity, might be involved. Contact sensitivity causes immediate systemic dispersion of the sensitizing hapten from the skin [7], and presumably hapten-protein and peptide complexes. Since there likely is release of other materials, we postulate that distant NK T cells are activated by hydrophobic, substances such as endogenous glycolipids that are released from the skin in the first hour following contact sensitization, perhaps as ‘danger’ signals emanating from damaged skin cells [8], to alert cytokine-producing NK T cells that express semi-invariant glycolipid-recognizing αβ-TCR [6]. A crucial effect of this NK T cell activation early in CS is the production of IL-4 [3], and likely other cytokines [5]. NK T cell-derived IL-4 has been demonstrated in the circulation within the first day of contact sensitization [9], and also rapidly following NK T cell activation by injection of anti-CD3 [10].
CS-initiating B-1 cells
The rapidly released NK T cell-derived IL-4 has a crucial role in the activation of a particular specific B cell subset that is required to elicit CS. This also occurs within the first hour following sensitization and depends on the NK T cells [3]. These are B-1 cells that reside mainly in the peritoneal cavity and the second-acting lymphocyte subset required to elicit CS. The B-1 cells produce IgM antibody to trigger a process that rapidly follows elicitation of the CS responses by local Ag challenge and is called CS-initiation. This process is marked by a 2-h ear swelling and overall is required to recruit the CS-effector T cells to mediate classical 24 h CS [5]. B-1 cells in the peritoneal cavity become activated within only 1 h after contact sensitization. It is suggested that some hapten conjugates dispersed from the skin site of sensitization [7] are rapidly drained to the peritoneal cavity where B-1 cell surface IgM receptors specific for the hapten cause stimulation of the B-1 cells. However, it is postulated that such antigen stimulation is necessary but not sufficient for full activation of the B-1 cells for their subsequent participation in CS. In addition, IL-4 is required.
NK T cell IL-4 activates the B-1 cells
The new preliminary results suggesting involvement of NK T cells are that CS responses are defective in mice that are deficient in NK T cells, via either deletion of Vα14 Jα281 or CD1d [3]. The defect of CS in NK T cell deficient mice results in partial but significantly impaired 24 h CS responses, and complete absence of the 2 h CS-initiating responses that are due to the B-1 cells. It was shown that CS-effector T cells are intact, and thus it was postulated that activation of CS-initiating B-1 cells is defective in NK T cell deficient mice, abrogating local recruitment of the CS-effector T cells. In fact, there indeed is defective CS-initiation in NK T cell deficient mice. Moreover, deficient CS can be reconstituted by injections of IL-4 at immunization, that normally is derived from early activated NK T cells [3]. The target of IL-4 appears to be the B-1 cell. Thus, CS in NK T cell deficient mice also can be reconstituted by transfer of immunized B-1 cells taken from sensitized wild type mice where NK T cell-derived IL-4 was available [3]. Therefore, we postulate that NK T cell released IL-4, and the TNP-antigens dispersed from the skin site of contact sensitization, both are needed to activate the B-1 cells. This results in migration of B-1 cells to the spleen and lymph nodes, likely to differentiate into plasma cells to produce specific antihapten IgM antibody within one day [5,11–13]. This formulation is similar to the explanation about findings of peritoneal B-1 cells migrating to the mesenteric lymph nodes of the GI tract to produce IgA antibodies [14]. In CS, this B-1 cell derived specific circulating IgM antibody plays a crucial role in the elicitation of the final CS response by enabling the local recruitment of the CS-effector αβ-T cells into the tissues at the site of Ag challenge (unpublished observation).
CS-assisting γδ-T cells
The third-acting lymphocyte subset involved in CS are γδ-T cells that play a crucial role by assisting the CS-effector αβ T cells. The γδ T cell-mediated assistance may be due to their γδ TCR recognizing activation markers in the tissues, perhaps on the αβ T cells, in order to optimize their responsiveness to the APC. The enigmatic CS-assisting role of the γδ-T cells will be discussed later in this review.
