Abstract
The thymus undergoes age-associated involution, with studies showing thymic size decreasing from birth at a rate of approximately 3% per year until middle age, and at a rate of 1% per year thereafter. The aim of this study was to determine the effect of thymic atrophy on T-lymphocyte production by the thymus, and to clarify the ongoing uncertainty regarding gender differences in thymic function. We quantified recent thymic emigrants (RTEs) in blood through the measurement of signal joint T-cell receptor rearrangement excision circles (sjTRECs), and showed that the decline in the number of RTEs in the blood with increasing age is gender-linked. Peripheral blood from females contained significantly higher levels of sjTRECs per CD3+ T cell than blood from males (P = 0·002), despite there being no significant gender difference in the absolute number of CD3+ T cells in the populations analysed (P > 0·10). Our findings suggest better thymic function in females compared with males, providing females with a higher number of recent thymic emigrants for longer periods of life. Such a finding provides a plausible explanation for the immunological gender differences observed in previous studies and possibly, for the general longer life expectancy in females compared with males.
Keywords: Thymus, sjTREC, gender, ageing, atrophy
INTRODUCTION
The central role of the thymus in the generation of T lymphocytes is well established [1], as is the process of age-related atrophy that leads to a reduction in this organ's function of T-lymphocyte generation [2]. The underlying causes of thymic atrophy remain to be defined, although several theories have been proposed. These theories include reduction in the number or function of the T-cell progenitors from the bone marrow [3], problems with the rearrangement of the TCRβ chain [2,4], adverse changes in the thymic microenvironment [5], or changes in the levels of hormones or growth factors [6,7]. Whatever the precise cause, the result is a reduction in the number of constituent thymocytes with age, a consequent shrinking of the thymus and a decline in its output. T-cell emigrants from the thymus contribute to the naïve T-cell pool, whose residents remain for a finite period and whose survival depends upon T-cell receptor (TCR) ligation with self MHC, whilst their expansion and differentiation into memory T cells requires successful recognition of foreign antigen [8].
Measurement of recent thymic emigrants (RTEs) has been achieved through the detection of extra-chromosomal DNA generated as by-products of the TCRα rearrangement process that occurs during intrathymic development. Such by-products do not replicate during cell division and so are present in higher concentrations in the most recent thymic emigrant population, after which they are diluted out by cell division. Previous studies clearly show an age-related decline in the number of these episomal DNA excision products within the peripheral T-cell pool, echoing the decline seen in thymic output [9,10].
One of the major differences between the naïve RTEs containing T-cell pool and memory T-cell pool is that the naïve T-cell pool may possess the capacity to recognize previously unencountered antigens for which no memory T-cell specificity exists. Thus, a reduction in the numbers of naïve T cells may decrease the number of potentially new antigens which can be recognized by the cell-mediated arm of the immune system. Such a functional consequence of thymic atrophy may explain why older individuals, who generally have fewer naïve T cells than younger individuals, show a less efficient ability to respond to vaccines [11,12].
This functional difference between young and old individuals, and its potential correlation with the size of the naïve T-cell pool, prompted our study on thymic performance according to age and gender. Several studies have shown that when immunological responses are analysed according to gender, females show better antibody and cell-mediated responses than males. Animal models reveal females to have the ability to reject allografts more rapidly, to have a greater ability to combat various viral and bacterial infections, and to show superior anti-tumour responses (reviewed in [13,14]). Rates of human thymic involution have been shown to differ according to gender, with male thymuses atrophying more rapidly than those of females [15,16], although this was refuted in a later study [3]. More recent support comes from a study showing gender differences in the rate of thymic involution in mice, with male mice showing more rapid involution than female mice [17]. To clarify this issue, we studied thymic performance as measured by thymic output in male and female volunteers as a function of age.
