Abstract
Persistent immune activation has been suggested to affect the subset composition and activation status of peripheral blood cells. In this study we have compared peripheral blood mononuclear cells (PBMC) from a group of Ghanaians living in an area with high prevalence of malaria, mycobacteria, EBV and helmintic infections to a group of European counterparts. Our hypothesis was that persistent challenge with microorganisms is associated with increased production of cytokines and increased susceptibility of periphery cells to undergo apoptosis. We observed an increased frequency of activated T cells and a higher frequency of IL-4- but not IFN-γ-producing cells in the periphery of the Ghanaians. The IL-4 was produced mainly by CD4+ cells, in contrast to IFN-γ which was produced equally by CD4+, CD8+ and TCR-γδ+ cells. The frequencies of cytokine-producing cells were highly correlated to the frequencies of activated cells. Finally, cells from Ghanaians were more susceptible to activation-induced apoptosis. These results may explain why some epidemic diseases seem to have a different mode of transmission in Africa compared to the western world, and may thus be of importance when vaccine strategies are considered in Africa.
Keywords: apoptosis, infection, T cells, Th1/Th2
Introduction
The ability of periphery blood mononuclear cells (PBMC) to proliferate, produce cytokines or undergo apoptosis upon stimulation may affect the clinical outcome of infections, proving investigations of lymphocyte responses from individuals living in areas with a high prevalence of infectious diseases to be of importance.
During studies on T- cell responses to Plasmodium falciparum malaria we have observed differences in terms of lymphocyte function when Danish and Ghanaian donors were compared. We have reported previously that healthy Africans had a higher percentage of circulating T expressing LFA-1 [1] and a higher number of γδ T cells [2,3] when compared to their European counterparts. Other studies have shown that peripheral cells from Africans have increased expression of TNF receptor and produce higher levels of IL-2, IL-4 and IL-10, but lower amounts of IFN-γ and IL-6 [4,5].
The differences in PBMC from African and European donors may be a consequence of differences in climate, socio-economics and nutritional status, but focus has been put upon infectious diseases such as malaria, tuberculosis and helmintic infections [6,7]. Our work on LFA-1 has shown that environmental differences, presumably exposure to infectious agents, affect the LFA-1 phenotype of circulating T cells in Africans [1]. A recent study on the up-regulation of CCR5 also suggests that immune activation in Africa is environmentally driven, and [8] other studies have shown that a high prevalence of infectious diseases may affect the composition of peripheral lymphocytes, especially CD4+ T cells [4,9–14]. Finally, another study suggests that cells from individuals in Uganda have increased susceptibility to antigen-induced cell death [15].
In this study we elaborate on the findings of differences in peripheral lymphocytes in terms of single-cell cytokine production and susceptibility to apoptosis between Ghanaians and Europeans. Our hypothesis was that due to the high prevalence of malaria, TB and helmintic infections in Africans induce a Th2 type of activation, and that PBMC from these individuals are primed for apoptosis upon activation.
Materials and methods
Donors and isolation of mononuclear cells
Heparinized, peripheral blood (20 ml) was collected from two groups of donors, a group of 10 clinically healthy Ghanaian individuals and a group of 10 clinically healthy Danes, matched for age and sex.
Peripheral blood mononuclear cells (PBMC) were isolated by Lymphoprep (Nyegaard, Oslo, Norway) density centrifugation. The cells were frozen, stored and transported in liquid nitrogen [16]. Before use, the cells were rapidly thawed and washed. The viability of the cells was ascertained by trypan blue exclusion. PBMC from five individuals had a complete analysis performed on fresh and frozen cells. Freezing did not change the number or composition of lymphocyte subsets in each group. The expression of cytokines or PHA-induced apoptosis did not change significantly when fresh and frozen cells were compared.