INTERIM SUMMARY
Four specific cells are involved in CS:
CS-inducing NK αβ-T cells probably activated by dispersed hydrophobic ligands generated during contact sensitization;
CS-initiating B-1 cells in the peritoneal cavity that probably are coactivated by dispersed specific hapten antigen complexes, together with IL-4 from the NK T cells, to produce IgM to mediate CS-initiation;
the recruited CS-effector αβ-T cells activated via hapten-self peptide MHC complexes on Langerhans dendritic cells;
CS-assisting γδ-T cells probably arizing in the spleen and activated at immunization to enter the circulation [15], and probably also are recruited locally to aid the CS-effector αβ-T cells.
The final elicitation of inflammatory responses by the CS-effector αβ-T cells in the skin following local secondary Ag challenge, thus depends on the cooperative activities of the three additional cells. Without the NK T, B-1, or γδ-T cells, despite the presence of the αβ-T cells, no CS is elicited (Fig. 1).
Required CS-initiation involves all four cells
At the time of secondary antigen challenge at a new skin site to elicit CS, local hapten self antigen complexes bind the IgM antibody derived from the circulation (unpublished observation), that was produced earlier by distant B-1 cells likely coactivated by dispersed TNP-Ag and by NK T cell-derived IL-4 [3]. The IgM-Ag complex activates complement causing local elaboration of C5a anaphylatoxin. Our results show that C5a is present in CS ear extracts within the first hour following Ag elicitation of CS [16]. The C5a binds to C5a receptors; perhaps on endothelium, but most importantly on local mast cells and platelets [17]. This causes the mast cells to release vasoactive TNF-α [16–18] and serotonin [19], and also serotonin from platelets [17]. These vasoactive mediators stimulate the local post capillary venules, inducing expression of adhesion molecules like ICAM-1 and VCAM-1 on the luminal surface [18]. Expression of adhesion molecules on the local vasculature enables passing, recently activated, recirculating CS-effector αβ-T cells to adhere via expressed integrins, and then be recruited locally into the tissues. Among these traversing activated T cells, a few have αβ-TCR specific for the hapten-peptide-MHC-complexes on the local APC, that were derived from the secondary hapten Ag challenge. Very few recruited Ag-specific effector T cells [20] likely need to be activated to produce Th1 cytokines like IFN-γ [16] that orchestrate the local inflammatory response. Inflammation is produced by IFN-γ via induction of local tissue cells like keratinocytes to produce chemokines, such as IP-10 [16], MIG, and I-Tac that act on CXCR3 leucocyte chemokine receptors. This attracts and activates nonspecific bone marrow-derived leucocytes to migrate into the site to constitute the perivascular infiltrate of subsequent tissue inflammation and swelling that characterizes these responses. We postulate that the CS-assisting γδ T cells are also recruited by CS-initiating mechanisms to optimize the effector αβ-T cell responses. Indeed, it has been shown that γδ-T cells are a major component of the cellular infiltrate in CS responses of lambs, that like other ruminants have large numbers of circulating and lymphoid γδ-T cells [21].
Others have suggested that processes akin to CS-initiation may be due to contact hapten induced direct skin activation; primarily stimulation of keratinocytes to release cytokines via nonantigen-specific activation of NF-κB proinflammatory gene transcription pathways [22]. Although this may apply to some situations, our data show that at sites of CS elicitation, local generation of C5a is Ag-specific [16]. Further, despite the appropriate specific hapten challenge that undoubtedly activates keratinocytes at elicitation, the presence of circulating sensitized T cells alone does not permit their local recruitment to elicit the CS responses [2,11–13, unpublished observation]. In fact, Ag-specific CS-initiation is required [unpublished observation,13].
