Abstract
This study evaluates serum CD26 (dipeptidyl peptidase IV, DPPIV) enzyme activity and serum levels of soluble CD30 as markers of T1 and T2 cytokine environments in HIV patients who achieved immune reconstitution after highly active antiretroviral therapy (HAART). Patients who had experienced inflammatory disease associated with pre-existent opportunistic infections after HAART (immune restoration diseases, IRD) were considered separately. Serum sCD30 levels and CD26 (DPPIV) enzyme activity were compared with IFN-γ production by PBMC cultured with cytomegalovirus (CMV) antigen in controls and patient groups. High sCD30 levels were associated with low IFN-γ production after antigenic stimulation in control subjects and, to a lesser extent, in immune reconstituted HIV patients. There was no association between serum CD26 (DPPIV) enzyme activity and IFN-γ production or sCD30 levels. Serum sCD30 levels and CD26 (DPPIV) enzyme activity were significantly increased in immune reconstituted patients with high HIV viral loads. Patients who had experienced CMV retinitis as an IRD had significantly higher sCD30 levels than all other patient groups. Hence, high sCD30 levels may be a marker of a T2 cytokine environment in HIV patients with immune reconstitution and are associated with higher HIV viral loads and a history of CMV associated IRD.
Keywords: CD26 (DPPIV), CD30, HIV, interferon-γ
Introduction
The progressive decrease in CD4+ T-cell counts in untreated HIV patients is associated with depression of cytokine production by type 1 (T1) cells (IFN-γ and IL-2), while type 2 (T2) cytokine production (IL-4, IL-5, IL-13) is relatively maintained [1]. Highly active antiretroviral therapy (HAART) results in a decrease in HIV viral load followed by an increase in CD4+ T-cell counts [2], but persistent immune defects, including dysregulation of T1/T2 cytokine production, may still be present 1 year after therapy is commenced [3]. The significance of this is unclear but persistent dysregulation of cytokine production may contribute to the pathogenesis of atypical presentations of opportunistic infections in patients responding to HAART. Current evidence suggests that these immune restoration diseases (IRD) are a consequence of the restoration of immune responses against pre-existent (often quiescent) infections by pathogens such as cytomegalovirus (CMV), Mycobacterium avium complex (MAC), varicella zoster virus (VZV) and herpes simplex virus (HSV) [4]. Evaluation of the T1/T2 cytokine balance in patients responding to HAART may therefore be informative. Serological markers are cheap and easy to apply in clinical practice, and avoid ambiguities intrinsic to methods based on in vitro culture of a patient's cells with mitogens and analysis of cytokine mRNA rather than protein. Here we evaluate serum levels of soluble CD30 (sCD30) and serum CD26 (DPPIV) enzyme activity as markers of T1 and T2 cytokine environment.
CD30 is a member of the tumour necrosis factor and nerve growth factor receptor superfamily. It is expressed on a subset of CD4+ T-cells and CD8+ T-cells producing predominantly T2 cytokines [5,6]. sCD30 is released by activated human T2 and T0 T-cell clones. sCD30 levels are increased in the serum of patients with T2-associated diseases, including Omenns' syndrome [7] and systemic sclerosis [8], but decreased in patients with T1-associated diseases, such as multiple sclerosis [9] and Crohn's disease [10]. In HIV-infected patients, high serum sCD30 levels are associated with primary HIV-1 infection [11], and we have shown that serum sCD30 levels are inversely related to cutaneous delayed-type hypersensitivity (DTH) responses [12].
CD26 is a cell surface glycoprotein with dipeptidyl peptidase IV (DPPIV) activity in its extracellular domain. It exhibits costimulatory activity in T-cell activation, and its expression is enhanced on T-cells following activation [13]. CD26 has been described as a marker of a T1 cytokine environment [14]. HIV Tat binds to CD26, inhibiting DPPIV enzyme activity, and increased plasma HIV RNA has been correlated with decreased DPPIV enzyme activity in HIV-1 infected patients [15].
