Abstract
Perinuclear antineutrophil cytoplasmic antibodies (p-ANCA) directed against cytoplasmic proteins of neutrophils have been studied extensively in patients with systemic vasculitides. Recent data indicate that antineutrophil antibodies in sera from patients with chronic inflammatory bowel diseases (IBD) or autoimmune liver disorders, currently called ‘atypical p-ANCA’, recognize a nuclear target antigen, rendering the term ‘ANCA’ inaccurate. Specific microscopic criteria to distinguish atypical p-ANCA from p-ANCA are lacking. We used planar and confocal laser scanning indirect immunofluorescence microscopy to examine the labelling characteristics of ethanol-, methanol- and formaldehyde-fixed neutrophils by antineutrophil antibodies in 153 serum samples from patients with IBD, autoimmune liver disorders, systemic vasculitides or healthy blood donors. On ethanol- or methanol-fixed neutrophils, multiple intranuclear fluorescent foci together with either a rim-like peripheral nuclear staining (‘type A’) or a combined cytoplasmic and peripheral nuclear staining (‘type B’) was noted exclusively with atypical p-ANCA in sera from patients with IBD or autoimmune liver disorders. Intranuclear foci, which probably corresponded to invaginations of the nuclear envelope, were not labelled by p-ANCA from patients with microscopic polyangiitis or cytoplasmic ANCA (c-ANCA) from patients with Wegener's granulomatosis. On formaldehyde-fixed neutrophils, atypical p-ANCA gave a fine rim-like staining of the nuclear periphery, whereas ANCA diffusely labelled the cytoplasm. To distinguish reliably between the patterns produced by atypical p-ANCA or p-ANCA, particularly p-ANCA, careful indirect immunofluorescence microscopy on ethanol- as well as on formaldehyde-fixed neutrophils is necessary, with particular emphasis on the presence of multiple intranuclear fluorescent foci.
Keywords: ANCA, autoimmunity, primary sclerosing cholangitis, autoimmune hepatitis, ulcerative colitis
Introduction
Antineutrophil cytoplasmic antibodies (ANCA) are autoantibodies directed against proteins of cytoplasmic granules or other cytoplasmic constituents of neutrophils [1,2]. The staining patterns produced by ANCA have been described as perinuclear (p-ANCA) or cytoplasmic (c-ANCA). While c-ANCA diffusely label the cytoplasm of ethanol-fixed neutrophils with or without interlobular accentuation, p-ANCA only label the perinuclear cytoplasm. C-ANCA are primarily present in sera from patients with Wegener's granulomatosis and mainly recognize proteinase 3. P-ANCA are frequently detected in patients with microscopic polyangiitis and most often react with myeloperoxidase. Labelling of the perinuclear cytoplasm by p-ANCA is actually an artefact of ethanol fixation, which causes a redistribution of the positively charged cytoplasmic granula proteins to collapse onto the surface of the negatively charged nucleus.
Antineutrophil antibodies have also been reported in high percentages of patients with ulcerative colitis (UC; 60–87%), primary sclerosing cholangitis (PSC; 60–92%), autoimmune hepatitis (AIH; 50–96%) and, to a lesser extent, Crohn's disease (CD; 5–25%) [3–9]. All attempts so far to identify molecularly a predominant cytoplasmic target antigen of antineutrophil antibodies in UC, CD, PSC and AIH have not been successful [10–14]. Thus, these antibodies are described frequently as ‘atypical p-ANCA’, ‘a-ANCA’ or ‘x-ANCA’ [15–17]. Our group has demonstrated that the majority of antineutrophil antibodies from patients with IBD and autoimmune liver disorders react with a myeloid cell-specific protein that is localized in the nuclear periphery [18,19]. Other groups have also obtained data, suggesting that a nuclear antigen is the target of these antibodies [20–23]. This nuclear localization of the recognized antigen renders the term ‘ANCA’ inaccurate for these autoantibodies and, as we propose, should be replaced by the term ‘perinuclear antineutrophil nuclear antibodies (p-ANNA)’ [19]. The suffix ‘perinuclear’ should always be used to avoid any confusion in terminology with ‘antineuronal nuclear antibodies (ANNA)’ [24]. However, as long as the molecular identification of the proposed nuclear target antigen has not been accomplished, a change in terminology would not be recommended. Accordingly, we will continue to use the term ‘atypical p-ANCA’ throughout this paper describing atypical p-ANCA as a serological subgroup marker of p-ANCA despite the putatively intranuclear localization of the antigen.
As long as the target antigen recognized by atypical p-ANCA remains unidentified, sensitive and specific solid phase assays cannot be developed, leaving immunofluorescence microscopy as the only widely available technique for the detection of these antibodies. However, uncertainty remains as to how to distinguish p-ANCA from atypical p-ANCA reliably. This distinction is complicated further by the fact that various heterogeneous fluorescence patterns are also commonly called ‘atypical p-ANCA’ [16]. For example, some investigators have described a ‘snow-drift-like’ fluorescence, characterized by a combined cytoplasmic and perinuclear fluorescence pattern [16,17], and others refer to a ‘very perinuclear’ fluorescence with or without nuclear fluorescence [6]. In the present study, we define simple microscopic features that distinguish the fluorescence pattern of atypical p-ANCA from that of p-ANCA using planar and confocal laser scanning indirect immunofluorescence microscopy.
