Abstract
To investigate the role of LFA-1 in the immune defects in DS patients, we analysed lymphocytes from DS patients in LFA-1 expression and LFA-1 mediated cell adhesion. DS patients less than 2 years of age expressed a higher level of LFA-1 when compared with age-matched controls. The difference in LFA-1 expression was much less significant in older DS patients when compared with age-matched children. Although older children (2–15-year-old groups) without DS tend to increase their expression of lymphocyte LFA-1 when compared with younger normal children (0–2 years old), DS patients showed no age-associated increase in lymphocyte LFA-1 expression. Two-colour analysis with CD4/CD8 and LFA-1 in patients and controls showed that proportions of CD4 + lymphocytes were comparable in DS patients and controls, while the proportion of CD8 + lymphocytes was higher in older DS patients. Expression levels of LFA-1 on both CD4 + and CD8 + lymphocytes in younger DS patients were higher when compared with age-matched controls and close to the expression levels in the older DS group. Proportions of memory lymphocytes expressing the CD45RO isoform were higher in both younger and older DS patients when compared with age-matched control groups. Noticeably, the LFA-1 expression levels on CD45RO lymphocytes from younger DS patients were higher than the levels of the controls and declined in the older DS group. We tested lymphocytes (EBV transformed B cells, resting and anti-CD3 stimulated T cells) for cellular adhesion to recombinant ICAM-1 and found that lymphocytes from DS patients were less adhesive, even though their β2 integrin expression was comparable with that of normal controls. These results suggest that more generalized pathological processes, such as early senescence of the immune system or ineffective lymphocyte activation, and subsequent integrin dysfunction may underlie the immune defects in DS patients.
Keywords: Down syndrome, cell adhesion molecules, lymphocytes, immunity
Introduction
Down syndrome (DS) is the most common chromosome anomaly in human beings. Approximately one in 1000 infants is born with DS. In addition to the characteristic malformations and mental retardation [1], impaired immune function is reported in DS patients [2,3]. The immunodeficiency results in a higher risk of viral infections and lower response rates to vaccination [4,5]. The immunodeficiency appears to start early in the development of the immune system and tends to worsen with ageing of DS individuals [6,7].
Lymphocyte function associated antigen-1 (LFA-1) is one of the lymphocyte cell surface adhesive proteins that are important for the activation and trafficking of lymphocytes [8]. Abnormalities that affect the function of LFA-1 can not only compromise lymphocyte activation in mature lymphocytes but also might disturb the subtle process of lymphocyte maturation [9]. Based on the observation that LFA-1 integrin β chain (β2, CD18) is encoded on chromosome 21 [10] and that LFA-1 expression is increased on the lymphocytes from DS patients [11], it was postulated that over-expression of LFA-1 integrin on lymphocytes due to gene over-dosage and the subsequent lymphocyte over-adhesiveness leads to abnormalities in lymphocyte development and function [9]. This hypothetical mechanism of immunodeficiency in DS gives hope of potential treatment for correcting the immunodeficiency in DS patients through suppressing the integrin-mediated adhesion in lymphoid organs, including thymus and lymph nodes, during fetal or early infantile stages.
Previous investigations revealed differences in the lymphocyte subpopulations in DS patients when compared with normal subjects [5]. The proportion of CD8 + lymphocytes was reported to be increased in DS patients while that of CD4 + lymphocytes was decreased. The decrease in the CD4 + subpopulation was due to the low proportion of CD4 + CD45RA + naïve lymphocytes in DS patients. These data suggested an abnormality in the development of the lymphocytes in DS patients. It was not clear whether the over-dosage of the CD18 gene plays a role in the abnormal development of lymphocyte subpopulations in the DS patients. On the other hand, it is tantalizing to speculate that the changes in the lymphocyte subpopulations may contribute to the over-expression of LFA-1 in DS patients. To investigate the role of the LFA-1 adhesion molecule on DS lymphocyte function, we analysed the expression of LFA-1 in lymphocytes from DS patients of different age groups. We also directly measured the adhesion function mediated by LFA-1 integrin by using an ICAM-1 recombinant protein in adhesion assays in order to elucidate the role of adhesion molecules in the immunodeficiency of DS.
Materials and methods
Subjects
DS patients were diagnosed by clinical evaluation and confirmed as trisomy 21 with cytogenetic testing at National Cheng-Kung University Hospital. DS patients, aged from 0 to 15 years old, without active infection at the time of blood sampling for a routine annual thyroid function test, were included in this study with the consent of their guardians. Age-matched healthy control donors were included for comparison.
Blood sampling
Heparinized venous blood (5 ml) was collected for flow cytometry cell surface marker analysis. For children older than 2 years of age, an additional 5 ml venous blood was collected for preparation of EBV transformed lymphocytes or purified peripheral blood lymphocytes used in adhesion assays.
Monoclonal antibodies
Fluorescein isothiocyanate (FITC)-labelled mouse anti-human CD4, CD8, CD45RO and LFA-1 (CD11a-CD18) monoclonal antibodies (MoAbs) and non-binding negative control MoAb were purchased from Southern Biotechnology Associates, Inc. (Birmingham, AL, USA). R-phycoerythrin (PE)-labelled anti-LFA-1 (CD11a-CD18) antibody was purchased from Caltag (Burlingame, CA, USA). OKT3 is a mouse IgG2a MoAb specific for human CD3. TS1/22 is an inhibitory mouse IgG1 MoAb specific for human LFA-1 [13]. 3C7 MoAb is an irrelevant mouse IgG1 antibody against Giardia muris trophozoites purchased from ATCC (ATCC# CRL-1959). Anti-ICAM-1 MoAb for Western blotting was purchased from R & D systems (Minneapolis, MN, USA). RFI2 MoAb for blocking adhesion mediated by human ICAM-1 was prepared using a method modified from a previous report [14] as follows. MoAbs were produced by immunizing BALB/cJ mice intraperitoneally with ICAM-1 recombinant protein three times. Three days after the final boost, the mice spleen cells were fused with FO myeloma cells in the presence of PEG. After fusion, cells were cultured in HAT selection medium. After 2 weeks, hybridoma supernatant fluids were screened with ELISA plates (Nalge Nunc International, NY, USA) coated with ICAM-1-Fc recombinant protein. The positive clones were established after two limiting dilutions. One of the MoAb clones, RF12, was found to be effective in blocking adhesion between human leucocytes and ICAM-1 recombinant protein, and was thus selected to be used in this study.