In this study, the serum sCD30 level and CD26 (DPPIV) enzyme activity were assessed in HIV patients who had achieved immune reconstitution after HAART, HIV patients who were untreated or unresponsive to HAART and normal controls. Results were correlated with cytomegalovirus (CMV) antigen-induced interferon-γ (IFN-γ) production by peripheral blood mononuclear cells (PBMC). In our cohort, 14 of 31 (45%) patients who had achieved immune reconstitution had experienced an IRD. We hypothesized that they carry stable immunological markers of disease risk and investigated serum sCD30 levels and serum CD26 (DPPIV) enzyme activity as markers of their T1/T2 cytokine balance, which can be monitored readily in clinical practice.
Materials and methods
Patients and controls
Adult HIV-infected patients (38 male and two female, median age 38 years, range 29–64 years) treated at Royal Perth Hospital, Western Australia, were selected on the basis of having had a CD4+ T-cell count < 100 cells/µl at some time prior to commencing HAART, which included two nucleoside analogue reverse transcriptase inhibitors and a protease inhibitor or a non-nucleoside reverse transcriptase inhibitor. Patients in Group 1 had achieved a CD4+ T-cell count of > 200 cells/µl or > fourfold increase over their lowest pretreatment level after HAART. Group 2 included five untreated patients and five patients who had failed to achieve immune reconstitution on HAART. At each visit, T-lymphocyte subsets were quantified by standard flow cytometric techniques and plasma HIV RNA was assessed by the AmplicorTM method (Roche, Branchburg, USA). Twenty-five HIV-seronegative controls (12 male and 13 female, median age 33 years, range 20–70 years) were also studied. Prior approval was obtained from the hospital ethics committee and informed consent was obtained from all participants.
Quantification of soluble CD30 in serum sCD30 levels were assayed by ELISA. Briefly, serum samples were stored at − 70°C. Half-volume (50 µl) 96-well ELISA plates (Costar, NY, USA) were coated with anti-CD30 antibody (Bender MedSystems, Austria) overnight at 4°C, blocked (1 h at room temperature) with phosphate buffered saline (PBS/1% bovine serum albumin (BSA) and washed with PBS/0·05% Tween 20. Samples, standards and anti-CD30 peroxidase conjugate were prediluted in PBS/BSA/0·05% Tween 20 and added to the plate together (3 h, room temperature on a plate shaker). The plate was washed and bound sCD30 was detected with 3,3′,5,5′ tetramethylbenzidine (TMB) substrate.
Quantification of CD26 (DPPIV) enzyme activity in serum
Half volume 96-well plates were coated overnight with anti-CD26 antibody (Pharmingen, CA, USA) at 4°C and blocked with PBS/1% BSA (1 h, at room temperature). Pre-diluted samples or a pooled serum standard were added (4 h, room temperature), and the plate was washed with PBS/0·05% Tween 20. Bound CD26 (DPPIV) enzyme activity was detected with a chromogenic substrate [Gly Pro pNA (Sigma, MI, USA) for 5 h at 37°C]. Absorbance was read at 405 nm.
Cytomegalovirus antigen
CMV strain ad 169 was propagated in human skin fibroblasts infected at a high multiplicity, harvested after 7 days, sonicated immediately in 0·1 m glycine buffer pH 9·5 [16], aliquoted and stored at − 70°C. Cells from CMV seronegative individuals (n = 18) did not display lymphoproliferation responses or IFN-γ production when stimulated with this antigen.
Lymphoproliferation assays and measurement of IFN-γ production by PBMC
PBMC were isolated by Ficoll Hypaque density gradient centrifugation and cultured for 5 days with CMV antigen for proliferation assays, as described previously [17]. Production of IFN-γ by CMV-stimulated PBMC was measured in cell culture supernatants by ELISA using antibody coated plates (CSL Biosciences, Australia), sensitivity 2·4 iu/ml, and by ELISpot assays using nitrocellulose backed microtitre plates (Millipore, Bedford, MA, USA), after overnight culture of PBMC, as described previously [17]. Phorbol myristate acetate (PMA, 0·05 µg/ml) with calcium ionophore (A23187, 14 µg/ml) (Sigma, MI, USA) was used as a positive control in all lymphoproliferation and IFN-γ assays. Optimal concentrations of CMV antigen and PMA/calcium ionophore were determined using lymphoproliferation assays with PBMC from healthy individuals.
Statistical analysis
Results are presented as median (range). Statistical differences between groups were determined by the non-parametric Wilcoxon Rank Sum Test, with P < 0·05 indicating a significant difference. The frequency of attainment of undetectable HIV viral loads was compared using Fisher's exact test. Correlations were evaluated using Spearman's rank correlation test.