Materials and methods
Patients
The study group consisted of 153 patients with CD (n = 13), UC (n = 29), PSC (n = 34), AIH (n = 26), Wegener's granulomatosis ([WG]; n = 19), microscopic polyangiitis ([mPAN]; n = 12), systemic lupus erythematosus ([SLE]; n = 10) and control sera from healthy blood donors without ANCA (n = 10). Clinical characteristics of the study population are given in Table 1. Diagnoses were based on established clinical, radiological, endoscopic, histological and serological criteria. The study was approved by the Columbia-Presbyterian Medical Centre Institutional Review Board.
Table 1.
Clinical characteristics of the study population
| CD | UC | PSC | AIH | WG | mPAN | SLE | Normals | |
|---|---|---|---|---|---|---|---|---|
| No. (total n = 153) | 13 | 29 | 34 | 26 | 19 | 12 | 10 | 10 |
| Sex (M/F) | 9/4 | 11/18 | 23/11 | 8/18 | 8/11 | 7/5 | 3/7 | 5/5 |
| Median age (years) | 36 | 40 | 40 | 41 | 56 | 63 | 41 | 34 |
| (range) | (17–49) | (10–82) | (12–62) | (9–71) | (33–72) | (56–77) | (26–59) | (21–43) |
| Concomitant IBDa | – | – | 16 | 0 | 0 | 0 | 0 | 0 |
| Concomitant PSCb | 0 | 0 | – | 6 | 0 | 0 | 0 | 0 |
| ANCA-positive | 13 | 29 | 34 | 26 | 19 | 12 | 10 | 0 |
| ANA-positive | 0 | 1 | 3 | 4 | 0 | 0 | 10 | 0 |
| Active/inactive disease | 10/3 | 25/4 | 28/6 | 21/5 | 12/7 | 10/2 | 4/6 | 0 |
| Immunosuppressionc | 7 | 17 | 12 | 18 | 13 | 10 | 5 | 0 |
After initial diagnosis of either PSC or AIH, the patient also developed either ulcerative colitis or Crohn's disease.
After initial diagnosis of IBD or AIH, the patient also developed PSC.
Corticosteroids, azathioprin or cyclosporin A were used for a monotherapy or combined immunosuppressive therapy.
Serum specimens
Serum samples were stored at − 20°C until analysis. All sera except 10 sera from healthy blood donors contained antineutrophil antibodies with serum endpoint titres > 1: 20 detected by conventional indirect immunofluorescence microscopy using fixed neutrophils (INOVA Diagnostics, La Jolla, CA, USA). All sera were also tested for the simultaneous presence of non-neutrophil-specific antinuclear antibodies (ANA) by indirect immunofluorescence microscopy using ethanol-fixed HepG2 cells (Kallestad, Chaska, MA, USA). Serum endpoint titres of > 1: 80 were considered positive for ANA. To exclude false positive results for antineutrophil antibodies due to the simultaneous presence of both ANA and antineutrophil antibodies, the serum endpoint titre of antineutrophil antibodies had to be more than twofold higher than the co-existing serum endpoint titre of ANA. To determine the serum endpoint titres, sera were diluted up to the highest dilution that still gave a characteristic fluorescence pattern. The sera were examined independently for their immunofluorescence patterns on fixed neutrophils by two investigators without knowledge of the underlying diseases of the patients.
Indirect immunofluorescence microscopy
Indirect immunofluorescence microscopy was performed using ethanol-, methanol- and formaldehyde-fixed human neutrophils. Slides with neutrophils spread as monolayers were purchased from Inova Diagnostics. Compared to using cytocentrifugation of neutrophils onto slides, this preparation results in a significant reduction of background fluorescence and the specific patterns produced by antineutrophil antibodies can be depicted more accurately. To exclude the possibility that differences in fluorescence patterns produced by antineutrophil antibodies were due to effects of this specific technique, we also prepared slides with neutrophils isolated from blood of healthy donors by density gradient centrifugation and attached to slides by cytocentrifugation, as described elsewhere [25]. The neutrophils were then fixed with either 98% (v/v) ethanol (15 min, − 20°C), 95% (v/v) methanol (15 min, − 20°C) or 4% formaldehyde (w/v) (10 min, 20°C) after permeabilizing with Triton X-100 (5 min, 20°C).
For indirect immunofluorescence microscopy, fixed neutrophils on slides were incubated with serum samples in a humidified chamber at room temperature for 30 min. Bound antibodies were detected by incubation (room temperature, 20 min) with fluorescein isothiocyanate (FITC)-conjugated goat antihuman IgG (H +) secondary antibodies (Inova Diagnostics) and counterstained with Evans blue. After mounting with an antifading medium (Slowfade Light Antifade Kit; Molecular Probes, Eugene, OR, USA), slides were viewed using a Leitz SM-Lux epi-illumination fluorescence microscope equipped with a 60× and 100× objective (Leica, Wetzlar, Germany). Slides were also examined by confocal laser scanning microscopy using a Zeiss CSM 410 confocal laser scanning system attached to an inverted Zeiss Axiovert 100 TV fluorescence microscope (Thornwood, NY, USA) and equipped with a 100× objective.