Cultivation of peripheral blood mononuclear cells
PBMC were resuspended in RPMI-1640, supplemented with 15% heat-inactivated pooled human serum, 20 IU/ml of penicillin and 20 µg/ml of streptomycin (Gibco, Paisley, UK) and seeded into 24-well multi-dish plates (Nunc, Roskilde, Denmark). Each well contained 1 × 106 PBMC in 1 ml of medium. The cells were cultured at 37°C in a humidified atmosphere with 5% CO2. For intracellular cytokine detection, the cells were incubated with 1·5 µm monensin (Sigma, St Louis, MO, USA), 1 µm ionomycin and 50 ng/ml PMA for 4 h. For detection of apoptosis, the cells were incubated with PHA in a final concentration of 40 µg/ml for 48 h.
Flow cytometry
For surface staining three-colour flow cytometry was applied using MoAb to the determinants listed below as either FITC, phycoerythrin or Cy5 conjugates; CD3 (UCHT1; Dako, Glostrup, Denmark), CD4 (MT310; Dako), CD8 (DK25; Dako), CD45R0 (F0800; Dako), CD95 (M019751, Pharmingen, CA, USA) and TCR γδ (11F2; Becton Dickinson). All samples were analysed on a FACScan flow cytometer (Becton Dickinson). Analysis of data was performed using Winlist software (Verity, Topsham, ME, USA). The absolute number of lymphocyte subset was found by multiplying the lymphocyte counts with the proportion of lymphocytes.
Intracellular CD4 and cytokine staining
Following surface staining and lysis the cells were washed twice in a freshly made saponin buffer (PBS/BSA/NaN3 containing 0·1% (w/v) saponin (Sigma)), and incubated with anticytokine antibody (IFN-γ or IL-4 from Pharmingen, CA, USA) and/or anti-CD4 (MT310; Dako) [17] for 30 minutes in the dark (4°C). Following cytokine labelling, the cells were washed twice in saponin buffer, twice in staining buffer, resuspended in the same buffer and analysed.
Analysis of apoptosis and necrosis by Annexin V and 7-aminoactinomycin D (7AAD) staining
PBMC were labelled with Annexin V (M046701, Pharmingen) and 7AAD (Sigma) in binding buffer (Pharmingen) according to the manufacturer's directions and analysed by flow cytometry.
Analysis of apoptosis by DNA fragmentation via TUNEL assay
PHA-induced DNA fragmentation was measured by Apo-Direct according to the manufacturer's directions (Pharmingen). In brief, the cells were labelled with FITC-dUTP in the presence of terminal deoxytransferase followed by counterstaining of total DNA in a reaction mixture containing PI and RNase A. The cells were analysed by flow cytometry.
Statistical analysis
Different groups were compared by Student's t-test; P values < 0·05 were considered significant. Results are presented as means and 95% confidence intervals. Correlation between parameters was tested by Pearson's product-moment correlation; values of P < 0·05 were considered significant.
Results
T cells from Africans exhibit an activated phenotype
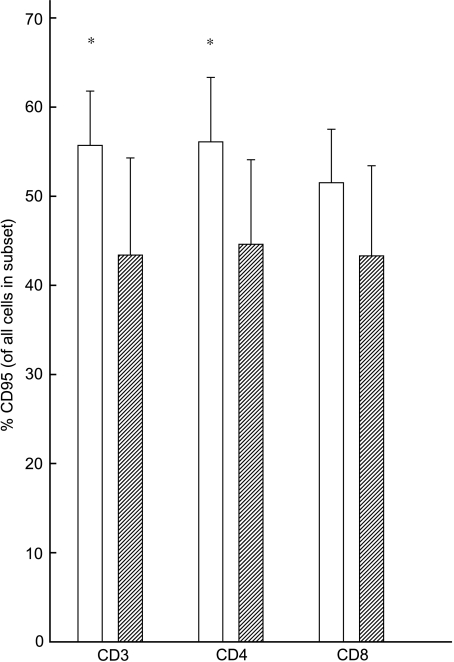
To investigate the activation status of T cells in the periphery, the frequencies of CD95+ T cells were measured. Ghanaian donors showed increased percentages of CD95+CD3+ T cells when compared to their Danish counterparts (P = 0·04). This increase was also found within CD4+ T cells (P = 0·04), but not within the CD8+ T cells (P = 0·1) (Fig. 1).