Results
Characteristics of HIV patients and controls
HIV patients were divided into two groups on the basis of their CD4+ T-cell responses to HAART (Table 1, See Materials and methods). Group 1 patients had higher CD4+ and CD8+ T-cell counts than Group 2 patients (P < 0·00001, Wilcoxon Rank Sum Test). A total of 17/31 Group 1 patients and 0/10 Group 2 patients achieved undetectable viral loads (plasma HIV RNA < 400 copies/ml). The median time on treatment for Group 1 patients was 34 months (range 2–45 months).
Table 1.
Parameters of HIV disease
| n | Nadir CD4+ T-cells/µl | Current plasma HIV RNA ( × 103 copies/ml) | Current CD4+ T-cells/µl | Current CD8+ T-cells/µl | |
|---|---|---|---|---|---|
| Group 1 | 31 | 18(0–50)* | < 0·4 (< 0·4–713)† | 280 (60–1107)† | 1056 (250–1890)† |
| Group 2 | 10 | 8 (0–45) | > 750 (9·6–> 750) | 11 (2–45) | 246 (22–700) |
Significantly different from Group 2 patients (P < 0·00001).
Median (range).
A total of 29/31 Group 1 patients, 7/10 Group 2 patients and 12/25 control subjects were seropositive for CMV. These individuals were used in analyses of T-cell responses to the CMV antigen.
Serum sCD30 levels and serum CD26 (DPPIV) enzyme activity are higher in Group 1 patients with high viral loads
There were no significant differences in serum sCD30 levels or CD26 (DPPIV) enzyme activity between control subjects and Group 1 or Group 2 HIV patients (Table 2). However, when Group 1 patients were stratified on the basis of their HIV viral loads, patients with detectable plasma HIV RNA (> 400 copies/ml) had significantly higher sCD30 levels (P = 0·03) and CD26 (DPPIV) enzyme activity (P = 0·004) than patients with undetectable HIV RNA.
Table 2.
sCD30, sCD26 (DPPIV) enzyme activity and CMV antigen-induced lymphoproliferation and IFN-γ responses in controls and patient groups
| Current plasma HIV RNA (× 103 copies/ml) | n | CD4+ T cells/µl | sCD30 (u/ml) | CD26 (DPPIV) enzyme activity (u/ml) | |
|---|---|---|---|---|---|
| Controls | 25 | N/A | 123 (11–780)* | 221 (136–572) | |
| Group 1 | 31 | 280 (60–1107) | 143 (20–7097) | 259 (125–1344) | |
| > 0·4 | 13 | 182 (60–392)† | 183 (60–7097)† | 400 (168–1344)† | |
| < 0·4 | 18 | 355 (60–1107) | 69 (30–2646) | 250 (153–696) | |
| Group 2 | 10 | 11 (2–45) | 84 (27–3527) | 212 (89–712) |
| Proliferation (DPM × 10−3) | IFN-γ in cell culture supernatants (U/ml) | IFN-γ ELISpot (per 2 × 105 cells) | |||
|---|---|---|---|---|---|
| Controls | 12 | 59(4–212) | 2·1 (0·2–38) | 80 (14–400) | |
| Group 1 | 29 | 5 (0·2–118)‡ | 0·4 (0·1–15)‡ | 33 (0–315) | |
| Group 2 | 7 | 0·8 (0·3–1·4)‡ | 0·2 (0·1–4)‡ | 1 (0–3)‡ |
Median (range).
Significantly different from Group 1 patients with < 400 copies HIV RNA/ml (P = 0·01 for CD4 + T-cell counts, P = 0·03 for CD26 (DPPIV) enzymeactivity and P = 0·003 for sCD30).
Significantly different from control subjects (P < 0·05).