MATERIALS AND METHODS
Sample collection
Forty-six healthy volunteers (21 males and 25 females) aged up to 62 years provided samples of peripheral blood. Blood samples were obtained with the subjects' informed consent and in accordance with the guidelines set by the Riverside Ethics Committee. Venous blood (25 ml) was collected from the volunteers into EDTA-K3 containers (Becton Dickinson, Oxford, UK) and peripheral blood mononuclear cells (PBMC) were isolated by density gradient centrifugation using Histopaque (Sigma, Poole, UK). DNA was extracted from 107 isolated PBMCs using the Puregene DNA purification kit (Gentra, Flowgen, Ashby de la Zouch, UK). β-actin polymerase chain reaction (PCR) amplification and agarose gel analysis were performed for each sample in order to validate the quality of the DNA.
sjTREC PCR amplification and quantification
sjTREC DNA was PCR amplified according to the previously published technique [18]. The product band was visualized on 1·2% agarose gels containing 0·005% ethidium bromide (Sigma), and the image captured using the Enhanced Analysis System (Scotlab, Coatbridge, UK). Image analysis was performed using the Scion Imager (Scion Corp., Maryland, USA). Quantification of sjTREC molecules per DNA band was determined according to the method of Aspinall et al. [18]. Briefly, a standard curve was constructed via the PCR amplification of known starting numbers of standard sjTREC molecules. PCR products were analysed on agarose gels using the Scion imager software. A best fit straight-line graph (R2 = 1·0) composed of the starting amount of sjTREC molecules used for the PCR against DNA band intensity was constructed (data not shown). For each of the test samples, the light intensity values of the amplified sjTREC DNA bands provided the number of molecules by reference to the standard curve. Results were considered valid if the light intensity value of the DNA band of the positive control differed from the actual values on the standard curve by no more than 10%.
FACS analysis of CD3 and CD45RA, CD62L T-lymphocyte populations
Peripheral blood (100 µl) obtained from subjects were treated with the following monoclonal antibodies: mouse anti-human CD3 conjugated to FITC (Sigma), mouse anti-human CD45RA conjugated to Quantum red (Pharmingen, Oxford, UK) and mouse anti-human CD62L conjugated to PE (Pharmingen), or to the appropriate isotype-matched negative controls, and incubated on ice for 30 min. The red cells were then lysed by the addition of 2 ml of Ortholyse (Ortho Diagnostic Systems, Amersham, UK) and the samples were analysed using the Absolute Ortho Cytotron flow cytometer.
Statistical analysis
The Mann–Whitney U-test was used to determine whether gender differences in sjTREC values were statistically significant, i.e. when the value of P was < 0·05. The Student's t-test was used to compare the ages of female and male volunteers. The line of best fit was drawn using the Microsoft Excel package, which also provided the value for R2 and the equation.
RESULTS
Thymic output and function progressively declines during ageing
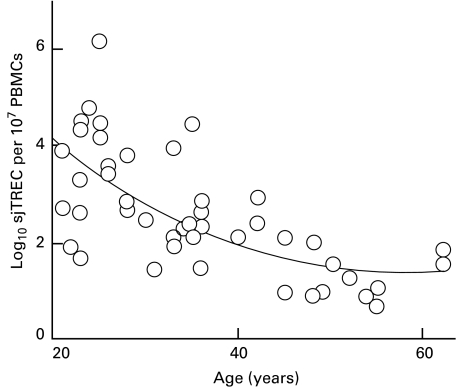
Measurement of sjTREC levels in peripheral blood mononuclear cells (PBMCs) from healthy female and male volunteers between the ages of 20 and 62 years (Fig. 1) revealed a progressive decline with age, indicating an age-related reduction in the contribution of RTEs within the cell population tested. The line of best fit (y = 0·002x2−0·2139x + 7·9681; R2 = 0·601) displays the classic bi-phasic rate of decline, with greater decline in sjTREC numbers prior to 40 years of age and slower decline in their numbers thereafter. Prior to 40 years of age, the rate of decline was approximately 2% per year but after 40 years, we calculated that the decline dropped to approximately 1·5% per year.
Fig. 1.
sjTREC numbers between the ages of 20 and 62 years. The number of sjTREC shows a progressive linear decline with age (R2 = 0·601), indicating a decrease in RTEs and therefore thymic output with increasing age.
