Abstract
In utero transmission of HIV-1 has been demonstrated and may account for around 10–20% of all materno–fetal HIV-1 transmission. The possible routes for such transmission are transannexial or transplacental. In both cases, the microenvironment (cytokines and chemokines) at the placental interface could be an important regulatory factor in viral transmission.
We therefore performed explant cultures of placental villi, and isolated purified trophoblasts, from term placentae obtained from HIV-1-seropositive and HIV-1-seronegative women in order to assess and compare the cytokine and chemokine secretion profiles using ELISA and semiquantitative RT-PCR.
No major differences could be seen in the secretions of cytokines and chemokines at the level of whole placental tissue in HIV-1-positive and HIV-1-negative women. However, variations were observed in the expression of inflammatory cytokines and chemokines from trophoblastic cells, depending on the status of HIV-1 infection of the mothers but not the babies, all of which remained uninfected. The significance of these data is discussed.
Keywords: cytokines, chemokines, placenta, materno–fetal HIV-1 transmission
INTRODUCTION
Materno–fetal transmission of HIV is increasing worldwide, especially in nondeveloped countries. The transmission rate is between 15 and 40% in the absence of prophylactic treatment, and falls to 5–8% with long-term AZT treatment [1] and to 2·5% in women undergoing bi-therapy [2]. Outside the developed countries, more feasible shorter-term treatments lower the transmission rate to around 13% [3].
The risk of transmission seems to be positively correlated with a high maternal viral load, and maternal clinical status. Epidemiological studies indicate that transmission can occur in utero either early or, for the most part, late in pregnancy and during delivery. Also important, and now well-documented, is transmission by milk during breast-feeding.
In early and late pregnancy, infection of the placenta, as well as fetal tissues such as the thymus, occurs [4–7]. While transmission could be transannexial, most HIV-1 transmission to the infant in utero is thought to occur through the placenta, based on indirect epidemiological evidence [8] and in situ detection [9,10]. This is considered a probable route by most researchers [11] although, unlike in monkeys [12], its existence cannot be proved directly. The possible (nonmutually exclusive) mechanisms that could be involved in such transplacental transmission are multiple. The first step, leakage through the trophoblastic barrier, could involve: a direct passage of infected maternal cells into the fetal circulatory system via microbreaches in the placental barrier, which is thinner and more vulnerable at the end of pregnancy [13]; the passage of virus complexed with maternal antibodies via placental FcγRs [14] or the recently cloned FcRn [15], which may be implicated in placental infection [16,17]; or the direct passage of maternal viruses by transcytosis [18]. Finally, there could also be a direct infection of the trophoblasts themselves, since infection of syncytiotrophoblasts has been found in vivo [4,19], although in vitro infection is much less easy to obtain with primary strains than originally seen with laboratory strains [16,20] and at best yields a poor viral production [21,22]. Irrespective of these initial mechanisms, subsequent successive infections of placental cells are required and indeed in vivo infection of Hofbauer cells [4], as well as in vitro infection of placental macrophages [23], has been demonstrated. The local microenvironment should play a fundamental regulatory role in this process, and cytokines and chemokines, as in other systems, could modulate transcytosis, cell-to-cell infection and permissivity towards virus entry of placental cells.
Several chemokines and cytokines, important in the placental microenvironment, could be major regulators in transplacental transmission. Three pro-inflammatory β chemokines, MIP-1α, MIP-1β and RANTES, known to act as chemo-attractants and activators for monocytes and T lymphocytes are also known to inhibit HIV-1 entry into target cells, since their cellular receptor is also the main coreceptor for primary (M-tropic) HIV-1 strains [24–27]. HIV-1 coreceptors, including CCR5, are expressed on early trophoblasts [28] and most likely on placental macrophages and Hofbauer cells as well.
Cytokines involved in placental and fetal growth and/or the regulation of HIV replication could also be important in transmission. Firstly, the pro-inflammatory cytokines IL-1β, IL-6 and TNF-α are known to be dysregulated during HIV-1 infection [29,30] and to enhance HIV-1 replication in vitro [31]. Secondly, the growth factors M-CSF and GM-CSF are involved in placental development [32] and the latter modulates HIV-1 replication in vitro [33]. Thirdly, for several authors, successful allopregnancy ‘is a Th2 phenomenon’, with selective down-regulation of local maternal antipaternal cellular immunity [34] and excess local production of Th1 cytokines compromising fetal survival. Finally, the chemokine IL-8 may be important as an attractant of immune cells, such as NK in mice [35] and probably humans [36], non-T non-B TGF-β2 secreting suppressor cells [37], as well as αβ and γδ T cells [38], seemingly essential for implantation and later on for decidual functions.
We have compared the level of expression of such cytokines and chemokines in the placentae of HIV-1-positive and HIV-1-negative women and found differential expression levels when comparing isolated trophoblastic cell cultures but not placental explants.
MATERIALS AND METHODS
Placentae
Human term placentae from HIV-1-negative women were obtained aseptically from programmed term caesarean deliveries (n = 15) (Table 1).
Table 1.
Characteristics of placentae
| Term seronegative | Term seropositive | |
|---|---|---|
| Number | 15 | 15 |
| Term | 38·5 WA* (37·5–39) | 38·5 WA (36–40·5) |
| Delivery | Programmed caesarean section | 2 emergency caesarean sections |
| 13 elective caesarean sections | ||
| CD4 number | – | 490 CD4/mm3 (130–778)† |
| Viraemia | – | 1780 copies/ml (< 50–12912)† |
| Treatment | – | AZT/AZT + 3TC or ddI/AZT + 3TC + protease inhibitor/d4T + 3TC or ddI + protease inhibitor‡ |
Weeks of amenorrhoea.
Median value (minimum–maximum).
Different types of treatment followed by HIV-1-seropositive mothers are described and separated by slash bars.
None of the term babies were infected.
Term placentae from HIV-1-seropositive women came from Rothschild, St Antoine, and Antoine Béclère Hospitals (n = 15). Two placentae were obtained from emergency caesarean sections, with the remaining 13 resulting from programmed elective caesarean delivery (Table 1).
It is important to note that none of the term babies proved to be infected.
Placental explants
Placental villi were isolated, minced, and a total weight of 3 g explant fragments of villi cultured in 20 ml of culture medium (see below). Reproducibility in cytokine and chemokine secretion between placental explants was always found to be around 90%. Placental tissues cultured for 48 h, from term (HIV− and HIV+) placentae, had good viability when tested by Trypan blue exclusion. The absence of cytokines and chemokines in the culture medium was verified.