Relationship between serum sCD30 levels and IFN-γ production by PBMC or serum CD26 (DPPIV) enzyme activity
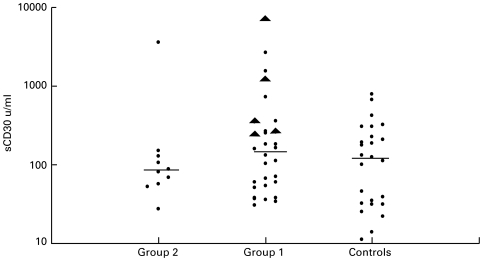
As serum CD30 levels varied considerably within the control subjects and Group 1 patients (Fig. 1), the groups were subdivided on the basis of a serum sCD30 level greater or less than the median value and compared with IFN-γ production by PBMC and CD26 (DPPIV) enzyme activity. CMV seropositive control subjects with a serum sCD30 level greater than or equal to 123 units/ml had significantly lower IFN-γ responses to CMV antigen in PBMC culture supernatants and ELISPOT assays than controls with sCD30 levels below 123 units/ml (Table 3). CMV seropositive Group 1 patients with high sCD30 levels also displayed lower IFN-γ production in the ELISpot assay, but the difference was not statistically different (Table 3). All Group 2 patients had low IFN-γ and proliferation responses to CMV antigen (Table 2), so no trends were apparent.
Fig. 1.
Distribution of soluble CD30 levels amofng HIV patients and control subjects. Horizontal lines indicate the median sCD30 levels of each group. CMV IRD patients (▴).
Table 3.
Serum CD26 (DPPIV) enzyme activity and CMV antigen-induced IFN-γ production in CMV-seropositive subjects stratified by high and low serum sCD30 levels
| n | sCD30 sub groups* | sCD30 (u/ml) | CD4+ T cells (cells/µl) | CD26 (DPPIV) enzyme activity (u/ml) | IFN-γ in cell culture supernatant (U/ml) | IFN-γ ELISpots (per 2 × 105 cells) | |
|---|---|---|---|---|---|---|---|
| Controls | 7 | H | 209(123–323)† | N/A | 280(147–572) | 0·5(0·23–2·1)‡ | 56 (14–84)§ |
| 5 | L | 31(14–101) | N/A | 203(136–275) | 5·6 (4·9–39) | 198 (100–400) | |
| Group 1 | 14 | H | 305(183–7097) | 321(60–700) | 322(153–1344) | 0·3(0·1–4·8) | 19(0–183) |
| 15 | L | 53(30–130) | 270(60–1107) | 254(125–594) | 0·7(0·1–15) | 52(1–315) | |
| Group 2 | 4 | H | 141(105–3527) | 23(5–45) | 190(177–221) | 0·3(0·11–4) | 0(0) |
| 3 | L | 69(27–81) | 12(3–18) | 118(89–302) | 0·1(0·1–0·25) | 1(0–3) |
H = high sCD30 (equal to or greater than median value), L = low sCD30 (lower than median value).
= median (range).
Significantly lower than controls with high sCD30 levels (P = 0·0043).
Significantly lower than controls with high sCD30 levels (P = 0·009).
Serum CD26 (DPPIV) enzyme activity did not differ between the high and low serum sCD30 subgroups in controls or patients (Table 3). In Group 1 patients, there was a weak negative correlation between serum CD26 (DPPIV) enzyme activity and IFN-γ production in supernatants of PBMC cultures (R = − 0·33, P = 0·07).
CD26 (DPPIV) enzyme activity is related to plasma HIV RNA and not to sCD30 levels or IFN-γ production
Fewer Group 1 patients with > 259 µ/ml CD26 (DPPIV) enzyme activity (median value) had undetectable plasma HIV RNA (< 400 copies/ml) than patients with < 259 µ/ml (6/16 patients vs. 12/15, Fisher's exact test, P = 0·02). For example, four patients in Group 1 with > 75 000 copies HIV RNA/ml had > 900 µ/ml of CD26 (DPPIV) enzyme activity. Therefore, CD26 (DPPIV) enzyme activity was higher in patients with a high HIV viral load.
Patients who had previously experienced a CMV IRD had high serum sCD30 concentrations
Group 1 patients who had previously experienced an IRD had similar CD4+ T-cell counts, sCD30 levels, CD26 (DPPIV) enzyme activity, lymphoproliferative responses and IFN-γ production to patients with no history of IRD (Table 4). However, a subgroup of IRD patients with a history of ‘relapsed’ CMV retinitis after HAART (denoted ‘CMV IRD’) had significantly higher serum sCD30 levels than other patients in the IRD group (P = 0·004, Table 4). As CMV IRD patients had higher CD4+ T-cell counts than HIV patients with other IRD, the high serum sCD30 concentration cannot be explained by a lesser degree of immune reconstitution. The CMV IRD group included some individuals with sCD30 values threefold higher than the highest values in the control group (Fig. 1). There was no significant difference in CD26 (DPPIV) enzyme activity, lymphoproliferation or IFN-γ production between patients with CMV IRD and any other group (Table 4).