Gender-related difference in sjTREC levels
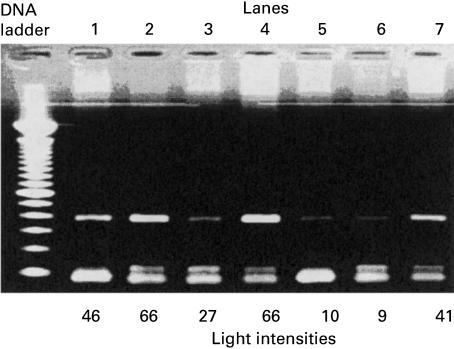
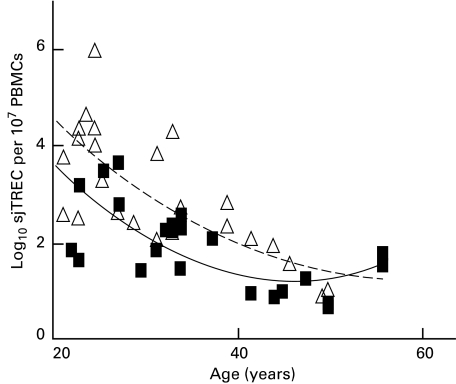
There was a higher intensity of DNA bands from females compared with males of comparative age (Fig. 2) which prompted further analysis of sjTREC numbers according to gender. This approach proved more successful and revealed an obvious difference in sjTREC levels, as well as a tighter correlation of the line of best fit, with R2 values for females and males being 0·81 and 0·63, respectively (Fig. 3) compared with 0·601 overall. Between the ages of 20 and 62 years, females were observed generally to have a greater number of sjTRECs than males, with the P-value calculated to be 0·004, indicating a gender difference that is statistically significant. Comparison of sjTREC levels obtained for subjects over the age of 30 years continued to reveal a significant difference between males and females with P = 0·014, and for subjects over 40, P = 0·036. Such P-values, while indicating a consistent statistically significant gender difference in sjTREC levels, also suggest that the difference becomes less apparent with age, so that thymic output eventually converges to some similar value.
Fig. 2.
Agarose gel analysis of sjTREC bands showing a correlation between band intensities and gender. Lane 2 containing sjTREC from a 23-year-old female has a greater intensity when compared with sjTREC of an age-matched male (lane 1). Lane 4 is the sjTREC for a 25-year-old female. Lane 7 contains a DNA band for a 42-year-old female and has a higher intensity than a 40-year-old male's DNA band (lane 3). Lanes 5 and 6 containing sjTREC bands for a 55-year-old female and a 48-year-old male, respectively, are shown to possess bands of almost equal intensities. The actual light intensity values for sjTREC DNA bands are shown below each lane.
Fig. 3.
sjTREC numbers in males (▪) and females (▵) at ages ranging from 20 to 62 years. Females have significantly higher sjTREC levels than age-matched males (P = 0·004) between the age of 20 and 62 years, with the difference becoming less apparent with increasing age. For females, the line of best fit formula was y = 0·0027x2 − 0·268x + 8·01, R2 = 0·81. For males, the line of best fit formula was y = 0·0017x2 − 0·2236x + 8·45, R2 = 0·63.
No gender differences in naïve T-lymphocyte population during ageing
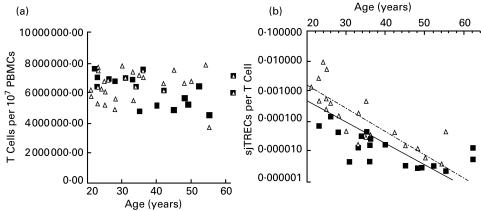
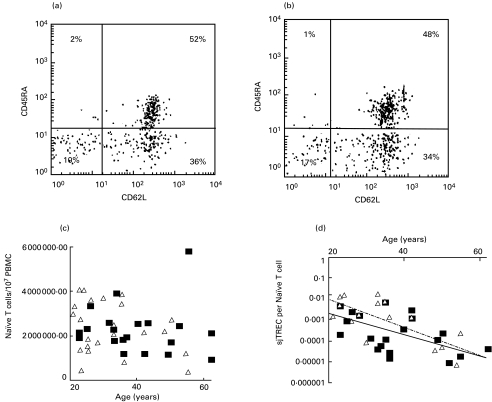
To counteract any bias due to T-cell numbers, we first calculated the number of CD3+ T cells in the PBMC samples analysed and observed no significant change in their number with age and no gender differences (P > 0·10). We then calculated the number of sjTREC per T cell (Fig. 4) and found the gender difference to remain statistically significant (P = 0·002). To refine our analysis further, we looked for differences in the number of naïve T cells (CD3+ CD45RA+ CD62L+) in our samples. Figure 5 shows two age-matched FACS profiles, one from the male and one from the female pool of samples, which are consistent with the overall result that revealed no significant gender differences in the number of these cells within the samples tested (P ≥ 0·10). However, when we calculated the number of sjTREC molecules per naïve T cell, we continued to find significant gender differences (P = 0·006), with higher levels of sjTREC molecules per naïve T cell in females than in males.