Preparation of trophoblastic cells
The enrichment procedure is a modified Kliman technique [39]. Briefly, villi fragments of term placentae were washed and digested for four cycles using 0·625 g of trypsin 1:250 (Difco, Detroit, MI, USA) in HBSS without Ca2+/Mg2+/phenol red, containing 2% penicillin-streptomycin, 2·5% HEPES buffer 1 m (HBSS and supplements: Gibco Ltd, Paisley, Scotland). The cells were separated by centrifugation (700 g) on a 5%–70% discontinuous Percoll gradient (Pharmacia, Uppsala, Sweden). Cells from the layer corresponding to the density 1·045–1·058 g/ml, known to be enriched for trophoblasts, were collected and immunodepletion was performed with monoclonal antibodies directed against human CD3 (T lymphocytes), human CD9 (fibroblasts), human CD31 (B lymphocytes, NK cells and endothelial cells) (Coulter Immunotech, Marseille, France), human CD14 (macrophages) or human CD45 (all leucocyte lineage) (Becton Dickinson, San Jose, CA, USA). Contaminating cells were then separated using sheep anti mouse IgG coated magnetic beads (Dynabeads, Dynal AS, Oslo, Norway). The purity of the cells was always immediately assessed by FACS (as described) and was always greater than 96% trophoblasts. The main contaminating cells (1–4%) were fibroblasts and macrophages [34]. Cells were then seeded at 2 × 105 viable trophoblasts per ml in the culture medium.
Culture conditions
Cultures were performed in RPMI 1640-glutamax medium (explants) or nutrient mixture F-10 HAM (trophoblasts) both supplemented with 10% heat inactivated fetal bovine serum (FBS), 1% penicillin-streptomycin and 1% sodium bicarbonate (all from Gibco Ltd, Paisley, Scotland) and maintained at 37°C in a 5% CO2 humid incubator. Supernatants were harvested after 3, 6, 12, 24, 48 or 72 h of culture. Each supernatant was centrifuged at 2000 g and stored at − 80°C for later analysis. For reproducibility and meaningful comparison of the samples, the same batch of FBS was used throughout.
Cell lines
BeWo choriocarcinoma (obtained from ATCC) and the CD4-transfected astrocytoma U87 cell line (provided by Drs Eva Maria Fenyö and Gabriella Scarlatti, Karolinska Institute, Sweden, and Institute San Raffaelo, Milano, Italy) were used as controls in FACS analysis.
Staining and flow cytometry analysis
To assess the purity of trophoblasts, we monitored the presence of trophoblasts and of possible contaminating lymphocytes, macrophages and fibroblasts by indirect and direct labelling. Indirect labelling of contaminating cells used the same primary monoclonal antibodies as for immune depletion (anti CD3, CD9, CD14, CD31, CD45 and CD163). Indirect labelling of trophoblasts used the GB25 trophoblast-specific monoclonal antibody [40] (a kind gift from Aster Biotechnologes, La Gaude, France) as the primary antibody. A GAM FITC (fluorescein-conjugated goat antimouse) F(ab′)2 fragment (Coulter Immunotech, Marseille, France) was used as the second antibody. All antibodies were used at a final concentration of 20 µg/ml in HBSS without Ca2+/Mg2+/phenol red, plus 1% bovine serum albumin and 0·1% sodium azide. Direct labelling was perfomed using fluorescein-conjugated antibodies directed against CD2 (T lymphocytes), CD11b (macrophages), CD20 (B lymphocytes), CD36 (monocytes, macrophages), and fluorescein-conjugated isotypic immunoglobulins (all from Coulter Immunotech, Marseille, France) at 20 µg/ml. At least 20 000 events were analysed on a FACScan cytofluorimeter (Becton Dickinson, San Jose, CA, USA).
Antibodies used for intracellular staining
We also used mouse antihuman cytokeratin 7, mouse antihuman vimentin (DAKO Copenhagen, Denmark), (FITC)-conjugated mouse antihuman cytokeratin 7 (Sigma, St Quentin Fallavier, France) monoclonal antibodies, goat antihuman cytokeratin 7 and goat antihuman vimentin polyclonal antibodies (Santa Cruz Biotechnology (USA). PE or FITC-conjugated rabbit antimouse F(ab′)2 and FITC-conjugated rabbit antigoat F(ab′)2 were used as secondary antibodies (also from Dako). Affinity purified (Texas Red)-conjugated antimouse IgG2 (γ2a chain specific) monoclonal antibody was purchased from Rockland (Gilbertsville, USA).
Intra- and extracellular staining for further monitoring the contamination of trophoblasts
When analysing trophoblastic cells by FACS, we used as controls BeW0 choriocarcinoma and vimentin + + + (CD4-transfected) U87 cells. In addition to membrane staining, intracellular staining was performed for cytokeratin 7 and vimentin intermediate filaments. After saturation at 4°C for 20 min with Normal Goat Serum, cells were incubated with a fixative reagent (Reactive A, Intrastain, DAKO) at 4°C for 15 min, washed and placed in a permeablization solution (Reactive B, Intrastain, DAKO) at 4°C for 10 min followed by incubation at 4°C for 20 min with antibodies against cytokeratin or human vimentin. After two washes with PBS/saponin (1% BSA, 0·01% NaN3, 0·03% saponin), PE- or FITC-conjugated rabbit antimouse F(ab′)2 secondary antibodies were incubated at 4°C for 20 min. Cells were then washed twice and conserved with 1% Paraformaldehyde/PBS before FACS analysis. In some experiments the polyclonal antibody against cytokeratin 7 or vimentin and the cytokeratin 7 FITC-coupled monoclonal antibody were also tested. When polyclonal antibodies were used, the blocking step was done with Normal Swine Serum and PE- or FITC-conjugated Rabbit antigoat monoclonal antibodies were used as the secondary antibodies. Nonspecific isotypic control monoclonal antibodies were always included for each specific antibody. In order to test the profile of markers between whole and purified trophoblast cells, contaminating cells were detached from magnetic beads after culture at 37°C for 24 h under 10% CO2 in an humidified atmosphere, and were processed for FACS analysis as indicated for trophoblast cells. Similarly, in order to test whether the remaining contaminating cells (particularly fibroblasts) could proliferate, purified trophoblast cells were cultured for 24 h in Ham-F10 medium (Gibco-BRL, UK) supplemented with 10% FCS, and the patterns of cytokeratin 7/vimentin immediately after purification and at 24 h were assessed.