Possible CS-initiation in clinical responses
Our unpublished results also indicate that related DTH responses to secondary challenge with soluble protein antigen also feature a B-1 cell mediated initiating cascade that similarly recruits DTH-effector T cells into the tissues. Therefore, the CS/DTH-initiating process may be a general phenomena and central to in vivo acquired T cell immunity in many instances. Thus, analogous DTH-initiation recruitment into the tissues of various effector T cell subsets, such as Th1 [16], Th2 [23] and Tc1 [24], may lead to local cytokine mediated inflammatory mechanisms that are the basis of microbial resistance, tumour immunity, organ specific autoimmunity and some allergies; including aspects of asthma and atopic dermatitis.
From a clinical perspective, our findings offer a new pathway for triggering these delayed hypersensitivity and related reactions, and hence potentially provide new routes for therapeutic intervention. B cells are already known to participate in a variety of T cell mediated disease models of mice, including autoimmunity in collagen arthritis [25], NOD diabetes [26], lupus-like lesions of lpr/lpr mice [27], encephalomyelitis [28], and T cell protection against infections [29,30]. To date the participation of B cells in these diseases has largely been interpreted as due to participation in afferent APC function [31]. However, our findings, in contrast, suggest that antibodies may participate in elicitation of the effector T cell responses in these diseases, particularly in models like collagen arthritis and encephalomyelitis, where B cells [25,28], antibodies [32,33], complement [34,35] and mast cells [37,38] have been implicated in the efferent T cell responses.
For CS and DTH responses elicited soon after immunization such as on day 4, early activated antigen-specific B-1 cells produce the initiating IgM, and by day 4 Ag-MHC-specific effector T cells become sensitized to mediate the subsequent effector limb that follows antigen challenge to elicit CS reactions in the skin. In responses occurring later, B-1 cells fade (unpublished observation), and B-2 cell-produced isotypes become responsible (unpublished observation) likely complement activating IgG2 antibodies. Even IgE and IgG1 antibodies, that in mice mediate T cell recruitment by directly activating mast cell release of vasoactive mediators, are able to mediate CS-initiation in a complement-independent process [25,38].
The CS-assisting γδ T cells
Yokozeki et al. [4] now confirm the important role of the CS-assisting γδ-T cell subset. They studied a novel CS system to the contact antigen para-phenylenediamine, which is widely used in hair dyes and is responsible for clinical contact dermatitis. BALB/c mice immunized multiply by topical painting with 2·5% solution and ear challenged on day 5, elicited small but significant 2 h CS-initiating responses, and larger 24 h CS-effector responses in a hapten-specific manner. Adoptive cell transfers suggested an early acting activated B cell population (B220+, Thy-1+, CD5+, αβ−, γδ−, CD3−, CD4−, CD8−), identical in phenotype to previously described CS-initiating B-1 cells [39–41] was responsible for the early responses, and CD4+ CS-effector αβ-T cells mediate the 24 h response; importantly with the aid of CS-assisting γδ-T cells [4]. As in PCl CS [2], depletion of the γδ-cells substantially diminished transferred CS responses mediated by the CS-effector αβ-T cells [4]. Also, as before [2], transferred CS was reconstituted by adding back the CS-assisting γδ T cells to the immune CS-effector αβ-T cells [4]. Again [2], the CS-assisting γδ-T cells were neither Ag-specific nor MHC restricted, and also γδ-T cells from normal spleen could assist isolated CS-effector αβ-T cells in adoptive transfer of CS [4,15]. RT-PCR determined the cytokine mRNA pattern in the CS-effector αβ CS-assisting γδ-T cells. The αβ-T cells strongly expressed IFN-γ as expected, while the γδ-T cells express not only IFN-γ but also IL-4 and IL-10 [4], suggesting a potential role for these cytokines in CS-assistance.