Fig. 1.
Differences in ex vivo expression of CD95 on CD3+, CD4+ and CD8+ cells from Ghanaians and Danes. Means and 95% confidence intervals are shown. *Significant difference between the groups (P < 0·05). □, Ghanaians;  , Danes.
, Danes.
The increase in CD95+CD3+ cells in Ghanaian donors was also found in absolute values (P = 0·03) (Table 2), indicating a real increase in activated T cells in the Ghanaian donors.
Table 2.
Mean values and range of absolute counts of lymphocyte subsets
| Cells | Ghanaians ounts/μl | Danes counts/μl | P-value |
|---|---|---|---|
| CD3+ CD95+ | 700 (412–1562) | 390 (98–774) | P = 0·03 |
| CD3+ IL-4+ | 74 (30–198) | 22 (8–70) | P = 0·003 |
| CD3+ IFN-γ+ | 176 (10–372) | 184 (74–575) | n.s. |
Circulating T cells from Africans are poised to produce Th2 cytokines
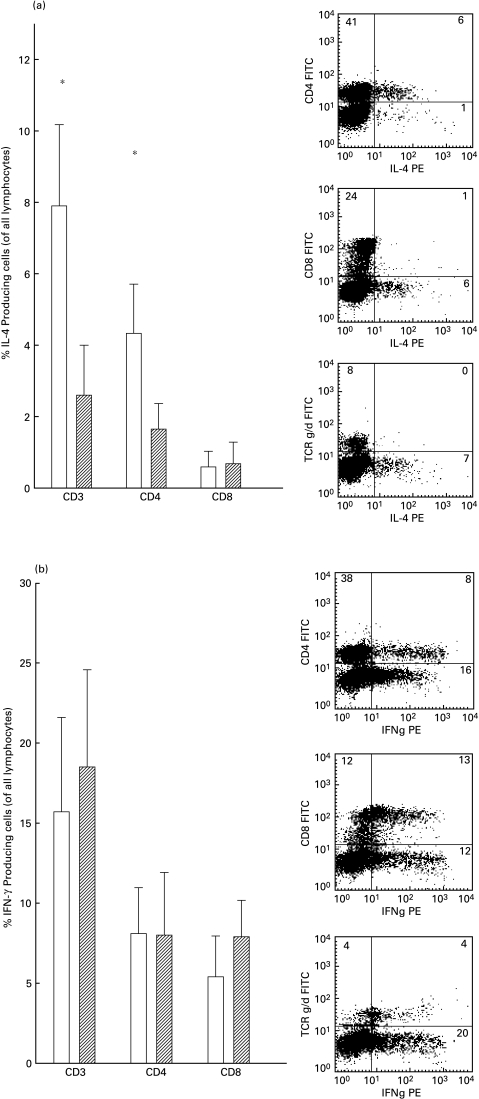
Intracellular staining of IL-4 and IFN-γ was performed to investigate the Th1/Th2 dichotomy. Ghanaians had an increase of IL-4-producing T cells in total when compared to their Danish counterparts (P = 0·02). This increase was also found in absolute values (P = 0·003) (Table 2), indicating a real increase in IL-4-producing T cells in the Ghanaian donors. The major part of the IL-4 was produced by the CD4+ subset (Fig. 2a). As a consequence, the increase of IL-4-producing cells was also found with the CD4+ subset (P = 0·001), but not with the CD8+ subset (P = 0·7). There was no difference in IFN-γ expression when CD3+, CD4+ or CD8+ T cells were compared between the two groups (P = 0·5, P = 0·9 and P = 0·1, respectively). In contrast to IL-4, IFN-γ was found to be produced in equal percentages of CD4+ and CD8+ cells in both groups and a large part of the γδ+ T cell subset also produced IFN-γ (Fig. 2b).
Fig. 2.