For double labelling immunofluorescence microscopy, neutrophils fixed on slides were incubated with sera containing antineutrophil antibodies, diluted 1: 20, and rabbit antibodies against lamin B1 [26] diluted 1: 100. Bound antineutrophil antibodies were detected with FITC-conjugated secondary antibodies as described above. Antibodies against lamin B1 were visualized by Texas red-conjugated goat antirabbit IgG (H + l) secondary antibodies, diluted 1: 100 (Jackson Immuno Research, West Grove, PA, USA). Confocal laser scanning microscopy was carried out using the system as described above with horizontal optical scanning performed in 1 µm steps. Images of the fluorescence patterns produced by antineutrophil antibodies, detected by FITC-conjugated secondary antibodies (525 nm wavelength), were superimposed optically with images produced by antilamin B1 antibodies, visualized by Texas red-conjugated secondary antibodies (570 nm wavelength). Microscopy images were processed on a Macintosh G3 computer (Apple Computer, Cupertino, CA, USA) using Photoshop version 4·0 software (Adobe Systems, San Jose, CA, USA).
Chemicals
Unless noted otherwise, routine chemicals were purchased either from Fisher Scientific (Pittsburgh, PA, USA) or Sigma (St Louis, MA, USA).
Statistical analysis
Data are provided as either medians or means ± standard deviations. Statistical analysis of the data was performed using the non-parametric Wilcoxon test. P-values < 0·05 were considered to be significant.
Results
Microscopic immunofluorescence patters produced by atypical p-ANCA compared to p-ANCA on ethanol-fixed neutrophils
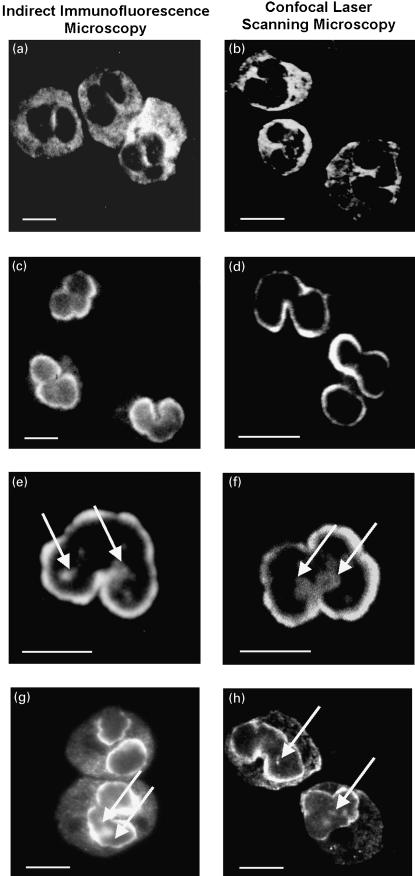
Three immunofluorescence patterns produced by antineutrophil antibodies were detected using indirect immunofluorescence microscopy. The fluorescence patterns of c-ANCA and p-ANCA in sera from patients with systemic vasculitides are well defined [1,2,16]. c-ANCA gave a diffuse fine granular labelling of the cytoplasm that was accentuated between the nuclear lobes (Fig. 1a,b). P-ANCA yielded a finely rimmed, homogeneous fluorescence staining of the perinuclear cytoplasm (Fig. 1c,d). The fluorescence pattern produced by atypical p-ANCA was clearly different from that of p-ANCA and was further subdivided into two subtypes, which we called ‘type A’ and ‘type B’. ‘Type A’ atypical p-ANCA were characterized by a broad, inhomogeneous ‘perinuclear’ staining of neutrophils (Fig. 1e,f), whereas ‘atypical p-ANCA type B’ showed a combined broad inhomogeneous ‘perinuclear’ fluorescence together with a diffuse coarse granular cytoplasmic staining (Fig. 1g,h). Contrary to the staining pattern produced by c-ANCA, the cytoplasmic fluorescence of atypical p-ANCA ‘type B’ was not accentuated between the nuclear lobes. According to the international consensus statement on testing and reporting on ANCA [16], this cytoplasmic staining has also been described as ‘flat’ cytoplasmic staining. As reported previously [18], we could demonstrate that the ‘perinuclear’ fluorescence of atypical p-ANCA actually reflects a labelling of the nuclear periphery. Multiple intranuclear fluorescent foci were regularly found with both types of atypical p-ANCA and were never detected in sera containing p-ANCA or c-ANCA (Fig. 1e–h). The intranuclear foci were detectable by planar indirect immunofluorescence microscopy as well as by high resolution confocal laser scanning microscopy. These scattered intranuclear fluorescent foci were highly sensitive (98%) and highly specific for atypical p-ANCA (96%) and their presence helped to reliably distinguish the fluorescence pattern produced by atypical p-ANCA from that given by p-ANCA. The fluorescence patterns of c-ANCA, p-ANCA and atypical p-ANCA were only detected on neutrophils and on monocytes, but not on fixed eosinophils or lymphocytes.
Fig. 1.
Immunofluorescence micrographs of staining patterns produced by different types of antineutrophil antibodies on ethanol-fixed human neutrophils examined by planar and confocal laser scanning indirect immunofluorescence microscopy. Labelling of ethanol-fixed neutrophils by antineutrophil antibodies was detected with FITC-conjugated goat antihuman IgG secondary antibodies. The micrographs in panels a, c, e and g have been visualized by indirect planar immunofluorescence microscopy, the images in panels b, d, f and h were taken by confocal laser scanning microscopy. (a and b) A diffuse cytoplasmic fluorescence, frequently accentuated between the nuclear lobes of the neutrophils, was characteristic of a serum with c-ANCA from a patient with Wegener's granulomatosis. (c and d) A fine homogeneous rim-like staining of the perinuclear cytoplasm was obtained with a serum containing p-ANCA from a patient with microscopic polyangiitis. (e and f) Atypical p-ANCA ‘type A’ gave a broad inhomogeneous rim-like staining of the nuclear periphery along with scattered intranuclear fluorescent foci (arrows). The atypical p-ANCA ‘type A’ were found in the serum of a patient with primary sclerosing cholangitis. (g and h) In addition to the characteristic staining pattern of atypical p-ANCA ‘type A’, atypical p-ANCA ‘type B’ produced a diffuse cytoplasmic fluorescence. The atypical p-ANCA ‘type B’ were detected in a serum from a patient with autoimmune hepatitis. Note that p-ANCA (c,d) can be distinguished from atypical p-ANCA (e–h) in that the atypical p-ANCA show multiple intranuclear fluorescent foci representing stained invaginations of the multilobulated neutrophil nuclear envelope. Size bars indicate 10 µm.