Possible mechanisms of CS assistance by γδ T cells
The mechanism of γδ T cell participation in CS remains obscure. There are at least three possibilities. Firstly, we postulate that some CS-assisting γδ T cells mobilized at immunization from the spleen into the circulation [15], are recruited locally at the site of challenge by the B-1 cell mediated CS-initiating process. The presence of CS-assisting γδ-T cells in normal spleen shows they are present before immunization. Their absence in nude or SCID mice shows they require thymic differentiation and rearrangement of TCR receptor genes. Since the γδ-T cells are neither Ag-specific nor MHC restricted [2,4], it is postulated that γδ -T cells assist by recognizing surface expressed self activation antigens on the co‐recruited CS-effector αβ-T cells, or on other cells such as APC or local tissue cells. Alternatively, or in addition, γδ-TCR recognition of activation antigens may induce the CS-assisting γδ-T cells to produce cytokines like IL-4 or IL-10 that somehow optimize the CS-effector αβ-T cell responses.
Secondly, the γδ-T cells may have a ‘contrasuppressive’ regulatory function by making the CS-effector T cells resistant to endogenous suppressor cells that maintain homeostasis in the normal recipients. Different pretreatments of recipients of transfers with the isolated CS-effector αβ-T cells (the γδ-T cells are removed) give insight into the mechanism of action of the CS-assisting γδ T cells. Recipients treated with Bordetella pertussis [15] do not need exogenous γδ T cells to express CS, since normal splenic γδ T cells became activated, and are mobilized into the circulation by the Bordetella injections, to then serve the CS-assisting function. Also, treatment of recipients with a low dose of cyclophosphamide thought to interfere with suppressor cells, or with mAb against determinants on suppressive cells, also restored transfers by CS-effector αβ-T cells in normal mice again without adding CS-assisting γδ T cells [15]. This suggested that suppressor cells normally present in the recipients ordinarily antagonize transfers mediated by CS-effector αβ-T cells, and that the CS-assisting γδ-T cells may serve a protective or contrasuppressive function to block this endogenous suppression, perhaps by rendering the αβ-T cells resistant to suppression.
A third possibility is that the γδ T cells, like the B-1 cells are activated by IL-4 or other cytokines [42], that perhaps also are derived from early glycolipid activation of the NK T cells [3,5,6], to help mobilize the γδ-T cells from their normal inactive site in the spleen to migrate into the circulation to be able to act as CS-assisting cells after recruitment to the local Ag elicitation site via CS-initiation. Note that these three formulations are not mutually exclusive. Further, a 1-day immune population described previously [43], has a mixed phenotype, that may represent a mixture of B-1 cells and NK T cells that together allowed cultured lines of mixed αβ and γδ T cells, that alone only transfer CS locally, to be able to systemically transfer CS [42–44,unpublished observations]; thereby also accounting for the four lymphocyte subsets that mediate CS. In these studies, the artificial procedure of local transfer of CS-effector αβ-T cell lines, which skips over the CS-initiation requirement, may also obviate the need for CS-assistance since so many effector T cells are transferred. This is compared to the very few Ag-specific CS-effector αβ-T cells that likely are recruited naturally [2], and thus their small numbers may require assistance in actively sensitized mice, or in recipients of systemic cell transfers.
Finally, the CS-assisting γδ-T cells preferentially use restricted variable region gene segments [44–46]. This preferential and unusual Vγ and Vδ-TCR gene expression leads to the hypothesis that particular host expressed antigens, such as activation markers or heat shock proteins, likely on the surface of the activated αβ-T cells (or APC, etc.), might serve as ligands for the γδ-TCR on the CS-assisting T cells. Thus, we suggest that CS-assisting γδ-T cells are recruited locally with the CS-effector αβ-T cells via the CS-initiation process that depends on NK T cell-derived IL-4, B-1 cell-derived IgM, and mast cell-derived TNF-α and serotonin. Then in the extravascular tissues, while the CS-effector αβ-T cells are interacting via cognate specificity with hapten-peptide-MHC complexes on APC, the γδ-TCR of CS-assisting cells interact via their γδ-TCR with either native activation proteins, or with low molecular weight phenyl pyrophosphate or alkamine‐like ligands, expressed via the activated αβ-T cells, or APC or keratinocytes, etc., to then assist the αβ-T cells. The surface expressed antigens that activate the γδ-TCR could be heat shock proteins or minor histocompatability antigens that become newly expressed via nonspecific skin cell activation via NF-κB pathways that are stimulated by the irritating nonspecific contact hapten challenge [21]. Binding of γδ-TCR to these surface complexes may then activate the γδ-T cells to positively influence the recruited CS-effector αβ-T cells, thereby optimizing their production of Th1 cytokines to subsequently generate full elicitation of CS inflammation.