Percentage of IL-4 (a) or IFN-γ (b) producing CD3+, CD4+ and CD8+ cells (of all lymphocytes) from Ghanaians or Danes. Mean and 95% confidence intervals are shown (left) and examples of cytokine profiles from CD4+, CD8+ and γδ TCR+ cells from one Ghanaian donor are shown (right). *Significant difference between the groups (P < 0·05). □, Ghanaians;  , Danes.
, Danes.
Percentages of activated T cells are correlated to percentages of cytokine-producing cells
To test whether a higher number of activated cells was associated with a higher number of cytokine-producing cells following PMA stimulation the number of activated (CD95+) cells within the different subsets was correlated to the percentages of IFN-γ- and IL-4-producing cells. The percentages of IFN-γ-producing cells were interrelated to expression of CD95 in all subsets but with a closer relation to the CD8+ (P = 0·01) than to CD4+ (P = 0·04). This was in contrast to IL-4 expression, which was closely interrelated with expression of CD95 on CD4+ cells (P = 0·002), but not with activated CD8+ cells (P = 0·3) (Table 1).
Table 1.
Correlation between expression of CD95 CD3+, CD4+ and CD8+ and percentage of cells expressing IFN-γ and IL-4
| IFN-γ | IL4 | |
|---|---|---|
| CD3+CD95+ | R = 0·7, P = 0·002 | R = 0·6, P = 0·03 |
| CD4+CD95+ | R = 0·5, P = 0·04 | R = 0·7, P = 0·002 |
| CD8+CD95+ | R = 0·6, P = 0·01 | R = 0·2, P = 0·3 |
R = Pearson's correlation coefficient.
Circulating T cells from Africans are poised to undergo apoptosis
Apoptotic cells were investigated by labelling with Annexin V and 7AAD or by DNA fragmentation.
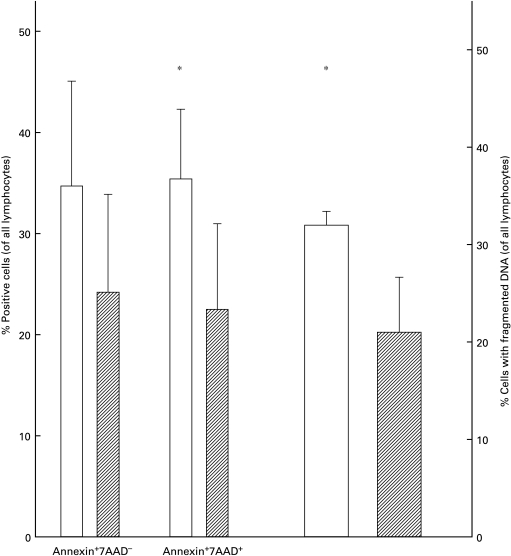
There were no differences in the percentages in Annexin V+/7AAD− cells between the two groups (P = 0·1), but the proportion of 7AAD+ cells was significantly increased in the cultures from Ghanaian donors (P = 0·02), indicating that more cells had died in these cultures (Fig. 3, left panel). In support of this observation, a significantly higher percentage of cells with fragmented DNA was found in the group with cells from Ghanaian donors (P = 0·02) (Fig. 3, right panel).
Fig. 3.
PHA-induced cell death measured by Annexin and 7AAD (left) or DNA fragmentation (right) from Ghanaians or Danes. *Significant difference between the groups (P < 0·05). □, Ghanaians;  , Danes.
, Danes.
Discussion
In this study we compared PBMC from individuals in Ghana to Danish donors in terms of the production of Th1 and Th2 cytokines and apoptosis. Our hypothesis was that a high prevalence of malaria, TB and helmintic infections leads to an increase in cytokine production as a consequence of activated cells in the periphery, and that the higher activation status makes peripheral cells more susceptible to activation-induced apoptosis. The major findings were that expression of the Type 2 cytokine IL-4 was increased significantly in the African individuals, in contrast to IFN-γ, which was produced by similar percentages when the two groups were compared. The production of cytokines was highly dependent on the activation status of the subset that was responsible for the production of the cytokines. Thus, IL-4 was produced almost exclusively by CD4+ cells and the number of IL-4-producing cells was highly correlated to the activation status of the CD4+ subset, but not to the CD8+ subset. Finally, cells from Ghanaians were more susceptible to induction of apoptosis than their Danish counterparts.