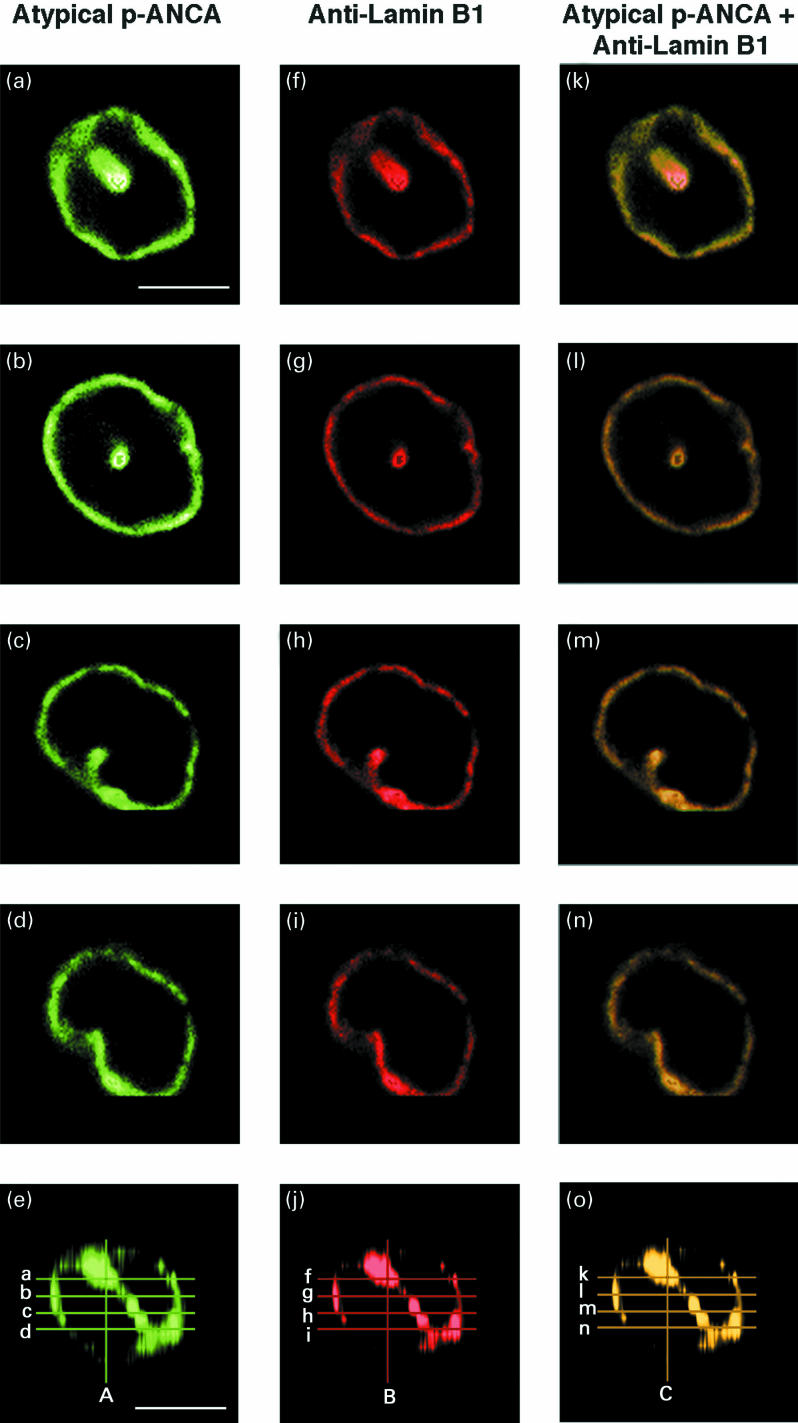
To exclude the possibility that the intranuclear fluorescence foci produced by atypical p-ANCA were caused by tangentially slicing the neutrophil surface, ethanol-fixed neutrophils were optically sectioned into multiple horizontal planes by confocal laser scanning microscopy. In these sequential sections, the multiple nuclear fluorescent foci could be assigned to the nucleus (Fig. 2a–e). In addition, double labelling with antibodies against the nuclear envelope protein lamin B1 (Fig. 2f–j) revealed a complete overlap of the fluorescence signals at the nuclear periphery as well as in the intranuclear foci (Fig. 2k–o). Three-dimensional reconstructions of optical sections obtained from neutrophils horizontally sectioned by confocal laser scanning microscopy showed that the intranuclear fluorescent foci were very likely invaginations of the nuclear envelope (Fig. 2e,j,o).
Fig. 2.