Table 4.
High serum sCD30 levels are associated with a history of CMV IRD
| History of IRD | n | CD4+ T cells (cells/µl) | sCD30 (u/ml) | CD26 (DPPIV) enzyme activity (u/ml) |
|---|---|---|---|---|
| All IRD | 11 | 221 (60–550)* | 183 (36–7097) | 365 (168–776) |
| No IRD | 17 | 272 (60–1107) | 130 (30–1496) | 255 (125–1344) |
| CMV IRD | 5 | 320 (154–429) | 348 (243–7097)† | 259 (168–707) |
| Other IRD | 6 | 153 (60–550) | 66 (36–183) | 471 (248–776) |
| Proliferation (DPM × 10−3) | IFN-γ in cell culture supernatant (U/ml) | IFN-γ ELISpot (per 2 × 105 cells) | ||
|---|---|---|---|---|
| All IRD | 10 | 5 (1–56) | 0·9 (0·1–4·8) | 68 (1–183) |
| No IRD | 16 | 5 (0–118) | 0·3 (0·1–15) | 21 (0–315) |
| CMV IRD | 5 | 3 (1–23) | 0·3 (0·1–4·8) | 72 (1–183) |
| Other IRD | 5 | 9 (2–56) | 1·7 (0·1–3·1) | 63 (1–148) |
Median (range).
Significantly higher than other IRD patients (P = 0·004).
Discussion
This study has investigated the clinical utility of measuring serum sCD30 levels and CD26 (DPPIV) enzyme activity as markers of T2 and T1 cytokine responses in patients with HIV infection, as these parameters can readily be assessed in clinical practice. We noted that control subjects with high sCD30 levels exhibited low antigen-induced IFN-γ production, consistent with sCD30 being a marker of a T2 cytokine environment. Overall, we found no significant differences in serum sCD30 levels between control subjects and the HIV patient groups but we identified factors that affected serum sCD30 levels.
Patients who had experienced immune reconstitution after receiving HAART but had HIV viraemia had higher serum sCD30 levels than aviraemic patients (Table 2). Pizzolo et al. [11] demonstrated increased serum sCD30 levels in patients with primary HIV-1 infection, with a return to normal levels after 35 months in six of 13 patients. The authors suggested that high sCD30 levels in primary HIV infection are a consequence of high viral replication. Rizzardi et al. [18] also correlated high serum levels of sCD30 (and also tumour necrosis factor-α [TNF-α] and soluble TNF receptors) with high plasma HIV-1 RNA and a poor clinical outcome. Here, we have shown that patients with very low CD4+ T-cell counts and high levels of HIV replication (Group 2) tended to have lower serum sCD30 levels than immune reconstituted patients (Group 1, see Fig. 1). Hence, high serum sCD30 levels appear to be a feature of persistent HIV replication without severe depletion of CD4+ T-cells. This suggests dysregulation of the cytokine balance with preferential production of T2 cytokines. These findings are consistent with our earlier study [12], where patients with very low CD4+ T-cell counts had low serum sCD30 levels and low DTH responses, indicating T-cell deficiency rather than a T1 cytokine bias.
When immune reconstituted patients were stratified according to their history of IRD, HIV patients with CMV IRD had significantly higher serum sCD30 levels than patients in the remainder of the cohort. This finding suggests that the pathogenesis of CMV IRD may be promoted by a T2 cytokine environment or that these patients have a persistent immune response against CMV that generates sCD30 production. Of note, CMV IRD patients were also different from other immune reconstituted patients in that they had distinct HLA profiles with a high frequency of carriage of HLA-A2, B44. The CMV IRD group did not have elevated IFN-γ responses to CMV antigen [19].