Conclusion
We have summarized recent data showing that in vivo T cell mediated immunity in contact sensitivity involves four specific lymphocyte subsets, including three types of T cells and also B-1 cells. Therefore, the required involvement of several previously unanticipated processes that take part in interactions between these lymphocyte subsets, raises the possibility that immune resistance, or deleterious allergic and autoimmune processes that are mediated by these mechanisms, may be susceptible to new treatment modalities that are based on this knowledge.
Acknowledgments
I am grateful for the administrative skills of Marilyn Avallone and for vigorous review of the manuscript by Cornelia Weyand, Regis Campos, and Vipin Paliwal. This work was supported by grants from the NIH (AI‐43372, HL‐56389, DK‐3498, AR‐41942) and a Rockefeller Bros. Fund Charles E. Culpepper Grant.
REFERENCES
- 1.Askenase PW. Effector and regulatory molecules and mechanisms in delayed-type hypersensitivity (DTH) In: Middleton Jr E, Reed CE, Ellis EF, Atkinson NF, Yunginger JW, Busse WW, editors. Allergy: Principles and Practice. 5. St. Louis: CV Mosby Co; 1998. [Google Scholar]
- 2.Ptak W, Askenase PW. γδ T cells assist αβ T cells in adoptive transfer of contact sensitivity. J Immunol. 1992;149:3503–8. [PubMed] [Google Scholar]
- 3.Campos RA, Szczepanik M, Iliopoulou P, Akahira-Azuma M, Askenase PWNK. T cell derived IL-4 activates B-1 cells to initiate contact sensitivity (CS) FASEB J. 2001;15:A693. #533.14. [Google Scholar]
- 4.Yokozeki H, Watanabe K, Igawa K, Miyazaki Y, Katayama I, Nishioka K. γδ T cells assist αβ T cells in the adoptive transfer of contact hypersensitivity to para-phenylenediamine. Clin Exp Immunol. 2001;125:351–359. doi: 10.1046/j.1365-2249.2001.01570.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Godfrey DI, Hammond KJL, Poulton LD, Smyth MJ, Baxter AG. NKT cells: facts, functions and fallacies. Immunol Today. 2000;21:573. doi: 10.1016/s0167-5699(00)01735-7. [DOI] [PubMed] [Google Scholar]
- 6.Burdin N, Kronenberg M. CD1-mediated immune responses to glycolipids. Current Opinion Immunology. 1999;11:326–31. doi: 10.1016/s0952-7915(99)80052-1. [DOI] [PubMed] [Google Scholar]
- 7.Pior J, Vogl T, Sorg C, Macher E. Free Hapten molecules are dispersed by way of the bloodstream during contact sensitization to fluorescein isothiocyanate. J Invest Derm. 1999;113:888–93. doi: 10.1046/j.1523-1747.1999.00770.x. [DOI] [PubMed] [Google Scholar]
- 8.Matzinger P. An innate sense of danger. Immunology. 1998;10:399–415. doi: 10.1006/smim.1998.0143. [DOI] [PubMed] [Google Scholar]
- 9.Dieli F, Taniguchi M, Askerson GL, Sireci G, Caccamo N, Scire E, Bonanno CT, Salerno A. Development of hapten-induced IL-4-producing CD4+ T lymphocytes requires early IL-4 production by alpha/beta T lymmphocytes carrying invariant V (alpha) 14 TCR alpha chain. Int Immunol. 1998;10:413420. doi: 10.1093/intimm/10.4.413. [DOI] [PubMed] [Google Scholar]
- 10.Yoshimoto T, Paul WE. CD 4pos, NK1.1pos T cells promptly produce interleukin 4 in response to in vivo challenged with anti-CD3. J Exp Med. 1994;179:1285–95. doi: 10.1084/jem.179.4.1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Van Loveren H, Askenase PW. Delayed-type hypersensitivity is mediated by a sequence of two different T cell activities. J Immunol. 1984;133:2397–401. [PubMed] [Google Scholar]