ELISAs
MIP-1α, MIP-1β, RANTES and M-CSF ELISAs were performed using Quantikine kits (R & D Systems Ltd, Oxon, UK) as per the manufacturer's instructions. IL-8, IL-1β, IL-6, TNF-α, GM-CSF, IL-10, IFN-γ, IL-4, IL-13 and IL-12 ELISAs were performed using Immunotech kits (Beckmann Coulter, Marseille, France). Sensitivities and assay precisions are described in Table 2.
Table 2.
ELISA
| Cytokine | Supplier | Sensitivity | Inter-assay precision | Intra-assay precision |
|---|---|---|---|---|
| MIP-1α | R & D System | 6 pg/ml | 5% | 2% |
| MIP-1β | R & D System | 4 pg/ml | 9% | 4% |
| RANTES | R & D System | 5 pg/ml | 6% | 2% |
| IL-8 | Immunotech | 8 pg/ml | 8% | 4% |
| IL-1β | Immunotech | 15 pg/ml | 7% | 5% |
| IL-6 | Immunotech | 3 pg/ml | 10% | 3% |
| TNF-α | Immunotech | 5 pg/ml | 8% | 6% |
| IL-4 | Immunotech | 5 pg/ml | 9% | 3% |
| IL-10 | Immunotech | 5 pg/ml | 7% | 4% |
| IL-13 | Immunotech | 2 pg/ml | 9% | 3% |
| IL-12 | R & D System | 5 pg/ml | 5% | 2% |
| IFN-γ | Immunotech | 0·08 IU/ml | 8% | 5% |
| GM-CSF | Immunotech | 5 pg/ml | 8% | 3% |
| M-CSF | R & D System | 9 pg/ml | 7% | 2% |
Detection of cytokine and chemokine mRNA by RT-PCR
RNA extraction
Total RNA was extracted as per the manufacturer's instructions using 1 ml of RNA-B (Bioprobe, Montreuil, France) for 5 × 106 cells. After isopropanol precipitation, the RNA was purified from contaminating DNA by a 30 min, 37°C, DNAse treatment (10 units of DNAse RNAse free, Boehringer, Mannheim, Germany) in Sodium Acetate 0·5 m, MgSO4 2·5 mm, in the presence of Human Placental Ribonuclease Inhibitor (10 units of HPRI, Boehringer Mannheim, Germany). The RNA was then extracted using the standard phenol-Sevag procedure (Sevag: Chloroform-Isoamylic Alcohol 24/1 volume). Total RNA (aqueous phase) was finally precipitated by the addition of Sodium Acetate 3M (1/12·5 volume) and Absolute Ethanol (2·2/1 volume).
Reverse transcription
mRNAs were reverse transcribed by use of an oligo-dT primer (Boehringer, Mannheim, Germany) and the M-MLV Reverse Transcriptase kit (Gibco BRL Ltd, Paisley, Scotland) in the presence of Human Placental Ribonuclease Inhibitor (40 units of HPRI, Boehringer, Mannheim, Germany). The reaction was performed at 42°C for 1 h.
Cytokine and chemokine cDNA amplification
Each cDNA was amplified by PCR using specific primers (Eurobio, Les Ulis, France) (Table 3). The PCR mixture contained 10 mm of each dNTP, 100 ng of each specific primer, reaction buffer 1X supplied by the manufacturer (Appligene, Pleasanton, CA, USA) and 1·25 University of Taq Polymerase (Appligene, Pleasanton, CA, USA). The PCRs were performed in a Croco II amplifier (Appligene, Pleasanton, CA, USA). cDNA extracted from PHA-P and IL-2 stimulated human mononuclear cells was used as a control in each PCR, and dilutions were performed to obtain a standard linear graph of amplification. Sample concentrations of cDNA were then chosen to ensure that the level of amplification would be in the linear zone of the graph. A negative control without DNA was always included.
Table 3.
Oligoprimers used for RT-PCR detection of cytokines and chemokines
| Cytokine | Primer's sequence | Product's length | T (°C) | Cycles | |
|---|---|---|---|---|---|
| GAPDH | 5′ | ACCACCATGGAGAAGGCTGG | 509 bp | 60 | 32 |
| 3′ | CTCAGTGTAGCCCAGGATGC | ||||
| MIP-1α | 5′ | TCGAGCCCACATTCCGTCAC | 618 bp | 60 | 41 |
| 3′ | GCTTTGGTGCCATGACTGCC | ||||
| MIP-1β | 5′ | CGTCGTGGTCAGAATCTGGG | 320 bp | 56 | 40 |
| 3′ | TCATTGCTACTGCCCTCTGC | ||||
| RANTES | 5′ | CCAAACCAAAAGAAGCAAGC | 373 bp | 58 | 42 |
| 3′ | AGAAACAGTGACAGTGGACC | ||||
| IL-1β | 5′ | ATGGCAGAAGTACCTGAGCTC | 810 bp | 60 | 40 |
| 3′ | TTAGGAAGACACAAATTGCATGGTGAA | ||||
| IL-6 | 5′ | ATGAACTCCTTCTCCACAAGC | 639 bp | 60 | 40 |
| 3′ | CTACATTTGCCGAAGAGCCCTCAGGCTGGACTG | ||||
| TNF-α | 5′ | ATGAGCCTGAAAGCATGATC | 702 bp | 60 | 40 |
| 3′ | TCACAGGGCAATGATCCCAAAGTAGACCTGC | ||||
| IL-10 | 5′ | ATGCCCCAAGCTGAGAACCAAGACCCA | 325 bp | 65 | 40 |
| 3′ | TCTCAAGGGGCTGGGTCAGCTATCCCA | ||||
| GM-CSF | 5′ | GAGGATGTGGCTGCAGAGC | 439 bp | 65 | 40 |
| 3′ | TCACTCCTGGACTGGCTCCC | ||||
| IFN-γ | 5′ | ATGAAATATACAAGTTATATCTTGGCT | 501 bp | 60 | 40 |
| 3′ | GCGACAGTTCAGCCATCACTTG |
PCR products were separated on a 1·5% agarose gel in TAE buffer, and levels of amplification were measured by integration of the intensity of bands with NIH imaging software. For each sample, the ratio (amplification of interest gene/amplification of GAPDH) was calculated.