Double immunofluorescence labelling of neutrophils by atypical p-ANCA and antibodies against lamin B1 examined by serial horizontal sectioning of human neutrophils using confocal laser scanning microscopy. Atypical p-ANCA were detected with FITC-conjugated goat antihuman IgG secondary antibodies on ethanol-fixed neutrophils. Antibodies against lamin B1 were visualized by Texas red-conjugated goat antirabbit IgG secondary antibodies. When the fluorescence signal of atypical p-ANCA (green) and the fluorescence labelling of antibodies against lamin B1 (red) were optically superimposed, areas of colocalization were indicated by a yellow labelling. Human neutrophils were optically sectioned from the top to the bottom of the cell into horizontal sections of 1 µm using confocal laser scanning microscopy. The left panels (a, b, c, d) show the different staining patterns produced by atypical p-ANCA with respect to the serial horizontal sections of the neutrophil; in the middle panels (f, g, h, i) the fluorescence labelling given by antibodies to lamin B1 is shown; in the right panels (k, l, m, n), the correspondig fluorescence signals produced by atypical p-ANCA and antilamin B1 are optically superimposed. The images of the panels e, j and o represent a three-dimensional reconstruction of the corresponding serial horizontal cell sections. The positions of the horizontal sections with respect to the images shown in the above panels are marked by alphabetic letters as indicated in the corresponding fluorescence images. An additional three dimensional reconstruction of serial vertical sections with particular respect to the intranuclear fluorescence is indicated by A, B and C in the panels e, j and o. The corresponding individual fluorescence images of vertically sectioned neutrophils are not shown. Panels a–e: ethanol-fixed neutrophils were incubated with the serum from a patient with PSC which contained atypical p-ANCA. An inhomogeneous rim-like staining pattern along with intranuclear fluorescent foci given by atypical p-ANCA was detected in each horizontal section of the neutrophils. The three dimensional reconstruction of these images (e) and the three-dimensional reconstruction of vertical sections of the same images clearly assigned the intranuclear labelling to an invagination of the nuclear periphery. Panels f–j: antibodies against lamin B1 gave a rim-like staining of the nuclear envelope along with intranuclear labelling which corresponded to an infolding of the nuclear envelope (j). Panels k–o: the fluorescence signals of atypical p-ANCA and antibodies to lamin B1 completely co-localized in the corresponding serial sections of the neutrophils as well as in the three-dimensional reconstructions. The size bars in the micrographs indicate 10 µm.
Atypical p-ANCA are closely associated with IBD and autoimmune liver disorders
In our study population of subjects with antineutrophil antibodies (n = 143), atypical p-ANCA (n = 99/143) were found almost exclusively in sera from patients with either inflammatory bowel diseases (CD n = 12/13[92%]; UC n = 26/29[90%]) or autoimmune liver disorders (PSC n = 30/34[88%]; AIH n = 25/26[96%]). Six of 10 sera from SLE patients also displayed atypical p-ANCA. Atypical p-ANCA were not present in sera from patients with Wegener's granulomatosis or microscopic polyangiitis. In sera containing atypical p-ANCA, the prevalence of ‘type B’ (CD 1/12[8%]; UC 5/26[19%]; PSC 7/30[23%]; AIH 2/25[8%]) was markedly lower than of ‘type A’ (CD 11/12[92%]; UC 21/26[81%]; PSC 23/30[77%]; AIH 23/25[92%], SLE 6/6[100%]). p-ANCA (n = 21) were present in patients with microscopic polyangiitis (n = 10/12[83%]), SLE (n = 3/10[30%]), WG (n = 1/19[5%] and were rarely detected in patients with IBD (n = 3) or autoimmune liver disorders (n = 4). The median serum endpoint titres of atypical p-ANCA ‘type A’ (1: 1280, n = 84) and ‘type B’ (1: 960, n = 15) were significantly higher (P < 0·05) than the median serum endpoint titres of p-ANCA (1: 160, n = 21), irrespective of the underlying disease. The presence of c-ANCA (n = 23) was closely associated with the diagnosis of Wegener's granulomatosis [n = 18/19(95%)]; however, sera from two patients with microscopic polyangiitis and one serum from a patient with SLE also appeared to contain c-ANCA. Two of the 102 sera (2%) from patients with IBD or autoimmune liver disorders contained antibodies that produced a c-ANCA fluorescence pattern. ANCA were detected in none of the sera from healthy blood donors (n = 10).
For each subject, the fluorescence patterns produced by antineutrophil antibodies were determined using serum samples obtained at least twice during a patient's disease course (mean of 6 ± 8 months apart). The observed patterns were always the same for the same patient. The differences in immunofluorescence patterns produced by p-ANCA and atypical p-ANCA ‘type A’ or ‘type B’ were not attributable to the preparation technique of the neutrophil slides as the staining patterns were the same when the neutrophils were either grown as monolayers or cytocentrifuged onto the slides (data not shown).