- 12.Van Loveren H, Kato K, Meade R, Green DR, Horowitz M, Ptak W, Askenase PW. Characterization of two different Ly1+ T cell populations that mediate delayed-type hypersensitivity. J Immunol. 1984;133:2402–11. [PubMed] [Google Scholar]
- 13.Ptak W, Herzog WR, Askenase PW. Delayed-type hypersensitivity initiation by early-acting cells that are antigen mismatched or MHC incompatible with late-acting, delayed-type hypersensitivity effector T cells. J Immunol. 1991;146:469–75. [PubMed] [Google Scholar]
- 14.Watanabe N, Ikuta K, Fagarasan S, Yazumi S, Chiba T, Honjo T. Migration and differentiation of autoreactive B-1 cell induced by activated γ/δ T cells in anti-erythrocyte immunoglobulin transgenic mice. J Exp Med. 2000;192:1577–86. doi: 10.1084/jem.192.11.1577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Askenase PW, Szczepanik M, Ptak M, Paliwal V, Ptak W. γδ T cells in normal spleen assist immunized αβ T cells in the adoptive cell transfer of contact sensitivity: effect of Bordetella pertussis, cyclophosphamide, and antisuppressor cells antibodies. J Immunol. 1995;154:3644. [PubMed] [Google Scholar]
- 16.Tsuji RF, Kawikova I, Ramabhadran R, Akahira-Azuma M, Taub D, Hugli TE, Gerard C, Askenase PW. Early local generation of C5a initiates the elicitation of contact sensitivity by leading to early T cell recruitment. J Immunol. 2000;165:1588–98. doi: 10.4049/jimmunol.165.3.1588. [DOI] [PubMed] [Google Scholar]
- 17.Geba GP, Ptak W, Anderson GA, Ratzlaff RE, Levin J, Askenase PW. Delayed-type hypersensitivity in mast cell deficient mice: dependence on platelets for expression of contact sensitivity. J Immunol. 1996;157:557–65. [PubMed] [Google Scholar]
- 18.McHale JF, Harari OA, Marshall D, Haskard DO. Vascular endothelial cell expression of intercellular adhesion molecule-1 and vascular cell adhesion molecule-1 at the onset of eliciting contact hypersensitivity in mice. Evidence for dominant role of TNF-α. J Immunol. 1999;162:1648–55. [PubMed] [Google Scholar]
- 19.Askenase PW, Bursztajn S, Gershon MD, Gershon RKT. cell dependent mast cell degranulation and release of serotonin in murine delayed-type hypersensitivity. J Exp Med. 1980;152:1358–74. doi: 10.1084/jem.152.5.1358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Marchal G, Seman M, Milon G, Truffa-Bachi P, Zilberfarb V. Local adoptive transfer of skin delayed-type hypersensitivity initiated by a single T lymphocyte. J Immunol. 1982;129:954–8. [PubMed] [Google Scholar]
- 21.Jorundsson E, Press CM, Ulvund M, Landsverk T. Prominence of γδ T cells in the elicitation phase of dinitrochlorobenzene-induced contact hypersensitivity in lambs. Vet Pathol. 1999;36:42–50. doi: 10.1354/vp.36-1-42. [DOI] [PubMed] [Google Scholar]
- 22.Grabbe S, Schwarz T. Immunoregulatory mechanisms involved in elicitation of allergic contact hypersensitivity. Immunol Today. 1998;19:37. doi: 10.1016/s0167-5699(97)01186-9. [DOI] [PubMed] [Google Scholar]
- 23.Askenase PW. Robinson D, editor. Proposing Th2 DTH Relevant to Asthma: Cutaneous Basophil Hypersensitivity (CBH) then, now. Symposium on Immunological Mechanisms in Asthma, Allergic Diseases. Chem Immunol. 2000;78:112–23. doi: 10.1159/000058821. [DOI] [PubMed] [Google Scholar]