We found no significant differences in serum CD26 (DPPIV) enzyme activity between controls and HIV patients, in contrast to work published by Hosono et al. [15], which demonstrated decreased sCD26 enzyme activity in immunodeficient HIV patients. The assays used in that study appear comparable to ours. We reported CD26 (DPPIV) enzyme activity in serum rather than plasma, but our preliminary studies suggest that this is not an important consideration. We therefore looked for correlations within our cohort. The breakdown of patients by sCD30 did not suggest an inverse relationship between sCD30 and CD26 (DPPIV) enzyme activity. Furthermore, there was no positive correlation between serum CD26 (DPPIV) enzyme activity and IFN-γ production in PBMC cultures from Group 1 patients. These findings do not support CD26 (DPPIV) enzyme activity as a T1 marker and suggest that elevated levels indicate generalized T-cell activation.
HIV Tat binds to CD26 partially inhibiting DPPIV enzyme activity, suppressing antigen-specific but not mitogen-induced activation of peripheral T-cells [20,21]. Our data (Table 2) provides no evidence of HIV-mediated inhibition of CD26 (DPPIV) enzyme activity in the serum of patients with high HIV viral loads, although direct measurements of HIV Tat were not undertaken.
Other markers of T1 and T2 environments in HIV patients have been evaluated but found to be unsuitable. For example, serum IFN-γ levels fluctuate substantially [22] and we have found that interleukin-5 is not detectable in PBMC culture supernatants from control subjects and in only a small minority of HIV patients after mitogen stimulation (unpublished data).
In conclusion, we present data from control subjects suggesting that serum sCD30 levels are inversely related to IFN-γ production by antigen-stimulated PBMC and hence mark a T2 cytokine environment. A similar trend was apparent in immune reconstituted patients on HAART but we also found that high serum sCD30 levels were associated with persistent HIV viraemia and a history of CMV IRD. Longitudinal studies will be necessary to determine if serum sCD30 levels are a useful prognostic marker of IRD. Our data do not support the use of assays for serum sCD26 (DPPIV) activity as a marker of a T1 cytokine environment in HIV patients on HAART.
Acknowledgments
We thank the patients and controls who have donated blood for this study. The work was supported by the National Health and Medical Research Council of Australia. This is publication no. 2000–30 of the Department of Clinical Immunology and Biochemical Genetics, Royal Perth Hospital.
References
- 1.Clerici M, Shearer GM. A TH1 to TH2 switch is a critical step in the etiology of HIV infection. Immunol Today. 1993;14:107–11. doi: 10.1016/0167-5699(93)90208-3. [DOI] [PubMed] [Google Scholar]
- 2.Connick E, Lederman MM, Kotzin BL, et al. Immune reconstitution in the first year of potent antiretroviral therapy and its relationship to virologic response. J Infect Dis. 2000;181:358–63. doi: 10.1086/315171. 10.1086/315171. [DOI] [PubMed] [Google Scholar]
- 3.Martinon F, Michelet C, Peguillet I, et al. Persistent alterations in T-cell repertoire, cytokine and chemokine receptor gene expression after one year of highly active antiretroviral therapy. AIDS. 1999;13:185–94. doi: 10.1097/00002030-199902040-00006. 10.1097/00002030-199902040-00006. [DOI] [PubMed] [Google Scholar]
- 4.French MA, Lenzo N, John M, et al. Immune restoration disease after the treatment of immunodeficient HIV-infected patients with highly active antoretroviral therapy. HIV Med. 2000;1:107–15. doi: 10.1046/j.1468-1293.2000.00012.x. [DOI] [PubMed] [Google Scholar]
- 5.Manetti R, Annunziato F, Biagiotti R, et al. CD30 expression by CD8+CD30+ T cell clones in human immunodeficiency virus infection. J Exp Med. 1994;180:2407–11. doi: 10.1084/jem.180.6.2407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Del Prete G, De Carli M, Almerigogna F, et al. Preferential expression of CD30 by human CD4+T cells producing Th2-type cytokines. FASEB J. 1995;9:81–6. [PubMed] [Google Scholar]