Statistical analysis
Different populations were compared using the nonparametric Mann and Whitney test with the Bonferonni correction factor when appropriate. All data are plotted in box plots; the horizontal lines in the boxes are the medians of each group of points. The square boxes contain 50% of the data centred around the median. The vertical lines begin at the 10th percentile and end at the 90th percentile. Each data element below the 10th percentile or above the 90th percentile is plotted individually.
RESULTS
Cytokines and chemokines produced by human term seronegative placenta
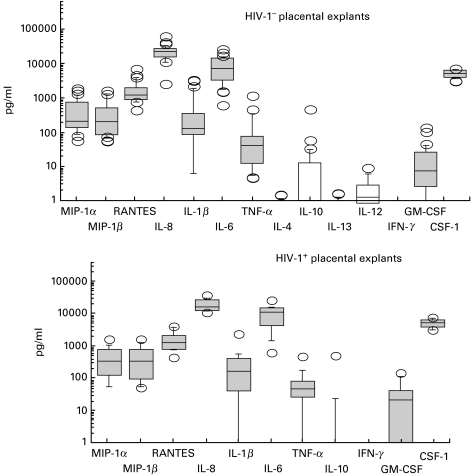
We monitored the secretion of several cytokines and chemokines in the supernatants of cultures of human term placental explants from HIV-1− women. Pro-inflammatory cytokines (IL-1β, IL-6 and TNF-α), growth factors (GM-CSF and M-CSF) and chemokines (MIP-1α, MIP-1β, RANTES and IL-8) were all detected (Fig. 1 and Table 4). In our culture conditions, the major components of the term placental microenvironment appeared to be IL-6, M-CSF, IL-8 and RANTES, all highly secreted by term chorionic villi. By contrast, and within the sensitivity limits of the assays, we were unable to trace significant production of any Th1 (IFN-γ, IL-12) or Th2 (IL-4, IL-13) cytokines at any time of culture in term placentae, save for a very low level of IL-10.
Fig. 1.
ELISA determination of cytokine and chemokine production by explants from term placentae from HIV-1-seropositive and HIV-1-seronegative women. The plotting method is explained in the materials and methods section.
Table 4.
Cytokine and chemokine production by human term seronegative placental explants
| Minima | Maxima | Median | |
|---|---|---|---|
| MIP-1α | 79 | 1825 | 171 |
| MIP-1β | 77 | 1625 | 155 |
| RANTES | 827 | 6968 | 1249 |
| IL-8 | 2566 | 63878 | 23689 |
| IL-1β | Undetectable | 3368 | 129 |
| IL-6 | 1635 | 20439 | 5046 |
| TNF-α | 5 | 1196 | 41 |
| GM-CSF | Undetectable | 39 | 6 |
| M-CSF | 3079 | 7570 | 5845 |
| IL-4 | Undetectable | Undetectable | Undetectable |
| IL-10 | Undetectable | 60 | 10 |
| IL-13 | Undetectable | Undetectable | Undetectable |
| IFN-γ | Undetectable | Undetectable | Undetectable |
| IL-12 | Undetectable | Undetectable | Undetectable |
Results of ELISA detection in supernatants of cultures of placental explants are expressed in pg/ml, except for IFN-γ (IU/ml).
Kinetics were performed for 10 term placentae and for all the cytokines, to determine whether the secretion of cytokines and β chemokines showed peaks or reached a plateau after a lag culture period. Except for TNF-α, whose secretion pattern appeared to be rather erratic and without any common kinetics between individuals, all cytokines were secreted in a time-dependent manner, secretions reaching a plateau after 24 h of culture (the data presented in all figures and Tables 4 and 5 are from 24 h of culture).
Table 5.
Comparison between term seronegative and term seropositive placentae
| Cytokine | HIV-1− term placentae (n = 15, pg/ml) | HIV-1+ term placentae (n = 15, pg/ml) |
|---|---|---|
| MIP-1α | 171 | 338 |
| MIP-1β | 155 | 334 |
| RANTES | 1249 | 1280 |
| IL-1β | 129 | 169 |
| IL-6 | 5046 | 14682 |
| TNF-α | 41 | 49 |
| IL-10 | 11 | 0 |
| IFN-γ | 0 | 0 |
| GM-CSF | 6 | 20 |
| CSF-1 | 5845 | 4893 |
| IL-8 | 23689 | 19437 |
Medians of secretion of cytokines and chemokines in supernatants of cultures of placental explants are expressed in pg/ml except for IFN-γ (IU/ml). Statistical tests were performed comparing early placentae with term HIV-1-negative placentae, or HIV-1-positive placentae with HIV-1-negative placentae.
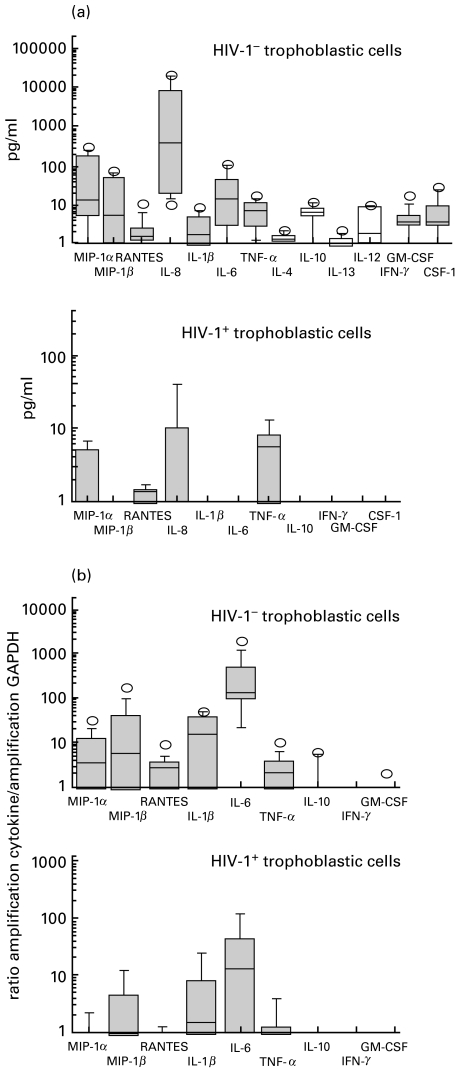
The production of cytokines and chemokines was also tested in supernatants of cultures of isolated trophoblasts. MIP-1α, MIP-1β, IL-6, IL-8, IL-1β, TNF-α, GM-CSF and M-CSF were detectable only at low levels and in relatively few supernatants (8/15 for MIP-1α, 7/15 for MIP-1β, 2/15 for IL-1β, 1/15 for TNF-α, 9/13 for IL-6, 1/12 for GM-CSF, 2/12 for M-CSF and 12/12 for IL-8) (Fig. 2a). For six preparations of trophoblasts from different placentae, the kinetics of the produced factors were tested. These secretions always reached a plateau after 24 h of culture.