- 24.Kalish RS, Askenase PW. Molecular mechanisms of CD8+ T cell-mediated delayed hypersensitivity: Implications for allergies, asthma, and autoimmunity. J All Clin Immunol. 1999;103:192–9. doi: 10.1016/s0091-6749(99)70489-6. [DOI] [PubMed] [Google Scholar]
- 25.Svensson L, Jirholt J, Holmdahl R, Jansson L. B cell-deficient mice do not develop type II collagen-induced arthritis (CIA) Clin Exp Immunol. 1998;111:521–6. doi: 10.1046/j.1365-2249.1998.00529.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Serreze DV, Chapman HD, Varnum DS, et al. B lymphocytes are essential for the initiation of T cell-mediated autoimmune diabetes: analysis of a new ‘speed congenic’ stock of NOD. Igµnull mice. J Exp Med. 1996;184:2049–53. doi: 10.1084/jem.184.5.2049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shlomchik MJ, Madaio MP, Donghui N, Trounstein M, Huszar D. The role of B cells in 1pr/1pr-induced autoimmunity. J Exp Med. 1994;180:1295–306. doi: 10.1084/jem.180.4.1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lyons JA, San M, Happ MP, Cross AH. B cells are critical to induction of experimental allergic encephalomyelitis by protein but not by short encephalitogenic peptide. Eur J Immunol. 1999;29:3432–9. doi: 10.1002/(SICI)1521-4141(199911)29:11<3432::AID-IMMU3432>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 29.Vordermeier HM, Venkataprasad N, Harris DP, Ivanyi J. Increase of tuberculous infection in the organs of B cell-deficient mice. Clin Exp Immunol. 1996;106:312–6. doi: 10.1046/j.1365-2249.1996.d01-845.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yang X, Brunham RC. Gene knockout B cell-deficient mice demonstrate that B cells play an important role in the initiation of T cell responses to Chlamydia trachmatis (mouse pneumonitis) lung infection. J Immunol. 1998;161:1439–46. [PubMed] [Google Scholar]
- 31.Falcone M, Lee J, Patstone G, Yeung B, Sarvetnick N. B lymphocytes are crucial antigen-presenting cells in the pathogenic autoimmune response to GAD65 antigen in nonobese diabetic mice. J Immunol. 1998;161:1163–8. [PubMed] [Google Scholar]
- 32.Litzenburger T, Fassler R, Bauer J, Lassmann H, Linington C, Wekerle H, Iglesias A. B lymphocytes producing demyelinating autoantibodies: development and function in gene-targeted transgenic mice. J Exp Med. 1998;188:169–80. doi: 10.1084/jem.188.1.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Linington C, Engelhardt B, Kapocs G, Lassman H. Induction of persistently demyelinated lesions in the rate following the repeated adoptive transfer of encephalitogenic T cells and demyelinating antibody. J Neuroimmunol. 1992;40:219–24. doi: 10.1016/0165-5728(92)90136-9. [DOI] [PubMed] [Google Scholar]
- 34.Wang Y, Rollins SA, Madri JA, Matis LA. Anti-C5 monoclonal antibody therapy prevents collagen-induced arthritis and ameliorates established disease. PNAS. 1995;92:8955–9. doi: 10.1073/pnas.92.19.8955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang Y, Kristan J, Hao L, Lenkoski CS, Shen Y, Matis LA. A role for complement in antibody-mediated inflammation: C5-deficient DBA/1 mice are resistant to collagen-induced arthritis. J Immunol. 2000;164:4340–7. doi: 10.4049/jimmunol.164.8.4340. [DOI] [PubMed] [Google Scholar]