Fig. 4.
Absolute number of CD3+ T cells in samples from male (▪) and female (▵) subjects at different ages shown in (a) reveal no gender difference (P > 0·10). Changes in the number of sjTREC molecules per CD3+ T cells for male (▪) and female (▵) subjects at different ages shown in (b) show a gender difference (P = 0·002), with females having higher sjTRECs than males. For females, the line of best fit formula was y = 0·0586e0·1798x and R2 = 0·72. For males, the line of best fit formula was y = 0·0143e−0·168x and R2 = 0·65.
Fig. 5.
An example of CD3+ cells FACS analysis from a 26-year-old male (a) and 26-year-old female (b) sample showing the naïve CD45RA+ CD62L+ T-cell population. Absolute numbers of naïve T cells per 107 PBMC at different ages for males (▪) and females (▵) are shown in (c). Changes in the number of sjTREC molecules per naïve T cells for male (▪) and female (▵) subjects at different ages are shown in (d), where they continue to show gender difference (P = 0·006), with females having more sjTRECs than males. For females the line of best fit formula was y = 0·1962e0·1516x and R2 = 0·53. For males the line of best fit formula was y = 0·0209e−0·1183x and R2 = 0·0.35.
DISCUSSION
Our result showing the progressive decline in sjTREC numbers with age agrees with previous studies showing a decline in the contribution of thymic emigrants to the peripheral T-cell pool with age [9,18,21]. However, the significant effect of gender in this process has not previously been established.
Comparison of sjTREC levels in males and females shows values to be persistently higher in females between the ages of 20 and 50, suggesting that thymic output is prolonged at higher levels in females compared with males.
There were some obvious and trivial explanations for our data, which we were able to discount. Firstly, the observed gender difference in sjTREC values was not due to a possible difference in the age range of the female and male volunteers employed in the study since comparison of the age ranges for each gender showed no significant difference (P = 0·095). Secondly, analysis of T-cell (CD3+) numbers in the volunteers' samples revealed no gender differences, dismissing the possibility that the observation could be due merely to differences in the absolute number of T cells in the samples. Calculation of the number of sjTREC molecules per CD3+ T cell, and the correlation of this number with age, again revealed significant gender differences, thus preventing any bias in the data due to individual variation in CD3+ T-cell numbers.
Other possible explanations were also considered. Survival times for T cells within the naïve T-cell pool may be greater in females than in males, producing the higher female sjTREC levels seen in this analysis. This would, however, be expected to produce a larger number and percentage of naïve T cells in females than in males. Our FACS analysis of T-lymphocyte subpopulations revealed no significant difference in the number of naïve (CD3+ CD45RA+ CD62L+) T cells between males and females, thus discounting this possibility. Calculation of the number of sjTREC per naïve T cell revealed a gender difference with a more rapid age-related drop in males compared with females. The naïve T-cell population has often been considered to be a non-dividing pool of cells dependant on thymic export to maintain its size [20]. However, an alternative reason for the observed gender difference in sjTREC levels may be that T cells with a naïve phenotype are turning over more rapidly in males than in females without changing phenotype, since sjTREC levels are a marker of division within a cell population, showing a reduced frequency in the dividing populations [9]. Another possibility is that there is a gender-related difference in the migratory pathways of T cells in the blood.