- 7.Chilosi M, Facchetti F, Notarangelo LD, et al. CD30 cell expression and abnormal CD30 serum accumulation in Omenn's syndrome: evidence for a T helper 2-mediated condition. Eur J Immunol. 1996;26:329–34. doi: 10.1002/eji.1830260209. [DOI] [PubMed] [Google Scholar]
- 8.Mavilia C, Scaletti C, Romagnnani P, et al. Type 2 Helper T cell predominance and high CD30 expression in systemic sclerosis. Am J Pathol. 1997;151:1751–8. [PMC free article] [PubMed] [Google Scholar]
- 9.D'Elios MM, Romagnani P, Scaletti C, et al. In vivo expression in human diseases with predominant activation of Th2-like T cells. J Leuk Biol. 1997;61:539–44. [PubMed] [Google Scholar]
- 10.Giancomelli R, Passacantando A, Parzanese I, et al. Serum levels of soluble CD30 are increased in ulcerative colitis (UC) but not in Crohn's disease. Clin Exp Immunol. 1998;111:532–5. doi: 10.1046/j.1365-2249.1998.00532.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pizzolo G, Vinante G, Nadali G, et al. High serum level of soluble CD30 in acute primary HIV-1 infection. Clin Exp Immunol. 1997;108:251–3. doi: 10.1046/j.1365-2249.1997.d01-1005.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Price P, Murray RJ, John M, French MA. High circulating levels of soluble CD30 correlate with impaired delayed-type hypersensitivity responses in HIV-infected patients. AIDS. 1999;13:2308–9. doi: 10.1097/00002030-199911120-00017. [DOI] [PubMed] [Google Scholar]
- 13.Morimoto C, Schlossman SF. The structure and function of CD26 in the T cell immune response. Immunol Rev. 1998;161:55–70. doi: 10.1111/j.1600-065x.1998.tb01571.x. [DOI] [PubMed] [Google Scholar]
- 14.Scheel-Toellner D, Richter E, Toellner KM, Reiling N, Wacker HH, Gerdes J. CD26 expression in leprosy and other granulomatous dieseases correlates with the production of interferon gamma. Lab Invest. 1995;73:685–90. [PubMed] [Google Scholar]
- 15.Hosono O, Homma T, Kobayashi H, et al. Decreased dipeptidyl peptidase IV enzyme activity of plasma soluble CD26 and its inverse correlation with HIV-1 RNA in HIV-1 infected individuals. Clin Immunol. 1999;91:283–95. doi: 10.1006/clim.1999.4711. 10.1006/clim.1999.4711. [DOI] [PubMed] [Google Scholar]
- 16.Kettering JD, Schmidt NJ, Lennette EH. Improved glycine-extracted complement-fixing antigens for human cytomegalovirus. J Clin Microbiol. 1977;6:647–9. doi: 10.1128/jcm.6.6.647-649.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Keane NM, Price P, Stone S, John M, Murray RJ, French MA. Assessment of immune function by lymphoproliferation underestimates lymphocyte functional capacity in HIV patients treated with highly active antiretroviral therapy. AIDS Res Human Retroviruses. 2000;16:1991–6. doi: 10.1089/088922200750054729. [DOI] [PubMed] [Google Scholar]
- 18.Rizzardi GP, Barcellini W, Tambussi FL, et al. Plasma levels of soluble CD30, tumour necrosis factor (TNF)-α and TNF receptors during primary HIV-1 infection: correlate with HIV-1 RNA and the clinical outcome. AIDS. 1996;10:F45–F50. doi: 10.1097/00002030-199611000-00001. [DOI] [PubMed] [Google Scholar]
- 19.Price P, Keane NM, Stone SF, Cheong KYM, French MA. MHC haplotypes affect the expression of opportunistic infections in HIV patients. Hum Immunol. 2001;62:157–64. doi: 10.1016/s0198-8859(00)00239-1. [DOI] [PubMed] [Google Scholar]
- 20.Schmitz T, Underwood R, Khiroya R, Bachovchin WW, Huber BT. Potentiation of the immune response in HIV-1+ individuals. J Clin Invest. 1996;97:1545–9. doi: 10.1172/JCI118577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Subramanyam M, Gutheil WG, Bachovchin WW, Huber BT. Mechanism of HIV-1 Tat induced inhibition of antigen-specific T cell responsiveness. J Immunol. 1993;150:2544–53. [PubMed] [Google Scholar]
- 22.Aziz N, Nishanian P, Taylor JMG, et al. Stability of plasma levels of cytokines and soluble activation markers in patients with human immunodeficiency virus infection. J Infect Dis. 1999;179:843–8. doi: 10.1086/314673. [DOI] [PubMed] [Google Scholar]