Influence of fixatives other than ethanol on the microscopic fluorescence patterns of atypical p-ANCA compared to p-ANCA
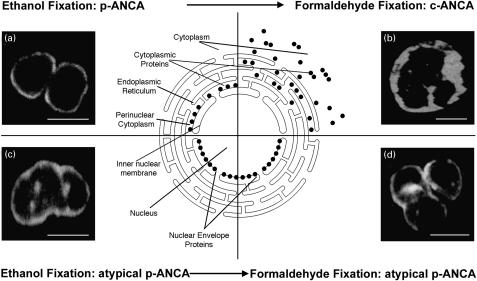
A solution of 98% ethanol is considered widely to be the standard agent to fix neutrophils for use in indirect immunofluorescence microscopy for the detection of antineutrophil antibodies [16,25]. We also assessed formaldehyde and methanol as fixative agents for the detection of antineutrophil antibodies by investigating the microscopic fluorescence features produced by the antibodies on formaldehyde- and methanol-fixed neutrophils [27] (Table 2). All sera tested that contained p-ANCA (n = 21) gave a cytoplasmic labelling of formaldehyde-fixed neutrophils, irrespective of the underlying disease (Fig. 3a,b). This is consistent with the known fact that the fluorescence pattern of p-ANCA is actually an artefact caused by a redistribution of positively charged cytoplasmic antigens to the negatively charged nucleus during ethanol fixation. In contrast, 96% (n = 81/84) of the sera containing atypical p-ANCA ‘type A’ did not label the cytoplasm of formaldehyde-fixed neutrophils as was observed for p-ANCA. Instead, a fine ‘perinuclear’ labelling and multiple intranuclear fluorescent foci were always observed when using confocal laser scanning microscopy (Fig. 3c,d). However, when lower resolution planar indirect immunofluorescence microscopy was used, this labelling was sometimes difficult to detect and required careful investigation of the entire slide, especially when the intensity of the fluorescence labelling varied within one slide. This may account for what has been termed ‘negative fluorescence produced by atypical p-ANCA on formaldehyde-fixed neutrophils’ by others [27]. In contrast to the ‘perinuclear’ labelling of neutrophils seen with atypical p-ANCA ‘type A’ when using formaldehyde-fixed neutrophils, the majority of atypical p-ANCA ‘type B’ (93%; n = 14/15) also diffusely labelled the cytoplasm of formaldehyde-fixed neutrophils (data not shown). Examination of formaldehyde-fixed neutrophils for a change in fluorescence patterns compared to ethanol-fixed neutrophils represented a reproducible (92%), specific (97%) and sensitive (98%) method to distinguish atypical p-ANCA from p-ANCA.
Table 2.
Microscopic features of different staining patterns produced by antineutrophil antibodies with respect to the neutrophil fixation
| Staining pattern | Staining pattern on ethanol-fixed neutrophils | Staining pattern on formaldehyde-fixed neutrophils |
|---|---|---|
| c-ANCA | Diffuse fine granular cytoplasmic staining,frequently accentuated between the nuclear lobes | Diffuse fine granular cytoplasmicstaining, rarely accentuated betweenthe nuclear lobes |
| p-ANCA | Fine homogeneous rim-like staining ofthe perinuclear cytoplasm | Diffuse fine granular cytoplasmic staining, rarely accentuated between the nuclear lobes |
| Atypical p-ANCA ‘type A’ | Broad inhomogeneous rim-like stainingof the nuclear periphery along with multiple fluorescent foci | Fine inhomogeneous rim-like staining of the nuclear periphery intranuclear and scattered intranuclear fluorescent foci |
| Atypical p-ANCA ‘type B’ | Broad inhomogeneous rim-like staining of thenuclear periphery, multiple intranuclearfluorescent foci and a diffuse coarse granularcytoplasmic staining, rarely accentuated betweenthe nuclear lobes | Fine inhomogeneous rim-like staining of the nuclear periphery, multiple intranuclear fluorescent foci and diffuse coarse granular cytoplasmicstaining, rarely accentuated between the nuclear lobes |
Fig. 3.
Microscopic immunofluorescence patterns of p-ANCA compared to atypical p-ANCA in relationship to the neutrophil fixation. Antineutrophil antibodies bound to their target proteins were detected by FITC-conjugated goat antihuman IgG secondary antibodies. To visualize the different fluorescence patterns, confocal laser scanning microscopy was used. The schematic diagram shows the subcellular distribution of the target antigen (•) of p-ANCA and atypical p-ANCA with respect to the used fixative agent. (a) On ethanol-fixed neutrophils, p-ANCA gave a fine perinuclear fluorescence labelling with the responsible target proteins collapsing in the perinuclear cytoplasm. (b) Using the cross-linking fixative formaldehyde, p-ANCA diffusely labelled the cytoplasm of ethanol-fixed neutrophils. A highlighting of the fluorescence between the nuclear lobes as frequently seen with c-ANCA on ethanol-fixed neutrophils was not observed. As depicted in the schematic diagram, the target proteins are diffusely distributed throughout the cytoplasm when formaldedhyde is used as fixative. (c) On ethanol-fixed neutrophils, atypical p-ANCA react with neutrophil-specific proteins in the nuclear periphery of neutrophils and produce a broad rim-like fluorescence pattern along with multiple intranuclear fluorescent foci. (d) The localization of the nuclear antigen recognized by atypical p-ANCA is not affected by the formaldehyde fixation. The staining pattern produced by atypical p-ANCA on formaldehyde-fixed neutrophils is identical to that on ethanol-fixed neutrophils. Size bars in the micrographs indicate 10 µm.
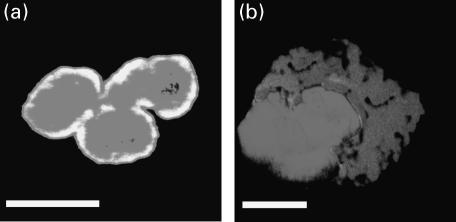
Double labelling immunofluorescence microscopy with atypical p-ANCA detected by FITC-conjugated secondary antibodies and propidium iodide, which labels DNA and marks the borders of the nucleus, demonstrated further that the ‘perinuclear’ fluorescence produced by atypical p-ANCA is different from that produced by p-ANCA. The fluorescence signals produced by atypical p-ANCA and propidium iodide overlapped at the nuclear periphery of formaldehyde-fixed neutrophils (Fig. 4a). In contrast, p-ANCA gave a diffuse cytoplasmic fluorescence labelling of formaldehyde-fixed neutrophils without co-localization of the perinuclear fluorescence to the propidium iodide fluorescence signal (Fig. 4b).