Fig. 2.
Production of the different cytokines and chemokines by cultures of purified trophoblastic cells from term placentae from HIV-1-seropositive and HIV-1-seronegative women. (a) Proteins were measured in culture supernatants by ELISA. (b) mRNA expression was measured using RT-PCR amplification and expressed as the ratio of cytokine or chemokine signals versus GAPDH signals. The plotting method is explained in the materials and methods section.
To confirm the ELISA data, mRNA was extracted from trophoblasts after 24 h of culture and the presence of mRNA for specific cytokines evaluated by semiquantitative RT-PCR. Under our conditions, we were able to detect the presence of mRNA for the three β chemokines, as well as those for IL-1β, IL-6, TNF-α and GM-CSF in trophoblastic cells, but mRNA for IL-10 was either rare or absent and mRNA for IFN-γ was never detected (Fig. 2b).
Differential expression patterns in HIV-1+ term placentae
When cytokines were assessed by ELISA, chorionic villi were found in vitro to secrete the same cytokines whether from the placentae of seropositive or seronegative women (Fig. 1 and Table 5). We could not see any correlation between the secretion of cytokines and chemokines and the CD4+-T cell counts or the viraemia of the seropositive mothers.
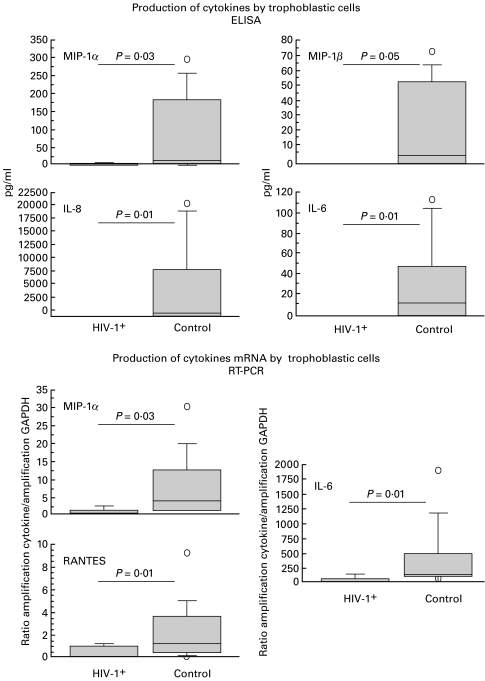
Even though few cytokines were detectable by ELISA in the supernatants of trophoblasts isolated from term placentae of HIV-1-seropositive mothers (2/6 for MIP-1α, 1/6 for MIP-1β, 1/6 for IL-10, 2/8 for IL-8 and 3/6 for TNF-α), we found significantly lower secretion of MIP-1α (P = 0·03), MIP-1β (P = 0·05), IL-8 (P = 0·01), and IL-6 (P = 0·01) by term trophoblasts from placentae of seropositive women compared with those of seronegative women (Figs 2a and 3).
Fig. 3.
Differences in production of cytokines and chemokines by trophoblastic cells isolated from term placentae of HIV-infected and noninfected women. Upper panel: ELISA determination in culture supernatants. Lower panel: cytokine mRNA levels versus GAPDH mRNA signals. P values are for the non-parametric Mann & Whitney test. The plotting method is explained in the materials and methods section.
Using RT-PCR, the same cytokines could be amplified by RT-PCR from the trophoblasts of seropositive and seronegative placentae but semiquantitative analysis of mRNA encoding MIP-1α (P = 0·03), RANTES (P = 0·01), and IL-6 (P = 0·01) showed decreased expression in trophoblasts from the placentae of seropositive as compared with seronegative women (Figs 2b and 3).
DISCUSSION
Despite abundant literature on the feto–maternal interface during HIV-1 infection, few studies detect and quantify by the same technique a large range of cytokines. We therefore established the pattern of expression of cytokines and chemokines with the same techniques for term placentae of both HIV-1-seropositive and HIV-1-seronegative women, using parallel cultures of explants of chorionic villi and isolated trophoblastic cells. While explants give an indication of which cytokines and chemokines are likely to be spontaneously released by chorionic tissues, the cultures of trophoblastic cells could help us to determine their source.
We tested trophoblast purity at various time intervals after purification, monitoring the expression of cytokeratin 7 (a marker highly expressed along the trophoblast lineage and thus recently proposed as an easily available cell surface marker highly specific for trophoblast cells, lacking vimentin markers). The pattern of cytokeratin 7 expression observed immediately after the purification protocol on trophoblast cells did not significantly differ following 24 h of culture (Fig. 4), regardless of the labelling technique or whether we used monoclonal or polyclonal antibody, thus indicating the purity of the trophoblast preparation.
CD36+ contaminant cells were always present in term trophoblast cell preparations but, using the macrophage specific CD163 marker, it was shown that monocytes/macrophages could not account for the CD36 contamination. Indeed, the profile of vimentin and CD36 expression confirms that the residual contamination is predominantly of fibroblast (or villous mesenchymal) lineage. It is important also to note that we confirmed the trophoblast specific pattern using the GB25 antibody.
Finally, using a fibroblast-specific monoclonal antibody, we were unable to trace significant fibroblast expansion after 24 h of culture, although this becomes apparent after 60 h and then regularly and rapidly increases. This was confirmed by intracellular staining. The validity of the markers used was assessed by using the control BeW0 and U87 cell lines in parallel. Thus we are confident that the 24 h cultures represent a highly purified trophoblast population without any significant involvement of nontrophoblast contaminants.
However, the isolation procedures used to prepare trophoblasts can modify the production of cytokines by these cells [41]. It thus seemed important to deal not only with isolated cells. With explant cultures, we obtained a good tissue viability following 48 h of culture, and the reproducibility of the results was verified. Also, there was no difference in viablilty beween explant cultures of tissues obtained from term placentae of HIV− or HIV+ women, nor when dealing with isolated trophoblastic cells from both term groups. Therefore, data obtained with this type of tissue culture could be the most accurate in vitro representation of the cytokine secretions effectively realized in situ in vivo.