Lymphocyte cell surface staining and flow cytometry analysis
Flow cytometry analysis was performed on isolated peripheral blood leucocytes as previously described [15] using the Epics XL/MCL flow cytometer (Beckman-Coulter). Lymphocytes were analysed after gating based on cell size and granularity. Primary and secondary antibodies were used at saturating concentrations. Isotype-matched irrelevant MoAbs were used as negative controls, while W6/32 antibody (mouse anti-human MHC class I) was used as a positive control. The mean fluorescence intensity (MFI) of negative controls was consistently less than 10 fluorescence units. For two-colour analysis, FITC-labelled CD4, CD8 or CD45RO MoAb and PE-labelled anti-LFA-1 were used to stain isolated leucocytes. Cells stained positive with FITC anti-CD4/CD8 or CD45RO were analysed for the mean fluorescence intensity (MFI) of anti-LFA-1 staining.
Preparation of ICAM-1 recombinant protein
ICAM-1-Fc recombinant protein was prepared as follows. The sequence encoding the entire extracellular portion of human ICAM-1 with additional 5′ BamHI and 3′ Nhe I restriction sites was amplified by PCR using Vent polymerase (New England Biolabs, Beverly, MA, USA) and ligated to the corresponding sites of the expression vector CD5lneg1 (kindly provided by Dr Brian Seed). This vector contains a genomic sequence encoding the hinge, CH2 and CH3 regions of human IgG1, and a sequence encoding the signal peptide from the cell surface molecule CD5. The selected clone was subjected to sequence analysis. The sequence encoding the entire fusion protein construct was removed by digestion with Xho I + Not I and transferred to the expression vector paNeo (Klickstein, unpublished) digested with the same enzymes, to yield the plasmid paNIC-1. This plasmid was introduced into CHO cells via electroporation, and a stable CHO cell clone was selected that expressed ICAM-1-Fc recombinant protein in the culture media. The recombinant protein was purified by protein A affinity chromatography from conditioned media. The protein was greater than 95% pure by SDS-PAGE and was aliquoted and stored at − 80°C. Fc protein was prepared similarly by transfer of the Xho I – Not I fragment of CD5lneg1 itself to paNeo, then CHO cells were transfected as above.
Protein analysis and Western blotting
The purified recombinant protein ICAM-1-Fc (IC1-Fc) that contains the entire extracelluar domain of the human ICAM-1 molecule and the Fc portion of human IgG1, and a control protein Fc which contains only the Fc portion of human IgG1, were diluted with sample buffer containing 10% glycerol, 3% SDS, with (for reducing analysis) or without (for non-reducing analysis) 5% 2-ME by boiling for 3 min and resolved by 10·5% SDS-polyacryamide gel electrophoresis as described [16]. For Western blotting analysis of the recombinant proteins, the resolved proteins were electroblotted to a PVDF membrane (Millipore, Bedford, MA, USA) and probed with primary MoAb (mouse anti-human ICAM-1 MoAb, R & D Systems Inc., Minneapolis, MN, USA) followed by a secondary antibody (goat anti-mouse HRP-labelled antibody, Upstate Biotechnology, Lake Placid, NY, USA). The blot was visualized with a chemoluminescence substrate (ECL, Amersham) and then exposed on an autoradiography film [17].
Lymphocyte purification and transformation with Epstein-Barr virus
Peripheral blood mononuclear cells were isolated by Ficoll-Hypaque (Pharmacia Fine Chemicals, Uppsala, Sweden) density gradient centrifugation. Adherent cells were removed by incubation in plastic tissue culture flasks. Purified T cells were collected according to a method previously described [18]. The purity of the isolated T cells was routinely more than 90% as assessed by anti-CD3 staining. For preparation of EBV-tansformed lymphocytes, mononuclear leucocytes isolated from 5 ml venous blood were cultured in growth medium (RPMI 1640 supplemented with 20% FCS, 1% glutamine, 2 µg/ml cyclosporin and 1% penicillin and streptomycin) and 15% culture supernatant fluid of EBV-secreting cell line B95–8. Phytohaemagglutinin (PHA, 45 µg/ml) was added to the culture medium on the third day. The concentration of cyclosporin was decreased to 1 µg/ml by adding an equal volume of cyclosporin-free medium after 1 week. The transformed lymphocytes formed cell clusters after 3–4 weeks. These transformed lymphocytes were generally negative for anti-CD3 MoAb staining.
Static lymphocyte-protein adhesion assay for lymphocyte cell lines and resting/anti-CD3 stimulated T lymphocytes
Cell-to-protein adhesion assays were performed with modifications of previously reported methods [14,19]. Recombinant protein-coated 96-well plates were prepared by incubating Fc or ICAM-1-Fc protein in PBS at a concentration of 50 µg/ml at 4°C overnight. After washing with PBS, the plates were kept at − 80°C before use. In antibody blocking experiments, the wells were incubated with 50 µl hybridoma culture supernatant fluid for 30 min before adding the suspension cells. Lymphocytes were labelled with 25 µg 2′,7′-bis-(2-carboxyethyl)-5 (and -6) carboxyfluorescein (BCECF-AM, Molecular Probes, Inc. Eugene, OR, USA) dissolved in 5 µl DMSO and added to complete culture medium for 30 min at 37°C. After washing with PBS, 40 000 labelled leucocytes were resuspended in 100 µl adhesion medium (50 mm Tris-HCl, pH 7·4, 150 mm NaCl and 1 mm CaCl2), with or without blocking antibodies, added to each well and incubated at 37°C for 30 min. For T-cell stimulation with anti-CD3 MoAb, 150 µl saturated OKT3 or irrelevant hybridoma supernatant fluid, both from a single preparation, were added to wells that contained assay medium during this incubation period. Unbound cells were then washed from the plates with adhesion medium (three or four washes). Bound cells were detected using a fluorescence plate reader (HTS7000 Bioassay reader, Perkin Elmer, Norwalk, CT, USA). The bound cells were read as fluorescence units detected on the reader. At least four replicates were performed in each experiment. These washing conditions remove approximately 90% of the leucocytes from wells coated with the negative control protein (Fc). Results were expressed as percentage of input cells bound.