To determine whether there were any functional implications for differences in the levels of sjTREC, we looked at mortality statistics, provided by the Office for National Statistics in the UK for the period 1993–98, for deaths due to pneumonia and influenza. Antigenic variation within the underlying causative organisms means that a less than competent response by the naïve T-cell pool could lead to disease susceptibility and eventual mortality. We hypothesized that greater thymic output would lead to a larger naïve T-cell pool repertoire, a more competent immune response and reduced mortality. Our analysis revealed that over the age range of our study subjects, the number of deaths in the male population was significantly higher (P = 0·001) compared with females (data not shown). The relationship between gender and death due to influenza and pneumonia, with more males dying in the sample age range than females, follows a trend similar to the results of thymic output and the expectation of improved efficiency in the immune response. Immune insufficiency due to reduced thymic output, particularly in males, may be a likely predisposing factor at the root of the gender difference in deaths.
Hormonal differences would appear to be the most probable cause of gender-related differences in the rate of thymic involution, with androgens such as the reproductive hormone testosterone being the most likely culprits. Standard tables for levels of testosterone show a difference between adult males and females of 10 nmol L−1 [22]. Furthermore, testosterone levels are known to decline with age in men, possibly accounting for the biphasic rate of decline seen in age-associated thymic atrophy. In addition, previous studies using animal models have shown that reduction in testosterone levels in males by chemical or surgical castration leads to a reversal of age-related thymic atrophy [6,23].
The consequences of prolonging higher levels of thymic output have been shown experimentally by the sequential grafting of syngeneic thymuses in an animal model. Analysis of a group of animals treated in this manner showed the procedure to result in the extension of mean life expectancy in these animals compared with the controls [24]. It is tempting to speculate that the consequence of improved thymic output in females compared with males may contribute towards the difference in life expectancy seen between the sexes.
Acknowledgments
This work was supported by the Welcome Trust (Grant Number 051541) and the Luard family (J.P-L is the Luard scholar). The authors would like to thank Chris von Roy for technical support, Prof. Frances Gotch for fruitful discussions and the Lnard family for their generosity.
REFERENCES
- 1.Berzins SP, Boyd RL, Miller JF. The role of the thymus and recent thymic migrants in the maintenance of the adult peripheral lymphocyte pool. J Exp Med. 1998;187:1839–48. doi: 10.1084/jem.187.11.1839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.George AJ, Ritter MA. Thymic involution with ageing: obsolescence or good housekeeping? [see comments] Immunol Today. 1996;17:267–72. doi: 10.1016/0167-5699(96)80543-3. [DOI] [PubMed] [Google Scholar]
- 3.Tyan ML. Age-related decrease in mouse T cell progenitors. J Immunol. 1977;118:846–51. [PubMed] [Google Scholar]
- 4.Aspinall R. Age-associated thymic atrophy in the mouse is due to a deficiency affecting rearrangement of the TCR during intrathymic T cell development. J Immunol. 1997;158:3037–45. [PubMed] [Google Scholar]
- 5.Haynes BF, Markert ML, Sempowski GD, et al. The role of the thymus in immune reconstitution in aging, bone marrow transplantation, and HIV-1 infection. Annu Rev Immunol. 2000;18:529–60. doi: 10.1146/annurev.immunol.18.1.529. [DOI] [PubMed] [Google Scholar]