Our data are in agreement with previous studies indicating that IL-4 production is up-regulated in individuals living in areas with a high prevalence of malaria, tuberculosis and helminth infections [13]. In contrast to previous reports we did not observe any difference in IFN-γ production when we investigated this on a single-cell level. However, the production of cytokines was clearly dependent on the number of activated cells in the individuals in both Danes and in Ghanaians.
One of the infections with a high prevalence in Africa is helmintic infection. Helminth infections can result in eosinophila, a high level of IgE, which suggests production of IL-4 and IL-5, thus shifting the balance from a Type 1 response to a Type 2 response [18], and the apparent shift in the Th1/Th2 cytokines seen in African donors in the present study may result from a high prevalence of helminth infections.
Malaria is also a common infection in Africa and is associated with elevated levels of a number of cytokines, soluble activation markers [19–21] and persistent B cell activation [22,23]. We have described recently activation and reallocation of T cells during acute malaria, and that these in vivo primed cells re-emerge into the periphery upon drug cure. These cells are poised for cytokine production and cell death (Kemp et al., submitted for publication). Malaria is also associated with increased numbers of γδ TCR+ T cells, and in agreement with recent studies [2] we also find that the percentages of γδ T cells are higher in Africans than in Europeans (not shown). These cells exhibit a Type 1 response upon activation with no production of IL-4. γδ T cells may be involved in regulating the B cell response [24,25], and the elevated numbers seen in the African donors may be a consequence of increased B cell activation induced by malaria.
Individuals challenged with many infections may respond differently to pathogens than individuals living in areas with a low transmission of infectious diseases. As such, HIV infection seems to have a different disease pattern and mode of transmission in Africa when compared to the situation in Europe and the United States [26,27], and increased numbers of activated cells and the shift towards a Type 2 response may leave individuals more susceptible to HIV. Thus, a changed lymphocyte function may be of importance to the clinical outcome of other infections and make individuals more susceptible to infections. This is supported by the high incidence of diseases such as tuberculosis [28].
Most of the infectious diseases with high prevalence in Africa are associated with elevated levels of plasma TNF-α and the constant effect of elevated TNF-α has been suggested to induce the changes in peripheral lymphocytes observed in the present and other studies [13]. TNF-α may have induced the increased susceptibility to apoptosis following stimulation, which was evident when the cells were investigated by DNA fragmentation and supported by the presence of more 7AAD+ cells in the cultures with cells from Ghanaian donors.
The presence of Type 2 cells and more apoptotic cells in the Ghanaians is contradictory, since IL-4-producing cells have been shown to be protected from apoptosis in vitro [29]. Since IL-4 production was seen only within the CD4+ subset, the increased apoptosis could be evident in other subsets.
The present study showed increased activation and potential to produce cytokines in peripheral mononuclear cells from Ghanaian donors. Furthermore, the cells were more susceptible to activation-induced cells death.
These findings could explain the different patterns observed when epidemic diseases are spread in Africa and Europe, and may be of importance when strategies of protective vaccines are developed.
Acknowledgments
This work was supported by grants from the Danish International Development Agency (RUF and the ENRECA programs), the Danish Biotechnology Program and the Novo Nordisk Foundation. G. Grauert is thanked for excellent technical support.