Statistical analysis
The Mann–Whitney non-parametric test was used to analyse the results of fluorescence intensity measurements by flow cytometry. Student's t-test was used to analyse the data of adhesion assays and percentage of lymphocyte subpopulations.
Results
Lack of age-associated lymphocyte LFA-1 up-regulation in DS patients
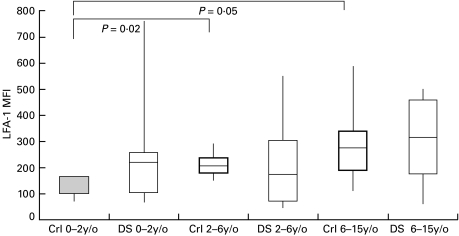
We analysed the LFA-1 expression in lymphocytes from DS patients by direct immunofluorescence with anti-CD11a MoAb and flow cytometry. DS patients less than 2 years of age expressed higher levels of LFA-1 when compared with age-matched controls (MFI medians: 225 versus 132; Fig. 1, first and second boxes). Although older children (2–6 and 6–15 year-old groups) without DS tend to increase their expression of lymphocyte LFA-1 when compared with normal younger children (0–2 years old) (dotted boxes on Fig. 1; P = 0·02 and P = 0·05, respectively), DS patients showed no age-associated increase in lymphocyte LFA-1 expression (clear boxes on Fig. 1). Further analyses of lymphocyte subpopulations were performed to elucidate the differences in lymphocyte LFA-1 expression in younger (< 2 years old) and older (> 2 years old) paediatric DS patients.
Fig. 1.
Expression of LFA-1 on DS patients and age-matched normal controls. Freshly-isolated lymphocytes were analysed for the expression of CD11a with cell-surface staining and flow cytometry analysis. For the 0–2 year-old group, five normal controls (Crl; MFI median: 132; mean age 12·0 months) and seven DS patients (DS; MFI median: 225; mean age 11·4 months) were compared. For the 2–6 year-old group, eight normal controls (MFI median: 195; mean age 4 years 1·7 month) and 23 DS patients (MFI median: 177; mean age 4 years 5·9 month) were included. The 6–15 year-old group comprised 16 controls (MFI median: 272; mean age 11 years 8·0 month) and five DS patients (MFI median: 310; mean age 9 years 3·2 month). Results are expressed as ranges of MFI values (maximal and minimal values with connecting vertical lines) and 75th percentiles, 25th percentiles and medians (50th percentiles), represented by upper and lower sides of boxes and the horizontal lines in the middle, respectively. Dotted boxes represent control groups while clear boxes represent DS patient groups. Statistical analyses were performed by the Mann–Whitney non-parametric method. Significant statistical differences are marked with brackets.
High proportion of CD8 + lymphocytes in older DS patients and failure of LFA-1 up-regulation with age in both CD4 + and CD8 + lymphocytes from DS patients
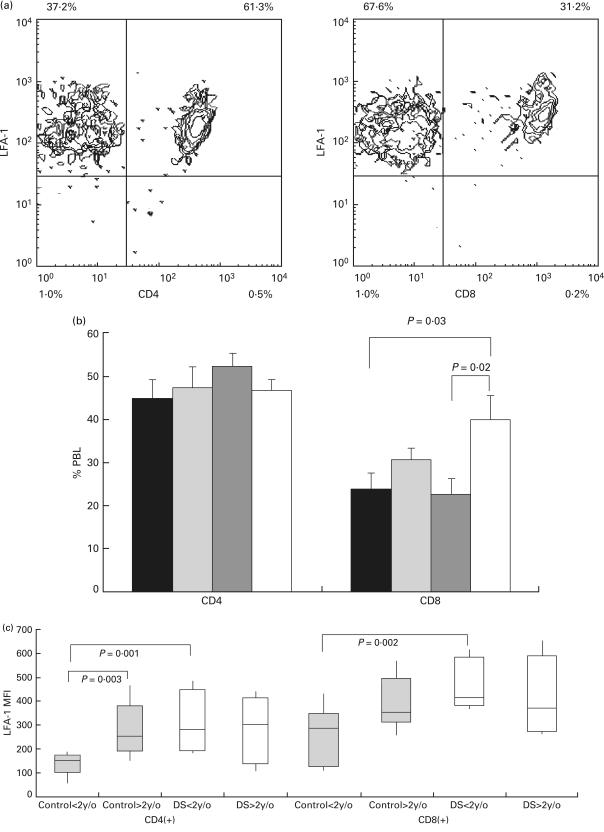
We investigated whether an abnormality in CD4 + and CD8 + subpopulations accounts for this LFA-1 expression pattern. Two-colour analysis was done with CD4/CD8 and LFA-1 staining on peripheral blood lymphocytes from controls and DS patients less than 2 years of age and DS patients between 2 and 15 years old. Proportions of cells expressing either CD4 or CD8 were measured. Cells expressing CD4/CD8 were further analysed for the expression of LFA-1 (Fig. 2a). We found that the proportion of CD4 + lymphocytes was comparable in younger and older DS children, and was not significantly different from controls (Fig. 2b). The proportions of CD8 + lymphocytes, however, was higher in older DS patients when compared with younger DS patients (39·8 ± 4·7% versus 22·3 ± 3·7%, P = 0·02) and younger controls (39·8 ± 4·7% versus 23·6 ± 4·1%, P = 0·03) (Fig. 2b). The expression levels of LFA-1 on CD4 + and CD8 + lymphocytes were analysed separately on these children. CD8 + cells tend to express a higher level of LFA-1 than CD4 + cells in all groups. Similar to the analysis on the whole lymphocyte population, expression levels of LFA-1 on both CD4 + and CD8 + lymphocytes were comparable in older controls, younger DS patients and older DS patients [MFI medians: 254, 278 and 298 (CD4 + lymphocytes); 334, 391 and 352 (CD8 + lymphocytes)]. The LFA-1 expression levels in the CD4 + or CD8 + subpopulations of lymphocytes from these three groups were higher then the levels of LFA-1 on the corresponding lymphocytes from the younger control group. On the CD4 + cells, significant differences were present between older controls and younger controls, and between younger DS patients and younger controls (MFI medians: 254 versus 152, P = 0·003 and 278 versus 152, P < 0·001, respectively) regarding LFA-1 expression. On the CD8 + cells, the difference on LFA-1 expression was significant between young DS patients and younger controls (MFI medians: 391 versus 275, P = 0·002) (Fig. 2c). The failure of age-associated LFA-1 up-regulation in lymphocytes from DS patients was thus apparent in both CD4 + and CD8 + subpopulations of lymphocytes.