- 36.Kakizoe ES, Li H, Kobayashi Y, Nishikori Y, Dekio S, Okunishi H. Increases in mast cells and chymase in fibroproliferative paws of collagen-induced arthritic mice. Inflamm Res. 1999;48:318–24. doi: 10.1007/s000110050467. [DOI] [PubMed] [Google Scholar]
- 37.Secor VH, Secor WE, Gutekunst C-A, Brown MA. Mast cells are essential for early onset and severe disease in a murine model of multiple sclerosis. J Exp Med. 2000;191:813–22. doi: 10.1084/jem.191.5.813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ptak W, Geba GP, Askenase PW. Initiation of delayed-type hypersensitivity by low doses of monoclonal IgE antibody. Mediation by serotonin and inhibition by histamine. J Immunol. 1991;146:3929–36. [PubMed] [Google Scholar]
- 39.Herzog WR, Ferreri NR, Ptak W, Askenase PW. The antigen-specific DTH-initiating Thy-1+ cell is double negative (CD4−, CD8−) and CD3 negative; and expresses IL-3 receptors, but no IL-2 receptors. J Immunol. 1989;143:3125–33. [PubMed] [Google Scholar]
- 40.Ishii N, Takahashi K, Nakajima H, Tanaka S, Askenase PW. DNFB contact sensitivity (CS) in BALB/c and C3H/He mice: requirement for early-occurring, early-acting, antigen-specific, CS-initiating cells with an unusual phenotype (Thy-1+, CD5+, CD3−, CD4−, CD8−, sIg−, B220+, MHC Class-II−, CD23+, IL-2R−, IL-3R+, Mel-14−, Pgp-1+, J11d+, MAC-1+, LFA-1+, and FcγIIR+) Invest Derm. 1994;102:321–7. doi: 10.1111/1523-1747.ep12371790. [DOI] [PubMed] [Google Scholar]
- 41.Ishii N, Sugita Y, Nakajima H, Tanaka S, Askenase PW. Elicitation of nickel sulfate (NiSO4)-specific delayed-type hypersensitivity requires early-occurring and early-acting, NiSO4-specific DTH-initiating cells with an unusual mixed phenotype for an antigen-specific cell. Cell Immunol. 1995;161:244–55. doi: 10.1006/cimm.1995.1033. [DOI] [PubMed] [Google Scholar]
- 42.Dieli F, Asherson GL, Romano GC, Sireci G, Gervasi F, Salerno A. IL-4 is essential for the systemic transfer of delayed hypersensitivity by T cell lines. Role of γδ cells. J Immunol. 1994;152:2698–704. [PubMed] [Google Scholar]
- 43.Salerno A, Dieli F, Sireci G, Bellavia A, Colizzi V, Ptak W, Asherson GL. Three cell subsets are required for the transfer of delayed-type hypersensitivity reaction by antigen-specific T cell lines. Cell Immunol. 1997;175:157–63. doi: 10.1006/cimm.1996.1034. [DOI] [PubMed] [Google Scholar]
- 44.Dieli F, Ptak W, Sireci G, Romano GC, Potestio M, Salerno A, Asherson GL. Cross-talk between Vβ8+ and γδ+ T lymphocytes in contact sensitivity. Immunology. 1998;93:469–77. doi: 10.1046/j.1365-2567.1998.00435.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dieli F, Asherson GL, Sireci G, Dominici R, Gervasi F, Vendetti S, Colizzi V, Salerno A. γδ cells involved in contact sensitivity preferentially rearrange the Vγ3 region and require interleukin-7. Eur J Immunol. 1997;27:206–14. doi: 10.1002/eji.1830270131. [DOI] [PubMed] [Google Scholar]
- 46.Ptak W, Szczepanik M, Ramabhadran R, Askenase PW. γδ T cells that assist αβ T cells in the adoptive cell transfer of contact sensitivity, preferentially employ Vγ5 and Vδ4 variable region gene segments. J Immunol. 1995;156:976–8648. [PubMed] [Google Scholar]