We detected the production of MIP-1α, MIP-1β, RANTES and IL-8 in agreement with other publications that have described the high secretion of IL-8 [42], MIP-1α (albeit in lesser amounts) [43], and RANTES [44] by chorionic tissues. The presence of inflammatory cytokines and growth factors in the placenta noted in our study is also consistent with the literature [45]. However, surprisingly, we could not detect significant secretion of the classical Th2 cytokines, except IL-10 in very small amounts. This discrepancy is reminiscent of a long-standing controversy between positive [46,47] and negative data [48] in mice and humans, our own group having detected placental IL-10 by immunohistochemistry.
We have observed that in our in vitro culture assay system, the cytokine expression profiles differed between the third trimester (see Results section) and the first trimester (independent experiments performed on such placentae, obtained quasi exclusively after RU 486 induced elective pregnancy termination, data not shown). RANTES and IL-6 were expressed to a greater degree by first trimester chorionic villi, while MIP-1β expression was lower. IL-6 is involved in hCG secretion by trophoblasts, which peaks during the first trimester of pregnancy [49], and higher production of IL-6 by first trimester placental explants may correlate with hCG regulation, whereas increased secretion of TNF-α by isolated first trimester trophoblasts may be related to regulation of trophoblast growth and invasion. These differences in cytokine expression profiles may therefore reflect physiological differences in cell migration and invasion between implantation and established pregnancy.
No major differences exist between the cytokine profiles of by explants from term placentae from HIV-1-negative and HIV-1-positive women. This may be related to the absence of infection in the babies. However, MIP-1α, MIP-1β, RANTES and IL-6 were found by ELISA and RT-PCR to be expressed at lower levels by trophoblasts from placentae of seropositive mothers. This is in contrast with the results of Lee et al. [50], who found IL-1β, IL-6 and TNF-α were secreted at significantly higher levels by trophoblasts from the placentae of seropositive mothers. However, their isolation and culture conditions are different from ours: dispase instead of trypsin to separate the trophoblasts; immunodepletions solely by anti-CD45; trophoblasts more concentrated in their cultures; and the use of human AB serum. Also, the populations of HIV-1-infected women differ in the two studies, as the placentae we collected were all from AZT, bi-therapy or tri-therapy treated women, whereas in the study by Lee et al. [50] 18/30 of their placentae were from AZT-treated women and 12/30 came from nontreated women. HIV sequences are readily detected in placentae of HIV+ women who do not undergo such treatments [51], but are absent in placentae from HIV+ women treated by AZT [52].
Since all HIV-1-seropositive women included in this study were undergoing antiretroviral treatment, we cannot exclude an effect of these drugs on cytokine and chemokine expression by placental cells. Moreover, the efficacy of the treatment on the prevention of HIV-1 transmission may be correlated with a normalization of the secretion of cytokines and chemokines in the placenta, hiding HIV-1-induced variations. This effect is difficult to evaluate in vitro, as the prophylaxis used is specific for each woman.
However, this study has highlighted that, even if no major differences exist in the global cytokine and chemokine profiles of the placentae of HIV-1-seropositive women, some cytokines and chemokines may be modulated around the trophoblastic layer, providing a rationale for the further study of the effect of these cytokines in several in vitro systems.
Acknowledgments
We thank all the staff of the Department of Obstetrics and Gynecology of Antoine Béclère Hospital (Clamart, France) for early and term HIV-1-seronegative placentae; and all the Departments of Obstetrics of Rothschild Hospital, of Tenon Hospital, of St Antoine Hospital and of Antoine Béclère Hospital for term HIV-1-positive placentae, as well as collaborators of the FAMA group. Thanks to Sylvie Dubanchet for technical assistance. This work was supported by ANRS, INSERM (France), an EEC BIOMED-2 contract (number PL951509) and CEFIPRA-IFCEPAR. Marlène Moussa was supported by a fellowship from the French Ministère de l'Enseignement Supérieur et de la Recherche.
REFERENCES
- 1.Connor EM, Sperling RS, Gelber R, et al. Reduction of maternal–infant transmission of human immunodeficiency virus type 1 with zidovudine treatment. New Engl J Med. 1994;331:1173–80. doi: 10.1056/NEJM199411033311801. [DOI] [PubMed] [Google Scholar]
- 2.Silverman NS, Watts DH, Hitti J, et al. Initial multicenter experience with double nucleoside therapy for human immunodeficiency virus infection during pregnancy. Infect Dis Obstet Gynecol. 1998;6:237–43. doi: 10.1002/(SICI)1098-0997(1998)6:6<237::AID-IDOG3>3.0.CO;2-E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Guay LA, Musoke P, Fleming T, et al. Intrapartum and neonatal single-dose nevirapine compared with zidovudine for prevention of mother-to-child transmission of HIV-1 in Kampala, Uganda: HIVNET 012 randomised trial. Lancet. 1999;354:795–802. doi: 10.1016/S0140-6736(99)80008-7. [DOI] [PubMed] [Google Scholar]
- 4.Lewis SH, Reynolds-Kohler C, Fox HE, et al. HIV-1 in trophoblastic and villous Hofbauer cells, and haematological precursors in eight-week fetuses. Lancet. 1990;335:565–8. doi: 10.1016/0140-6736(90)90349-a. [DOI] [PubMed] [Google Scholar]
- 5.Courgnaud V, Lauré F, Brossard A, et al. Frequent and early in utero HIV-1 infection. AIDS Res Hum Retro. 1991;7:337–41. doi: 10.1089/aid.1991.7.337. [DOI] [PubMed] [Google Scholar]