Fig. 2.
Proportions and expression levels of LFA-1 on CD4 + and CD8 + lymphocytes in DS patients and control groups. (a) Two-colour flow cytometric analysis of peripheral blood lymphocytes in a typical experiment. The proportion of CD4 + lymphocytes (left panel) and CD8 + lymphocytes (right panel) were measured. Because essentially all lymphocytes expressed LFA-1, double positive populations (right upper quadrants) of lymphocytes for CD4/LFA-1 and CD8/LFA-1 staining were analysed for the level of LFA-1 expression. (b) The percentage of CD4 (left panel) and CD8 (right panel) expressing cells in lymphocytes were analysed in younger control children (▪, n = 10; mean age 7 years 6 months, CD4 percentage: 44·9 ± 4·4, CD8 percentage: 23·6 ± 3·8), older (more than 2 years of age) control group ( , n = 8; mean age 8 years 3 month, CD4 percentage: 47·3 ± 4·9, CD8 percentage: 30·3 ± 2·8), younger (less than 2 years of age) DS patient group (
, n = 8; mean age 8 years 3 month, CD4 percentage: 47·3 ± 4·9, CD8 percentage: 30·3 ± 2·8), younger (less than 2 years of age) DS patient group ( , n = 6; mean age 13·1 months, CD4 percentage: 52·1 ± 3·0, CD8 percentage: 22·3 ± 3·8), and older DS patient group (□, n = 6, mean age 11·5 months, CD4 percentage: 46·6 ± 2·4, CD8 percentage: 39·8 ± 5·4). (c) The expression of LFA-1 on lymphocytes on CD4 (left panel) and CD8 (right panel) expressing lymphocytes were analysed on the younger control group (LFA-1 MFI medians on CD4 + cells and CD8 + cells: 152 versus 275), older control group (LFA-1 MFI medians on CD4 + cells and CD8 + cells: 254 versus 334), younger DS patient group (LFA-1 MFI medians on CD4 + cells and CD8 + cells: 278 and 391), and older DS patient group (LFA-1 MFI medians on CD4 + cells and CD8 + cells: 298 and 352). Results are expressed as ranges of values, 25th percentiles, medians and 75th percentiles as in Fig. 1.
, n = 6; mean age 13·1 months, CD4 percentage: 52·1 ± 3·0, CD8 percentage: 22·3 ± 3·8), and older DS patient group (□, n = 6, mean age 11·5 months, CD4 percentage: 46·6 ± 2·4, CD8 percentage: 39·8 ± 5·4). (c) The expression of LFA-1 on lymphocytes on CD4 (left panel) and CD8 (right panel) expressing lymphocytes were analysed on the younger control group (LFA-1 MFI medians on CD4 + cells and CD8 + cells: 152 versus 275), older control group (LFA-1 MFI medians on CD4 + cells and CD8 + cells: 254 versus 334), younger DS patient group (LFA-1 MFI medians on CD4 + cells and CD8 + cells: 278 and 391), and older DS patient group (LFA-1 MFI medians on CD4 + cells and CD8 + cells: 298 and 352). Results are expressed as ranges of values, 25th percentiles, medians and 75th percentiles as in Fig. 1.