Fig. 4.
Microscopic immunofluorescence patterns of p-ANCA and atypical p-ANCA on formaldehyde-fixed neutrophils with respect to propidium iodide counterstaining. p-ANCA and atypical p-ANCA were detected using FITC-conjugated goat antihuman IgG secondary (dark grey). Propidium iodide counterstaining, was used to label the nucleus (light grey). When both images were optically superimposed, a co-localization of both fluorescence signals was indicated by a bright white staining. The fluorescence patterns were visualized by confocal laser scanning microscopy. (a) Atypical p-ANCA gave a rim-like labelling of the nuclear periphery along with scattered intranuclear fluorescent foci which both colocalized with the propidium iodide staining. (b) p-ANCA showed a diffuse labelling of the cytoplasm which did not overlap with the nuclear propidium iodide staining. Size bars indicate 10 µm.
We also examined the effects of methanol fixation of neutrophils on the fluorescence patters produced by antineutrophil antibodies. The labelling patterns produced by c-ANCA, p-ANCA and atypical p-ANCA on methanol-fixed neutrophils did not differ markedly from the fluorescence patterns produced by these antibodies on ethanol-fixed neutrophils (data not shown), irrespective of the underlying disease. However, some slides showed a more intense non-specific background staining compared to ethanol-fixed neutrophils.
Discussion
The definition of so-called ‘atypical p-ANCA’ or, as we proposed recently, the more accurate term ‘p-ANNA’ antineutrophil nuclear antibodies [19], in patients with IBD and autoimmune liver disorders has been based primarily on the heterogeneous microscopic labelling features of ethanol-fixed neutrophils examined by planar indirect immunofluorescence microscopy. We have tried to define reliable microscopic criteria by using indirect immunofluorescence microscopy and confocal laser scanning microscopy as well as various fixatives. We have shown that the presence of multiple intranuclear fluorescent foci is a reproducible, highly specific and sensitive microscopic feature to differentiate atypical p-ANCA from p-ANCA on ethanol- and methanol-fixed neutrophils. These intranuclear fluorescent foci are not observed when using sera containing p-ANCA or c-ANCA. On formaldehyde-fixed neutrophils, atypical p-ANCA give a fine, rim-like labelling of the nuclear periphery. In contrast, the perinuclear labelling of ethanol-fixed neutrophils by p-ANCA always converts to a diffuse cytoplasmic fluorescence pattern on formaldehyde-fixed cells. These immunofluorescence criteria can be used to facilitate the difficult distinction between ANCA, particularly p-ANCA, and atypical p-ANCA. As atypical p-ANCA are found at high prevalences in patients with IBD or autoimmune liver disorders, they represent a potentially valuable diagnostic seromarker for these disorders [3–9] and should be differentiated reliably from p-ANCA that are present primarily in patients with systemic vasculitides.
The scattered intranuclear fluorescent foci characteristic of atypical p-ANCA likely results from labelling of invaginations of the multi-segmented nuclear envelope of neutrophils. This was supported by our results using confocal laser scanning microscopy. Compared to conventional planar indirect immunofluorescence microscopy, out-of-focus light obscuring a point of interest is inhibited, resulting in an increased spatial resolution. Additional elimination of the light above and below the plane of focus allows for the optical sectioning of the specimen. Using this type of high-resolution microscopy, we were able to demonstrate that the intranuclear fluorescent foci are actually part of the neutrophil nucleus and most probably correspond to invaginations of the multi-lobulated nuclear envelope. Fricker et al. [28] have also described invaginations of the nucleus in various cell types using high-resolution microscopic techniques.
Accurate distinction of the staining pattern produced by atypical p-ANCA from the labelling pattern produced by p-ANCA is facilitated when different agents are used to fix the neutrophils [27,29]. This is based on the fact that the ‘perinuclear’ staining pattern of p-ANCA results from the artefactual redistribution of the recognized antigen to the perinuclear cytoplasm during ethanol-fixation, resulting in a perinuclear fluorescence. This redistribution of positively charged cytoplasmic antigens does not occur when cross-linking fixatives such as formaldehyde are used. Consistent with our previous findings that the target antigen of atypical p-ANCA is localized in the nuclear periphery of neutrophils [18,19], virtually all the sera containing atypical p-ANCA showed a rim-like labelling of the nuclear periphery on formaldehyde-fixed neutrophils. The staining produced by atypical p-ANCA overlapped with the staining given by the nuclear dye propidium iodide and with the fluorescence staining of nuclear envelope antibodies. This observation confirmed that the ‘perinuclear’ rim-like staining of atypical p-ANCA on formaldehyde-fixed neutrophils actually reflects a labelling of the nuclear envelope. In contrast, the ‘perinuclear’ staining given by p-ANCA on ethanol-fixed neutrophils regularly converted into a cytoplasmic staining on formaldehyde-fixed neutrophils. The high specificity of this phenomenon (97%) and its reproducibility (92%) renders the formaldehyde fixation of neutrophils another powerful tool to distinguish the staining given by atypical p-ANCA from the staining produced by p-ANCA.