- 6.Brossard Y, Aubin JT, Mandelbrot L, et al. Frequency of early in utero HIV-1 infection: a blind DNA polymerase chain reaction study on 100 fetal thymuses. AIDS. 1995;9:359–66. [PubMed] [Google Scholar]
- 7.De Andreis C, Simoni G, Rossella F, et al. HIV-1 proviral DNA polymerase chain reaction detection in chorionic villi after exclusion of maternal contamination by variable number of tandem repeats analysis. AIDS. 1996;10:711–5. doi: 10.1097/00002030-199606001-00004. [DOI] [PubMed] [Google Scholar]
- 8.Chouquet C, Burgard M, Richardson S, et al. Timing of mother-to-child HIV-1 transmission and diagnosis of infection based on polymerase chain reaction in the neonatal period by a non-parametric method. AIDS. 1997;11:1183–99. doi: 10.1097/00002030-199709000-00015. [DOI] [PubMed] [Google Scholar]
- 9.Katz JM, Fox CH, Eglinton GS, et al. Relationship between human immunodeficiency virus-1 RNA identification in placenta and perinatal transmission. J Perinat. 1997;17:119–24. [PubMed] [Google Scholar]
- 10.Sheikh AU, Polliotti BM, Miller RK. Human immunodeficiency virus infection: in situ polymerase chain reaction localization in human placentas after in utero and in vitro infection. Am J Obstet Gynecol. 2000;182:207–13. doi: 10.1016/s0002-9378(00)70514-x. [DOI] [PubMed] [Google Scholar]
- 11.Miller RK, Thiede HA, editors. Trophoblast research. University of Rochester Press; 1994. HIV, perinatal infection and therapy. The role of the placenta. [Google Scholar]
- 12.Martin Amedee A, Lacour N, Martin LN, Clements JE, Bohm RB, Jr, Davison B, Harrison R, Murphey-Corb M. Genotypic analysis of infant macaques infected transplacentally and orally. J Med Primatol. 1996;25(3):225–35. doi: 10.1111/j.1600-0684.1996.tb00020.x. [DOI] [PubMed] [Google Scholar]
- 13.Burton GJ, O'Shea S, Rostron T, et al. Physical breaks in the placental trophoblastic surface: significance in vertical transmission of HIV. AIDS. 1996;10:1294–5. doi: 10.1097/00002030-199609000-00020. [DOI] [PubMed] [Google Scholar]
- 14.Saji F, Samejima Y, Kamiura S, et al. Dynamics of immunoglobulins at the feto–maternal interface. Rev Reprod. 1999;4:81–9. doi: 10.1530/ror.0.0040081. [DOI] [PubMed] [Google Scholar]
- 15.Simister NE, Story CM, Chen HL, et al. An IgG-transporting Fc receptor expressed in the syncytiotrophoblast of human placenta. Eur J Immunol. 1996;26:1527–31. doi: 10.1002/eji.1830260718. [DOI] [PubMed] [Google Scholar]
- 16.David FJE, Tran HC, Serpente N, et al. HIV infection of choriocarcinoma cell lines derived from human placenta: the role of membrane CD4 and Fc-Rs into HIV entry. Virology. 1995;208:784–8. doi: 10.1006/viro.1995.1212. [DOI] [PubMed] [Google Scholar]
- 17.Athanassakis I, Protopapadakis E, Vassiliadis S. Localisation of pepstatin's inhibitory action during Fc-mediated antibody internalisation: Possible implication in antibody-mediated viral transmission. Cell Immunol. 2000;199:81–8. doi: 10.1006/cimm.1999.1606. [DOI] [PubMed] [Google Scholar]
- 18.Bomsel M, Barré-Sinoussi F, Menu E, et al. A selection of maternal viral quasispecies occurs during penetration of HIV across a human trophoblastic tight barrier. Keystone symposium. 1999.
- 19.Backé E, Jimenez E, Unger M, et al. Demonstration of HIV-1 infected cells in human placenta by in situ hybridisation and immunostaining. J Clin Pathol. 1992;45:871–4. doi: 10.1136/jcp.45.10.871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Douglas GC, Fry GN, Thirkill T, et al. Cell-mediated infection of human placental trophoblast with HIV in vitro. AIDS Res Hum Retro. 1991;7:735–40. doi: 10.1089/aid.1991.7.735. [DOI] [PubMed] [Google Scholar]
- 21.Zachar V, Spire B, Hirsch I, et al. Human transformed trophoblast-derived cells lacking CD4 receptor exhibit restricted permissiveness for human immunodeficiency virus type 1. J Virol. 1991;65:2102–7. doi: 10.1128/jvi.65.4.2102-2107.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zachar V, Norskov-Lauritsen N, Juhl C, et al. Susceptibility of cultured human trophoblasts to infection with human immunodeficiency virus type 1. J Gen Virol. 1991;72:1253–560. doi: 10.1099/0022-1317-72-6-1253. [DOI] [PubMed] [Google Scholar]
- 23.Kesson AM, Fear WR, Kazazi F, et al. Human immunodeficiency virus type 1 infection of human placental macrophages in vitro. J Infect Dis. 1993;168:571–9. doi: 10.1093/infdis/168.3.571. [DOI] [PubMed] [Google Scholar]
- 24.Cocchi F, DeVico AL, Garzino-Demo A, et al. Identification of RANTES, MIP-1α, and MIP-1β as the major HIV-suppressive factors produced by CD8+ cells. Science. 1995;270:1811–5. doi: 10.1126/science.270.5243.1811. [DOI] [PubMed] [Google Scholar]
- 25.Deng HK, Liu R, Ellmeier W, et al. Identification of a major co-receptor for primary isolates of HIV-1. Nature. 1996;381:661–7. doi: 10.1038/381661a0. [DOI] [PubMed] [Google Scholar]
- 26.Dragic T, Litwin V, Allaway GP, et al. HIV-1 entry into CD4+ cells is mediated by the chemokine receptor CD-CKR5. Nature. 1996;381:667–73. doi: 10.1038/381667a0. [DOI] [PubMed] [Google Scholar]
- 27.Alkhatib G, Combadiere C, Broder CC, et al. CC CKR5: a RANTES, MIP-1α, MIP-1β receptor as a fusion cofactor for macrophage-tropic HIV-1. Science. 1996;272:1955–8. doi: 10.1126/science.272.5270.1955. [DOI] [PubMed] [Google Scholar]
- 28.Mognetti B, Moussa M, Croitoru J, et al. HIV-1 co-receptor expression on trophoblastic cells from early placentas and permessivity to infection by several HIV-1 primary isolates. Clin Exp Immunol. 2000;119:486–92. doi: 10.1046/j.1365-2249.2000.01149.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sinicco A, Biglino A, Sciandra M, et al. Cytokine network and acute primary HIV-1 infection. AIDS. 1993;7:1167–72. doi: 10.1097/00002030-199309000-00003. [DOI] [PubMed] [Google Scholar]