Increased proportion of CD45RO expressing lymphocytes from DS patients and age-associated decrease in LFA-1 expression in their CD45RO-negative cells
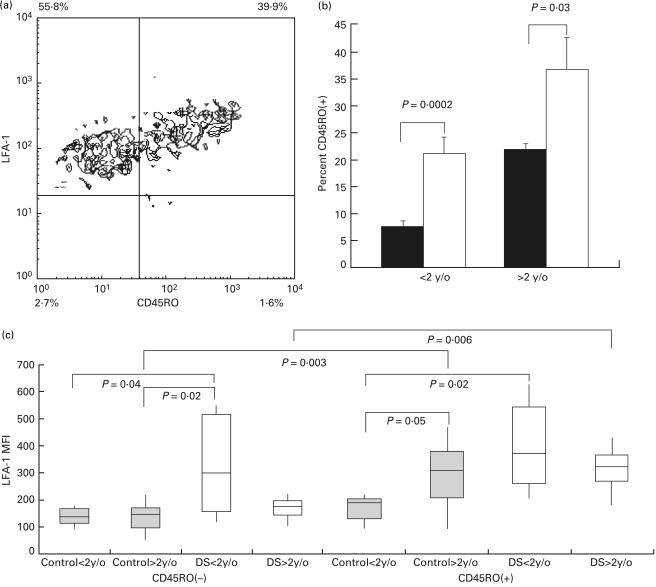
Lymphocytes from DS and normal controls were analysed for expression of CD45RO, which is expressed on the memory or activated lymphocytes. Proportions of cells stained positive or negative for CD45RO were measured. These subpopulations of lymphocytes were further analysed for their expression of LFA-1 (Fig. 3a). For lymphocytes from both younger and older patient groups, the percentage of CD45RO + cells was significantly higher than those in age-matched normal controls (Fig. 3b). While CD45RO + lymphocytes expressed higher levels of LFA-1 when compared with CD45RO – lymphocytes in the older control group and the older DS group (MFI medians: 332 versus 143, P = 0·003 and 347 versus 166, P = 0·006, respectively), LFA-1 expression on CD45RO + and CD45RO – lymphocytes from the younger DS group and younger control group were not significantly different. The lack of difference between CD45RO – and CD45RO + lymphocytes in the younger DS group was due to the high level of LFA-1 expression in CD45RO – cells, which was significantly higher than the expression levels of the same subpopulation in the younger and older control groups (MFI medians: 294 versus 131, P = 0·04 and 294 versus 143, P = 0·02, respectively). The LFA-1 expression level on CD45RO – cells from older DS patients, however, was not different from the levels of younger and older control groups (MFI medians: 166, 131 and 143). The failure of CD45RO – lymphocytes from older DS patients to maintain the high level of LFA-1 expression in the younger DS group correlated with the lack of age-associated up-regulation of lymphocyte LFA-1 in DS patients. Among the CD45RO + lymphocytes, cells from the older control group, and the younger and older DS groups, expressed LFA-1 at comparable levels (MFI medians: 332, 398 and 347), which were higher than the level of the younger control group (Fig. 3c).
Fig. 3.
Proportions and expression levels of LFA-1 on CD45RO + and CD45RO – lymphocytes in DS patients and control groups. (a) Two-colour flow cytometric analysis of peripheral blood lymphocytes in a typical experiment. The proportions of CD45RO + lymphocytes and CD45RO – lymphocytes were measured. Single positive population for LFA-1 (left upper quadrant) and double positive population (right upper quadrant) of lymphocytes for CD45RO/LFA-1 staining were analysed for the level of LFA-1 expression. (b) The percentage of CD45RO + expressing cells in lymphocytes were analysed in younger control children (▪, n = 9; mean age 6·5 months, CD45RO + 7·4 ± 1·0%), older control group (□, n = 8; mean age 8 years 1 month, CD45RO + 21·8 ± 1·1%), younger DS patient group (▪, n = 5; mean age 5·4 months, CD45RO + 21·1 ± 2·9%), and older DS patient group (□, n = 8, mean age 7 years 6 months, CD45RO + 36·5 ± 6·0%). (c) The expression of LFA-1 on lymphocytes on CD45RO – (left panel) and CD45RO + (right panel) expressing lymphocytes were analysed on the younger control children (LFA-1 MFI medians on CD45RO – cells and CD45RO + cells: 131 and 202), older control group (LFA-1 MFI medians on CD45RO – cells and CD45RO + cells: 143 and 332), younger DS patient group (LFA-1 MFI medians on CD45RO – cells and CD45RO + cells: 294 and 398), and older DS patient group (LFA-1 MFI medians on CD45RO – cells and CD45RO + cells: 166 and 347). Results are expressed as ranges of values, 25th percentiles, medians and 75th percentiles as in Fig. 1.
Lymphocyte adhesiveness to ICAM-1 is impaired in DS patients
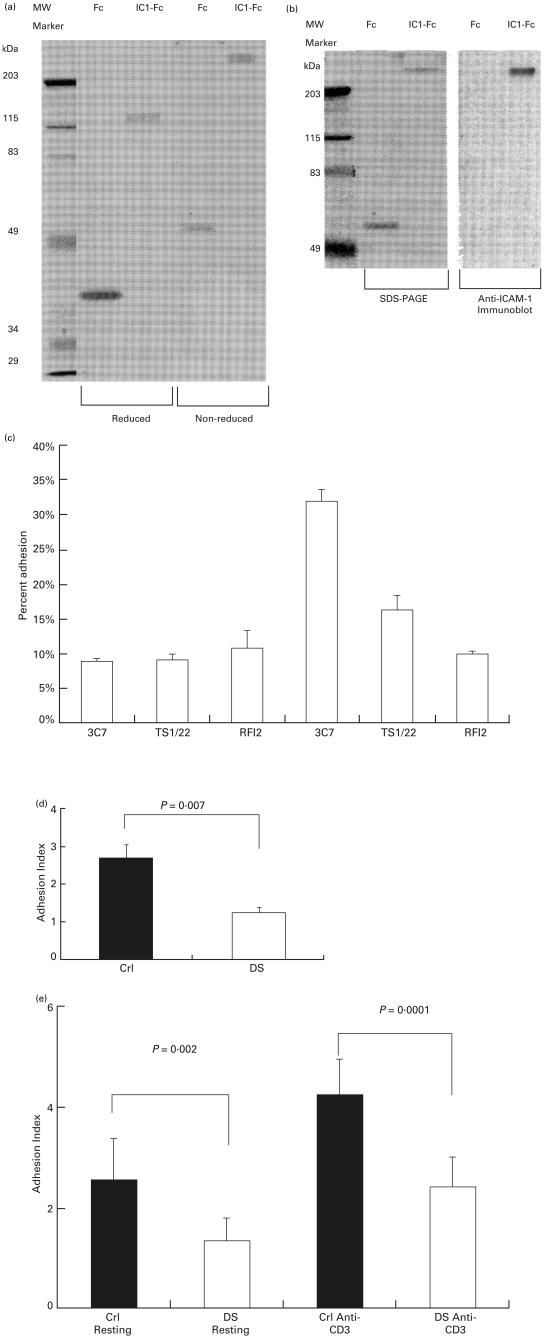
We tested lymphocytes from older DS patients and age-matched normal controls for CD18 containing integrin-mediated binding to ICAM-1 with a recombinant protein ICAM-1-Fc (IC1-Fc), which was resolved as a 120 kDa protein reduced and a 250 kDa protein non-reduced, and a control protein Fc, which was resolved as a 38 kDa protein reduced and a 65 kDa protein non-reduced (Fig. 4a). The identity of the ICAM-1 portion of the IC1-Fc protein was proved by immunoblotting with anti-ICAM-1 MoAb (Fig. 4b).