References
- 1.Hviid L, Elhassan IM, Dodoo D, et al. Differential T-cell expression of LFA-1 in residents from Africa and Denmark. Description of the phenomenon and its possible basis. Immunol Lett. 1994;39:147–51. doi: 10.1016/0165-2478(94)90099-x. [DOI] [PubMed] [Google Scholar]
- 2.Hviid L, Akanmori BD, Loizon S, et al. High frequency of circulating γδ Tcells with dominance of the Vδ1 subset in a healthy population. Int Immunol. 2000;12:797–805. doi: 10.1093/intimm/12.6.797. [DOI] [PubMed] [Google Scholar]
- 3.Hviid L, Kurtzhals JAL, Adabayeri V, et al. Perturbation and pro-inflammatory type activation of V1+ T cells in African children with Plasmodium falciparum malaria. Infect Immun. 2001;69:3190–6. doi: 10.1128/IAI.69.5.3190-3196.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kalinkovich A, Weisman Z, Burstein R, Bentwich Z. Standard values of T-lymphocyte subsets in Africa. J AIDS Hum Retrovirol. 1998;17:183–5. doi: 10.1097/00042560-199802010-00017. [DOI] [PubMed] [Google Scholar]
- 5.Kalinkovich A, Livshits G, Engelmann H, et al. Soluble tumour necrosis factor receptors (sTNF-R) and HIV infection: correlation to CD8+ lymphocytes. Clin Exp Immunol. 1993;93:350–5. doi: 10.1111/j.1365-2249.1993.tb08184.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Annan A, Crompton DW, Walters DE, Arnold SE. An investigation of the prevalence of intestinal parasites in pre-school children in Ghana. Parasitology. 1986;92:209–17. doi: 10.1017/s0031182000063563. [DOI] [PubMed] [Google Scholar]
- 7.Hengster P, Schmutzhard E, Fuchs D, Hofbauer J, Wachter H, Dierich MP. Evaluation on HIV serology and immune-stimulation on patients in Tanzania. Int J STD AIDS. 1991;2:180–4. doi: 10.1177/095646249100200306. [DOI] [PubMed] [Google Scholar]
- 8.Clerici M, Butto S, Lukwiya M, et al. Immune activation in africa is environmentally-driven and is associated with upregulation of CCR5. Italian–Ugandan AIDS Project. AIDS. 2000;14:2083–92. doi: 10.1097/00002030-200009290-00003. 10.1097/00002030-200009290-00003. [DOI] [PubMed] [Google Scholar]
- 9.Anglaret X, Sylla-Koko F, Diagbouga S, Combe P, Van De PP, Dabis F. CD4 count and CD4% in African HIV-infected people. Int J Epidemiol. 1998;27:928–9. doi: 10.1093/ije/27.5.928. [DOI] [PubMed] [Google Scholar]
- 10.Anglaret X, Diagbouga S, Mortier E, et al. CD4+ T-lymphocyte counts in HIV infection: are European standards applicable to African patients? J AIDS Hum Retrovirol. 1997;14:361–7. doi: 10.1097/00042560-199704010-00009. [DOI] [PubMed] [Google Scholar]
- 11.Zekeng L, Sadjo A, Meli J, et al. T lymphocyte subset values among healthy Cameroonians. J AIDS Hum Retrovirol. 1997;14:82–3. doi: 10.1097/00042560-199701010-00016. [DOI] [PubMed] [Google Scholar]
- 12.Kalinkovich A, Weisman Z, Greenberg Z, et al. Decreased CD4 and increased CD8 counts with T cell activation is associated with chronic helminth infection. Clin Exp Immunol. 1998;114:414–21. doi: 10.1046/j.1365-2249.1998.00736.x. 10.1046/j.1365-2249.1998.00736.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bentwich Z, Kalinkovich A, Weisman Z. Immune activation is a dominant factor in the pathogenesis of African AIDS. Immunol Today. 1995;16:187–91. doi: 10.1016/0167-5699(95)80119-7. [DOI] [PubMed] [Google Scholar]