- 6.Kendall MD, Fitzpatrick FT, Greenstein BD, et al. Reversal of ageing changes in the thymus of rats by chemical or surgical castration. Cell Tissue Res. 1990;261:555–64. doi: 10.1007/BF00313535. [DOI] [PubMed] [Google Scholar]
- 7.Binz K, Joller P, Froesch P, et al. Repopulation of the atrophied thymus in diabetic rats by insulin-like growth factor I. Proc Natl Acad Sci USA. 1990;87:3690–4. doi: 10.1073/pnas.87.10.3690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tanchot C, Lemonnier FA, Perarnau B, et al. Differential requirements for survival and proliferation of CD8 naive or memory T cells. Science. 1997;276:2057–62. doi: 10.1126/science.276.5321.2057. [DOI] [PubMed] [Google Scholar]
- 9.Douek DC, McFarland RD, Keiser PH, et al. Changes in thymic function with age and during the treatment of HIV infection. Nature. 1998;396:690–5. doi: 10.1038/25374. [DOI] [PubMed] [Google Scholar]
- 10.Kong F, Chen CH, Cooper MD. Thymic function can be accurately monitored by the level of recent T cell emigrants in the circulation. Immunity. 1998;8:97–104. doi: 10.1016/s1074-7613(00)80462-8. [DOI] [PubMed] [Google Scholar]
- 11.Bernstein ED, Gardner EM, Abrutyn E. Cytokine production after influenza vaccination in a healthy elderly population. Vaccine. 1998;16:1722–31. doi: 10.1016/s0264-410x(98)00140-6. [DOI] [PubMed] [Google Scholar]
- 12.Burns EA, Lum LG, L'Hommedieu G, et al. Specific humoral immunity in the elderly: in vivo and in vitro response to vaccination. J Gerontol. 1993;48:B231–6. doi: 10.1093/geronj/48.6.b231. [DOI] [PubMed] [Google Scholar]
- 13.Miller L, Hunt JS. Sex steroid hormones and macrophage function. Life Sci. 1996;59:1–14. doi: 10.1016/0024-3205(96)00122-1. [DOI] [PubMed] [Google Scholar]
- 14.Olsen NJ, Kovacs WJ. Gonadal steroids and immunity. Endocr Rev. 1996;17:369–84. doi: 10.1210/edrv-17-4-369. [DOI] [PubMed] [Google Scholar]
- 15.Simpson JG, Gray ES, Beck JS. Age involution in the normal human adult thymus. Clin Exp Immunol. 1975;19:261–5. [PMC free article] [PubMed] [Google Scholar]
- 16.Smith SM, Ossa Gomez LJ. A quantitative histologic comparison of the thymus in 100 healthy and diseased adults. Am J Clin Pathol. 1981;76:657–65. doi: 10.1093/ajcp/76.5.657. [DOI] [PubMed] [Google Scholar]
- 17.Aspinall R, Andrew D. Gender related differences in the rates of age-associated thymic atrophy. Dev Immunol. 8:95–107. doi: 10.1155/2001/17064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Aspinall R, Pido-Lopez J, Andrew DA. simple method for the measurement of sjTREC levels in blood. Mech Ageing Dev. 2001;121:59–69. doi: 10.1016/s0047-6374(00)00197-4. [DOI] [PubMed] [Google Scholar]
- 19.Mackall CL, Fleisher TA, Brown MR, et al. Age, thymopoiesis, and CD4+ T-lymphocyte regeneration after intensive chemotherapy. N Engl J Med. 1995;332:143–9. doi: 10.1056/NEJM199501193320303. [DOI] [PubMed] [Google Scholar]
- 20.Tanchot C, Rocha B. The peripheral T cell repertoire: independent homeostatic regulation of virgin and activated CD8+ T cell pools. Eur J Immunol. 1995;25:2127–36. doi: 10.1002/eji.1830250802. [DOI] [PubMed] [Google Scholar]
- 21.Whitacre CA, Reingold SC, O'Looney PA Task Force. A gender gap in autoimmunity. Science. 1999;283:1277–8. doi: 10.1126/science.283.5406.1277. [DOI] [PubMed] [Google Scholar]
- 22.Kumar PJ, Clark ML. Clinical Medicine. 2. Bailliere Tindall; 1990. [Google Scholar]
- 23.Greenstein BD, Fitzpatrick FT, Adcock IM, et al. Reappearance of the thymus in old rats after orchidectomy: inhibition of regeneration by testosterone. J Endocrinol. 1986;110:417–22. doi: 10.1677/joe.0.1100417. [DOI] [PubMed] [Google Scholar]
- 24.Hirokawa K, Utsuyama M. The effect of sequential multiple grafting of syngeneic newborn thymus on the immune functions and life expectancy of aging mice. Mech Ageing Dev. 1984;28:111–21. doi: 10.1016/0047-6374(84)90157-x. [DOI] [PubMed] [Google Scholar]