Fig. 4.
Measurement of lymphocyte adhesion to ICAM-1. (a) Protein analysis for recombinant proteins ICAM-1-Fc (IC1-Fc) and Fc. Purified proteins were analysed under reducing or non-reducing conditions on 10·5% SDS-PAGE and visualized by staining with Coomassie blue. (b) Coomassie blue-stained non-reduced SDS-PAGE gel (left panel) and anti-ICAM-1-probed immunoblot (right panel). (c) EBV-transformed lymphocyte cell lines from DS patients and normal controls were tested for binding to immobilized recombinant Fc and ICAM-1-Fc proteins. A typical adhesion assay with lymphocytes from a normal control is shown. Each bar represents the mean of four wells. Error bars represent standard deviations (s.d.). The ratio of cells bound to ICAM-1-Fc and to Fc was calculated as an adhesion index (SI). (d) The adhesion indexes of transformed B cells from normal controls (▪, Crl, mean age 5 years 2·8 months, n = 7, SI = 2·70 ± 0·92) and DS patients (□, DS, mean age 4 years 11·2 months, n = 5, SI = 1·24 ± 0·23) are compared. Results of adhesion indexes are expressed as mean ± standard deviation (s.d.). (e) The adhesion indexes of T cells from normal controls (▪, mean age 7 years 10 months, n = 8) and DS patients (□, mean age 9 years 10 months, n = 8) in resting condition (first and second columns, adhesion indexes: 2·55 ± 0·82 and 1·33 ± 0·46) or with anti-CD3 stimulation (third and fourth columns, adhesion indexes: 4·22 ± 0·71 and 2·40 ± 0·59).
We used these recombinant proteins (IC1-Fc and Fc control protein) to establish a cell adhesion assay to measure the binding of EBV-transformed lymphocytes to ICAM-1. The Fc recombinant protein was used as a negative adhesion control. In a typical experiment, we found that these transformed lymphocytes bound to ICAM-1 Fc more efficiently than to Fc protein. The binding of lymphocytes to ICAM-1-Fc could be blocked with MoAb to LFA-1 (TS1/22) and MoAb to ICAM-1 (RFI2) (Fig. 4c). We measured ICAM-1-mediated lymphocyte binding by calculating the lymphocyte adhesion indexes (ratios of binding to ICAM‐1-Fc and Fc proteins) for these transformed lymphocytes from different individuals. We found that lymphocytes from DS patients were less adhesive to ICAM-1 (Fig. 4d; mean ± standard deviation (s.d.), 1·24 ± 0·23 for DS patients versus 2·70 ± 0·92 for age-matched normal controls), even though their LFA-1 expression levels were similar [mean MFI: 250 (DS lymphocytes) versus 283 (control lymphocytes)].
To test the LFA-1-mediated binding of fresh T cells to ICAM-1, we used purified T cells from peripheral blood from DS patients and age-matched normal controls to test the cellular binding to ICAM-1 in the resting condition and after anti-CD3 stimulation. While the cellular expression levels of LFA-1 were comparable, we found that the binding of T cells from DS patients was significantly lower than T cells from normal controls (Fig. 4e) in resting states (adhesion index: 1·33 ± 0·46 versus 2·55 ± 0·82). After TCR stimulation with anti-CD3, although binding to ICAM-1 increased in T cells from both DS and control groups, the binding of DS T cells was still significantly lower than T cells from the control group (adhesion index: 2·40 ± 0·59 versus 4·22 ± 0·31). The expression level of LFA-1 did not significantly change after the 30 min treatment with anti-CD3 used in these experiments.
Discussion
With the significant progress in caring for DS patients, understanding the underlying causes of immunodeficiency in DS patients remains a challenge. Given the fact that LFA-1 integrin β chain (β2, CD18) is encoded on chromosome 21 [10] and the observation that LFA-1 expression is increased on the lymphocytes from DS patients [11], the over-expression of LFA-1 integrin on T lymphocytes due to gene over-dosage was previously postulated to be the culprit that leads to aberrant T-cells maturation [9]. The increased expression of ICAM-1 (the counter-receptor of LFA-1) on thymic epithelium [9] is postulated to be compatible with the theory that the immunodeficiency in DS patients is due to excessive thymic deletion caused by over-expression of adhesion molecules. If this mechanism indeed plays an important role in immunodeficiency in DS children, it is reasonable to treat DS children early in their life in order to decrease the adhesiveness of their lymphocytes and facilitate the normal development of their lymphocyte repertoire. However, the chromosomal anomaly in DS involves many genes that encode molecules that are potentially important for the immune responses and lymphocyte development in these patients. Interaction between these genes that are affected by the chromosomal anomaly of trisomy 21 might lead to immunological phenotypes that are not expected by simply assuming the gene over-dosage of a single molecule.
In this study, we first investigated the lymphocytes from different age groups of DS patients in order to understand the effect of maturation on the lymphocyte LFA-1 expression. The over-expression of LFA-1 in DS children is evident only in patients younger than 2 years of age. In older DS patients, although lymphocyte LFA-1 expression is maintained at a high level, the level is not higher than the expression of LFA-1 in older controls who have up-regulated their lymphocyte LFA-1 levels with age. It was previously suggested that the increased expression of LFA-1 in DS patients might be related to the increased proportion of CD8 + T cells, which tend to express higher levels of LFA-1 [20]. The results in this study suggest that the change in the proportion of CD8 + T cells is not the only factor that affects LFA-1 expression in DS patients. Although older DS patients have a higher proportion of CD8 + T cells, and CD8 + T cells did express higher levels of LFA-1 when compared with CD4 + T cells, both CD4 + and CD8 + lymphocytes from DS patients failed to increase LFA-1 expression with age. The proportion of CD45RO + memory cells was higher in lymphocytes from younger DS patients than in age-matched controls and increased moderately with age. The expression level of LFA-1 in CD45RO – lymphocytes from younger DS was markedly higher than the levels of the same subpopulation of lymphocytes from control groups and older DS patients. Failure of a more significant increase in the proportion of CD45RO + lymphocytes and the age-associated decline of LFA-1 expression in CD45RO – lymphocytes appeared to contribute to the failure of up-regulation of LFA-1 in lymphocytes from DS patients. These observations thus suggested a more generalized anomaly involving the naïve and memory phenotypes in the DS lymphocytes regarding LFA-1-related immune maturation.