- 14.Messele T, Abdulkadir M, Fontanet AL, et al. Reduced naive and increased activated CD4 and CD8 cells in healthy adult Ethiopians compared with their Dutch counterparts. Clin Exp Immunol. 1999;115:443–50. doi: 10.1046/j.1365-2249.1999.00815.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rizzardini G, Piconi S, Ruzzante S, et al. Immunological activation markers in the serum of African and European HIV-seropositive and seronegative individuals. AIDS. 1996;10:1535–42. doi: 10.1097/00002030-199611000-00012. [DOI] [PubMed] [Google Scholar]
- 16.Hviid L, Albeck G, Hansen B, Theander TG, Talbot A. A new portable device for automatic controlled-gradient cryopreservation of blood mononuclear cells. J Immunol Methods. 1993;157:135–42. doi: 10.1016/0022-1759(93)90079-m. [DOI] [PubMed] [Google Scholar]
- 17.Kemp K, Bruunsgaard H. Identification of IFN-producing CD4+ T cells following PMA stimulation. J Interf Cytok Res. 2001;21:503–6. doi: 10.1089/10799900152434376. [DOI] [PubMed] [Google Scholar]
- 18.Villa OF, Kuhn RE. Mice infected with the larvae of Taenia crassiceps exhibit a Th2-like immune response with concomitant anergy and downregulation of Th1-associated phenomena. Parasitology. 1996;112:561–70. doi: 10.1017/s0031182000066142. [DOI] [PubMed] [Google Scholar]
- 19.Kwiatkowski D, Hill AV, Sambou I, et al. TNF concentration in fatal cerebral, non-fatal cerebral, and uncomplicated Plasmodium falciparum malaria. Lancet. 1990;336:1201–4. doi: 10.1016/0140-6736(90)92827-5. [DOI] [PubMed] [Google Scholar]
- 20.Wenisch C, Parschalk B, Narzt E, Looareesuwan S, Graninger W. Elevated serum levels of IL-10 and IFN-γ in patients with acute Plasmodium falciparum malaria. Clin Immunol Immunopathol. 1995;74:115–7. doi: 10.1006/clin.1995.1017. [DOI] [PubMed] [Google Scholar]
- 21.Nguyen-Dinh P, Greenberg AE. Increased levels of released interleukin-2 receptors in Plasmodium falciparum malaria. J Infect Dis. 1988;158:1403–4. doi: 10.1093/infdis/158.6.1403. [DOI] [PubMed] [Google Scholar]
- 22.Cohen S, McGregor IA, Carrington S. Gammaglobulin and acquired immunity to human malaria. Nature. 1961;192:733–7. doi: 10.1038/192733a0. [DOI] [PubMed] [Google Scholar]
- 23.Banic DM, Viana-Martins FS, De Souza JM, Peixoto TD, Daniel-Ribeiro C. Polyclonal B-lymphocyte stimulation in human malaria and its association with ongoing parasitemia. Am J Trop Med Hyg. 1991;44:571–7. doi: 10.4269/ajtmh.1991.44.571. [DOI] [PubMed] [Google Scholar]
- 24.Born W, Cady C, Jones-Carson J, Mukasa A, Lahn M, O'Brien R. Immunoregulatory functions of γδ T cells. Adv Immunol. 1999;71:77–144. [PubMed] [Google Scholar]
- 25.Bonneville M, Fournié JJ. γδ T lymphocytes, a link between immunity and homeostasis? Microbe Infect. 1999;1:173. [Google Scholar]
- 26.Gilks CF. The clinical challenge of the HIV epidemic in the developing world. Lancet. 1993;342:1037–9. doi: 10.1016/0140-6736(93)92885-w. [DOI] [PubMed] [Google Scholar]
- 27.Anzala OA, Nagelkerke NJ, Bwayo JJ, et al. Rapid progression to disease in African sex workers with human immunodeficiency virus type 1 infection. J Infect Dis. 1995;171:686–9. doi: 10.1093/infdis/171.3.686. [DOI] [PubMed] [Google Scholar]
- 28.Orme IM. Prospects for new vaccines against tuberculosis. Trends Microbiol. 1995;3:401–4. doi: 10.1016/s0966-842x(00)88987-8. [DOI] [PubMed] [Google Scholar]
- 29.Carbonari M, Tedesco T, Del Porto P, Paganelli R, Fiorilli M. Human T cells with a type-2 cytokine profile are resistant to apoptosis induced by primary activation: consequences for immunopathogenesis. Clin Exp Immunol. 2000;120:454–62. doi: 10.1046/j.1365-2249.2000.01243.x. 10.1046/j.1365-2249.2000.01243.x. [DOI] [PMC free article] [PubMed] [Google Scholar]