- 30.Salazar-Gonzalez JF, Martinez-Maza O, Aziz N, et al. Relationship of plasma HIV-RNA levels and levels of TNF-alpha and immune activation products in HIV infection. Clin Immunol Immunopathol. 1997;84:36–45. doi: 10.1006/clin.1997.4364. [DOI] [PubMed] [Google Scholar]
- 31.Israel N, Hazan U, Alcami J, et al. Tumor Necrosis Factor stimulates transcription of HIV-1 in human T lymphocytes, independently and synergistically with mitogens. J Immunol. 1989;143:3956–60. [PubMed] [Google Scholar]
- 32.Garcia-Lloret MI, Morrish DW, Wegmann TG, et al. Demonstration of functional cytokine–placental interactions. M-CSF and GM-CSF stimulate human cytotrophoblast differentiation and peptide hormon secretion. Exp Cell Res. 1994;214:46–54. doi: 10.1006/excr.1994.1232. [DOI] [PubMed] [Google Scholar]
- 33.Kedzieska K, Rainbird MA, Lopez AF, et al. Effect of GM-CSF on HIV-1 replication in monocytes/macrophages in vivo and in vitro: a review. Vet Immunol Immunopathol. 1998;63:111–21. doi: 10.1016/s0165-2427(98)00087-7. [DOI] [PubMed] [Google Scholar]
- 34.Wegmann TG, Lin H, Guilbert L, et al. Bidirectional cytokine interactions in the maternal–fetal relationship: is successful pregnancy a Th2 phenomenon? Immunol Today. 1993;14:353–6. doi: 10.1016/0167-5699(93)90235-D. [DOI] [PubMed] [Google Scholar]
- 35.Guimond MJ, Wang B, Croy BA. Engraftment of bone marrow from severe combined immunodeficient (SCID) mice reverses the reproductive deficits in natural killer cell-deficient tg epsilon 26 mice. J Exp Med. 1998;187:217–23. doi: 10.1084/jem.187.2.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Loke YW, King A. Immunological aspects of human implantation. J Reprod Fertil Suppl. 2000;55:83–90. [PubMed] [Google Scholar]
- 37.Lea RG, Underwood J, Flanders KC, et al. A subset of patients with recurrent spontaneous abortion is deficient in transforming growth factor β2 producing suppresor cells in uterine tissue near the placental attachment site. Am J Reprod Immunol. 1995;34:52–63. doi: 10.1111/j.1600-0897.1995.tb00919.x. [DOI] [PubMed] [Google Scholar]
- 38.Vassiliadou N, Bulmer JN. Quantitative analysis of T lymphocyte subsets in pregnant and nonpregnant human endometrium. Biol Reprod. 1996;55:1017–22. doi: 10.1095/biolreprod55.5.1017. [DOI] [PubMed] [Google Scholar]
- 39.Kliman HJ, Nestler JE, Sermasi E, et al. Purification, characterization, and in vitro differentiation of cytotrophoblasts from human term placentae. Endocrinology. 1986;118:1567–82. doi: 10.1210/endo-118-4-1567. [DOI] [PubMed] [Google Scholar]
- 40.Hsi BL, Yeh CJG. Monoclonal antibody GB25 recognizes human villous trophoblasts. Am J Reprod Immunol Microbiol. 1986;12:1–3. doi: 10.1111/j.1600-0897.1986.tb00049.x. [DOI] [PubMed] [Google Scholar]
- 41.Kauma SW, Walsh SW, Nestler JE, et al. Interleukin 1 is induced in the human placenta by endotoxin and isolation procedures for trophoblasts. J Clin Endocr Metab. 1992;75:951–5. doi: 10.1210/jcem.75.3.1517391. [DOI] [PubMed] [Google Scholar]
- 42.Shimoya K, Matsuzaki N, Taniguchi T, et al. Human placenta constitutively produces interleukin-8 during pregnancy and enhances its production in intrauterine infection. Biol Reprod. 1992;47:220–6. doi: 10.1095/biolreprod47.2.220. [DOI] [PubMed] [Google Scholar]
- 43.Dudley DJ, Edwin SS, Dangerfield A, et al. Regulation of cultured human chorion cell chemokine production by group B streptococci and purified bacterial. Am J Reprod Immunol. 1996;36:264–8. doi: 10.1111/j.1600-0897.1996.tb00175.x. [DOI] [PubMed] [Google Scholar]
- 44.Denison FC, Kelly RW, Calder AA, et al. Cytokine secretion by human fetal membranes, decidua and placenta at term. Hum Reprod. 1998;13:3560–5. doi: 10.1093/humrep/13.12.3560. [DOI] [PubMed] [Google Scholar]
- 45.Kauma S, Matt D, Strom S, et al. Interleukin 1 beta, human leukocyte antigen HLA-DR alpha, and transforming growth factor-beta expression in endometrium, placenta and placental membranes. Am J Obstet Gynecol. 1990;163:1430–7. doi: 10.1016/0002-9378(90)90601-3. [DOI] [PubMed] [Google Scholar]
- 46.Roth I, Corry DB, Locksley RM, et al. Human placental cytotrophoblasts produce the immunosuppressive cytokine interleukin 10. J Exp Med. 1996;184:539–48. doi: 10.1084/jem.184.2.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lin H, Mosmann TR, Guilbert L, et al. Synthesis of T helper 2-type cytokines at the maternal–fetal interface. J Immunol. 1993;151:4562–73. [PubMed] [Google Scholar]
- 48.Delassus S, Coutinho GC, Saucier C, et al. Differential cytokine expression in maternal blood and placenta during murine gestation. J Immunol. 1994;152:2411–20. [PubMed] [Google Scholar]
- 49.Masuhiro K, Matsuzaki N, Nishino E, et al. Trophoblast-derived interleukin 1 stimulates the release of human chorionic gonadotropin by activating IL-6 and IL-6R system in first trimester human trophoblasts. J Clin Endocr Metab. 1991;72:594–601. doi: 10.1210/jcem-72-3-594. [DOI] [PubMed] [Google Scholar]
- 50.Lee BN, Ordonez N, Popek EJ, et al. Inflammatory cytokine expression is correlated with the level of human immunodeficiency virus (HIV) transcripts in HIV-infected placental trophoblastic cells. J Virol. 1997;71:3628–35. doi: 10.1128/jvi.71.5.3628-3635.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Menu E, Mbopi-Keou FX, Lagaye S, et al. Selection of maternal human immunodeficiency virus type 1 variants in human placenta. European network for in utero transmission of HIV-1. J Infect Dis. 1999;179:44–51. doi: 10.1086/314542. [DOI] [PubMed] [Google Scholar]
- 52.Tscherning-Casper C, Papadogiannakis N, Anvret M, et al. The trophoblastic epithelial barrier is not infected in full-term placentae of human immunodeficiency virus-seropositive mothers undergoing antiretroviral therapy. J Virol. 1999;73:9673–8. doi: 10.1128/jvi.73.11.9673-9678.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]