The adhesion of leucocyte integrins is regulated to fulfil the unique requirements of cell trafficking and functioning in diverse microenvironments. Although the expression level of lymphocyte adhesion molecules is an important factor in determining the adhesion function of cells, integrin-mediated adhesion is regulated at other levels as well. For the integrins on the cell surface to bind to their specific ligands, inside-out signals activate their ligand-binding domains and enable these molecules to establish tight adhesion with their ligands [21,22]. Intracellular signalling may also determine the association between the cytoplasmic domain of integrins and cytoskeleton, and determine the adhesion function of integrins [23]. In this study, we used a recombinant protein, ICAM-1-Fc, to perform the protein–cell adhesion assay and directly measure integrin-mediated binding at the cellular level. We used freshly-isolated T cells and EBV-tranformed lymphocyte cell lines to measure ICAM-1-mediated lymphocyte binding. ICAM-1 is the ligand of β2 integrins, including LFA-1, Mac-1 and αxβ2 [24,25]. As LFA-1 is the major integrin counter-receptor of ICAM-1 on lymphocytes, our measurements should reflect the LFA-1-mediated adhesion on these lymphocytes. Even though the expression of LFA-1 on lymphocytes is not significantly different in the older DS patients and normal controls, cells from DS patients bound less tightly to ICAM-1. This defective binding by LFA-1 might be due to either failure in TCR-dependent or TCR-independent T-cell activation, or defective intracellular signal transduction. It was reported that lymphocytes from older DS patients are defective in the signal transduction triggered by T-cell receptor/CD3 [26]. This defective T-cell receptor signalling that fails to adequately activate LFA-1 may underlie the poor integrin-mediated T-cell binding in DS patients. However, our observation that both T cells and transformed B cells bound weakly to ICAM-1 again suggested that a more general defect in the mechanism for activating lymphocyte LFA-1 integrins exists in DS patients. A recently described gene named AIRE (autoimmune regulator), identified from chromosome 21q22.3 by positional cloning, encodes a protein that contains DNA-binding domains and is located in both the nucleus and cytoplasm. Nonsense or missense mutations of this AIRE product lead to a familial immunodeficiency and autoimmune endocrinopathy [27]. Whether over-expression of this gene or other genes that are involved in regulation of immune responses in DS patients contributes to the impaired LFA-1 up-regulation and activation of integrin-mediated adhesion, merits further investigation.
In summary, our study on DS children of different age groups revealed high expression of lymphocyte LFA-1 in younger DS patients. There was no significant increase in LFA-1 expression level with the growing of the children. Moreover, direct measurement of T- and B-lymphocyte adhesion showed lower binding to an ICAM-1 recombinant protein. The results of this investigation suggest that a generalized pathological process, such as early senescence of the immune system and ineffectiveness in activating lymphocytes, rather than pure lymphocyte hyperadhesiveness, may underlie the immune function defects in older DS patients.
Acknowledgments
The authors thank all the patients and their families for participating in this research. We want to thank Dr Leonard E. Maroun for helpful discussions and Ms Yan-Shio Goh and Ya-Lan Yang for excellent technical assistance. This study was supported partly by a research grant (NHRI-GT-EX89S839C) from the National Health Research Institute, Taiwan, an intramural research grant from NCKU Hospital to C.-C. Shieh and a research grant from the National Science Council, Taiwan, to S.-J. Lin.
References
- 1.Lin SJ, Huang MC, Chen SH, Tsai CJ, Shu SF. A follow-up study of genetic counseling in Down syndrome. Pediatrica Taiwanica. 1995;36:192–6. [PubMed] [Google Scholar]
- 2.Cuadrado E, Barrena MJ. Immune dysfunction in Down's syndrome: primary immune deficiency or early senescence of the immune system? Clin Immunol Immunopathol. 1996;78:209–14. doi: 10.1006/clin.1996.0031. 10.1006/clin.1996.0031. [DOI] [PubMed] [Google Scholar]
- 3.Novo E, Garcia MI, Lavergne J. Non-specific immunity in Down syndrome. Am J Med Genet. 1993;46:384–91. doi: 10.1002/ajmg.1320460408. [DOI] [PubMed] [Google Scholar]
- 4.Ahman L, Back E, Bensch K, Olcen P. Non-efficiency of low-dose intradermal vaccination against hepatitis B in Down's syndrome. Scand J Infect Dis. 1993;25:16–23. doi: 10.1080/00365549309169664. [DOI] [PubMed] [Google Scholar]
- 5.Barrena MJ, Echaniz P, Garcia-Serrano C, Cuadrado E. Imbalance of the CD4+ subpopulations expressing CD45RA and CD29 antigens in the peripheral blood of adults and children with Down syndrome. Scand J Immunol. 1993;38:323–6. doi: 10.1111/j.1365-3083.1993.tb01733.x. [DOI] [PubMed] [Google Scholar]
- 6.Lockitch G, Singh VK, Puterman ML, Godolphin WJ. Age related changes in humoral and cell-mediated immunity in Down syndrome children living at home. Pediatr Res. 1987;22:536–40. doi: 10.1203/00006450-198711000-00013. [DOI] [PubMed] [Google Scholar]
- 7.Thilaganathan B, Tsakonas D, Nicolaides K. Abnormal fetal immunological development in Down's syndrome. Br J Obs Gyn. 1993;100:60–2. doi: 10.1111/j.1471-0528.1993.tb12952.x. [DOI] [PubMed] [Google Scholar]
- 8.Larson RS, Springer TA. Structure and function of leukocyte integrins. Immunol Rev. 1990;114:181–217. doi: 10.1111/j.1600-065x.1990.tb00565.x. [DOI] [PubMed] [Google Scholar]
- 9.Murphy M, Insoft RM, Pike-Nobile L, Derbin KS, Epstein LB. Overexpression of LFA-1 and ICAM-1 in Down syndrome thymus. J Immunol. 1993;150:5696–703. [PubMed] [Google Scholar]
- 10.Soumalainen HA, Gahmberg CG, Patarroyo MM, Beatty PG, Shroeder J. Genetic assignment of GP90 leukocyte adhesion glycoprotein to human chromosome 21. Somat Cell Mol Genet. 1986;12:297–302. doi: 10.1007/BF01570789. [DOI] [PubMed] [Google Scholar]
- 11.Taylor GM, Haigh H, Williams A, D'Souza SW, Harris R. Down's syndrome lymphoid cell lines exhibit increased adhesion due to the over-expression of lymphocyte function-associated antigen (LFA-1) Immunology. 1988;64:451–6. [PMC free article] [PubMed] [Google Scholar]
- 12.Kung PC, Goldstein G, Reinherz EL, Schlossman SF. Monoclonal antibodies defining distinctive human T cell surface antigens. Science. 1979;206:347–59. [PubMed] [Google Scholar]
- 13.Sanchez-Madrid F, Krensky AM, Ware CF, et al. Three distinct antigens associated with human T-lymphocyte-mediated cytolysis. LFA-1, LFA-2, and LFA-3. Proc Natl Acad Sci USA. 1982;79:7489–93. doi: 10.1073/pnas.79.23.7489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shieh CC, Sadasivan BK, Russell GJ, Schon MP, Parker CM, Brenner MB. Lymphocyte adhesion to epithelia and endothelia mediated by the lymphocyte endothelial-epithelial cell adhesion molecule glycoprotein. J Immunol. 1999;163:1592–601. [PubMed] [Google Scholar]
- 15.Parker CM, Groh V, Band H, et al. Evidence for extrathymic changes in the T cell receptor gamma/delta repertoire. J Exp Med. 1990;171:1597–612. doi: 10.1084/jem.171.5.1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–5. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 17.Harlow E, Lane D. Antibodies: a laboratory manual. Cold Spring Harbor: Cold Spring Harbor Laboratory Press; 1988. Immunoblotting; pp. 471–510. [Google Scholar]
- 18.Arroyo AG, Garcia-Vicuna R, Marazuela M, Yednock TA, Gonzalez-Amaro R, Sanchez-Madrid F. Expression and functional significance of an activation-dependent epitope of the beta 1 integrins in chronic inflammatory diseases. Eur J Immunol. 1995;25:1720–8. doi: 10.1002/eji.1830250635. [DOI] [PubMed] [Google Scholar]
- 19.Cepek KL, Shaw SK, Parker CM, et al. Adhesion between epithelial cells and T lymphocytes mediated by E- cadherin and the alpha E beta 7 integrin. Nature. 1994;372:190–3. doi: 10.1038/372190a0. [DOI] [PubMed] [Google Scholar]
- 20.Murphy M, Epstein LB. Down syndrome (DS) peripheral blood contains phenotypically mature CD3+TCR alpha, beta+ cells but abnormal proportions of TCR alpha, beta+, TCR gamma, delta+, and CD4+ CD45RA+ cells: evidence for an inefficient release of mature T cells by the DS thymus. Clin Immunol Immunopathol. 1992;62:245–51. doi: 10.1016/0090-1229(92)90079-4. [DOI] [PubMed] [Google Scholar]
- 21.Hynes RO. Integrins: versatility, modulation, and signaling in cell adhesion. Cell. 1992;69:11–25. doi: 10.1016/0092-8674(92)90115-s. [DOI] [PubMed] [Google Scholar]
- 22.Dustin ML, Springer TA. T-cell receptor cross-linking transiently stimulates adhesiveness through LFA-1. Nature. 1989;341:619–24. doi: 10.1038/341619a0. [DOI] [PubMed] [Google Scholar]
- 23.Kassner PD, Alon R, Springer TA, Hemler ME. Specialized functional properties of the integrin alpha 4 cytoplasmic domain. Mol Biol Cell. 1995;6:661–74. doi: 10.1091/mbc.6.6.661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dustin ML, Springer TA. Role of lymphocyte adhesion receptors in transient interactions and cell locomotion. Annu Rev Immunol. 1991;9:27–66. doi: 10.1146/annurev.iy.09.040191.000331. [DOI] [PubMed] [Google Scholar]
- 25.Miller J, Knorr R, Ferrone M, Houdei R, Carron CP, Dustin ML. Intercellular adhesion molecule-1 dimerization and its consequences for adhesion mediated by lymphocyte function associated-1. J Exp Med. 1995;182:1231–41. doi: 10.1084/jem.182.5.1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Scotese I, Gaetaniello L, Matarese G, Lecora M, Racioppi L, Pignata C. T cell activation deficiency associated with an aberrant pattern of protein tyrosine phosphorylation after CD3 perturbation in Down's syndrome. Pediatr Res. 1998;44:252–8. doi: 10.1203/00006450-199808000-00019. [DOI] [PubMed] [Google Scholar]
- 27.Bjorses P, Halonen M, Palvimo JJ, et al. Mutations in the AIRE gene: effects on subcellular location and transactivation function of the autoimmune polyendocrinopathy-candidiasis-ectodermal dystrophy protein. Am J Hum Genet. 2000;66:378–92. doi: 10.1086/302765. [DOI] [PMC free article] [PubMed] [Google Scholar]