Data published previously about the reactivity of atypical p-ANCA on formaldehyde-fixed neutrophils in IBD and autoimmune liver disorders are rare and controversial. Some investigators have reported a ‘formalin-positive’ reactivity in a high proportion of their sera tested [6,30], whereas others could not detect any fluorescence on formaldehyde-fixed neutrophils in patients with IBD or hepatobiliary disorders [31]. Consistent with our results, Billing et al. [20] demonstrated a retained perinuclear fluorescence on formaldehyde-fixed neutrophils with antineutrophil antibodies from patients with UC. Variations in the formaldehyde fixation techniques [27,29,32–34] and the resolution of the used immunofluorescence microscope might be responsible for the controversial reports about the microscopic features of atypical p-ANCA on formaldehyde-fixed neutrophils. Due to inconsistent results, recent reports even suggest to cease determining ANCA reactivity on formaldehyde-fixed neutrophils [32,34]. However, these reports refer only to p-ANCA testing of sera from patients with systemic vasculitides. Reactivity of atypical p-ANCA on formaldehyde-fixed neutrophils has not been studied systematically and a final consensus statement of atypical p-ANCA testing on formaldehyde-fixed neutrophils of the International ANCAWorkshop is pending [16]. With respect to our data, p-ANCA and atypical p-ANCA labelling of formaldehyde-fixed neutrophils was reproducible and highly specific, provided that the entire neutrophil slide was carefully examined with a high magnification objective by experienced investigators when using lower resolution immunofluorescence microscopy. Therefore, we would recommend testing for p-ANCA and atypical p-ANCA on formaldehyde-fixed neutrophils in the clinical laboratory.
Some serum samples containing atypical p-ANCA gave a combined peripheral nuclear and cytoplasmic staining. This dual labelling, which we called ‘atypical p-ANCA type B’ was observed for about 8% of serum samples containing atypical p-ANCA. Such a pattern probably results from antibody reactivity with two antigens with different subcellular localizations. The antigen recognized by atypical p-ANCA is probably localized to the nuclear envelope while another antigen(s) recognized by other antibodies in the sera is localized to the cytoplasm. This is consistent with the fact that many individuals with autoimmune disorders often have autoantibodies of more than one specificity. In previous studies, a similar staining pattern has been sometimes described as a ‘snow-drift-like’ fluorescence pattern [16,17].
In conclusion, indirect immunofluorescence microscopy remains the only currently available standard detection method to detect atypical p-ANCA. Carefully performed immunofluorescence microscopy paying attention to the various features described in this paper can be used to differentiate atypical p-ANCA from p-ANCA reliably. In particular, laser scanning confocal microscopy can be used to highlight intranuclear foci recognized by atypical p-ANCA and fixation with formaldehyde can be performed to confirm a nuclear envelope localization of the antigen. The ultimate molecular identification of the antigen recognized by atypical p-ANCA will make the development of simple diagnostic assays possible and may obviate the need for using these somewhat cumbersome techniques.
Acknowledgments
This study was supported by grants from the German Academic Exchange Association (to B. T.) and the Crohn's and Colitis Foundation of America (to H. J. W.). The confocal microscopy facility used for this work was established by NIH grant 1S10-RR10506 and supported by NIH grant 5 P30-CA13696 as part of the Herbert Irving Cancer Center at Columbia University. The authors are very grateful to Ian McFarlane MD, King's College London, London, UK, Christian Scheurlen MD, University of Bonn, Bonn, Germany and Ulrich Beuers MD, University of Munich, Munich, Germany, for providing serum samples.
References
- 1.Woude FJ, Lobatto S, Permin H, et al. Autoantibodies against neutrophils and monocytes: tools for diagnosis and marker of disease activity in Wegener's granulomatosis. Lancet. 1985;1:425–9. doi: 10.1016/s0140-6736(85)91147-x. [DOI] [PubMed] [Google Scholar]
- 2.Falk RJ, Jennette JC. Anti-neutrophil cytoplasmic autoantibodies with specificity for myeloperoxidase in patients with systemic vasculitis and idiopathic necrotizing and crescentic glomerulonephritis. N Engl J Med. 1988;318:1651–7. doi: 10.1056/NEJM198806233182504. [DOI] [PubMed] [Google Scholar]
- 3.Duerr RH, Targan SR, Landers CJ, et al. Neutrophil cytoplasmic antibodies: a link between primary sclerosing cholangitis and ulcerative colitis. Gastroenterology. 1991;100:1385–91. [PubMed] [Google Scholar]
- 4.Oudkerk-Pool M, Ellerbroek PM, Ridwan BU, et al. Serum antineutrophil cytoplasmic autoantibodies in inflammatory bowel disease are mainly associated with ulcerative colitis. A correlation study between perinuclear antineutrophil cytoplasmic autoantibodies and clinical parameters, medical and surgical treatment. Gut. 1993;34:46–60. doi: 10.1136/gut.34.1.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mulder AHL, Horst G, Haagsma EB, Kleibeuker JH, Kallenberg CGM. Antineutrophil cytoplasmic antibodies in autoimmune liver diseases. Hepatology. 1993;17:411–7. [PubMed] [Google Scholar]
- 6.Hardarson S, LaBrecque DR, Mitros FA, Neil GA, Goeken JA. Antineutrophil cytoplasmic antibody in inflammatory bowel and hepatobiliary diseases. Am J Clin Pathol. 1993;99:277–81. doi: 10.1093/ajcp/99.3.277. [DOI] [PubMed] [Google Scholar]
- 7.Targan S, Landers C, Vidrich A, Czaja AL. High-titer antineutrophil cytoplasmic antibodies in type 1 autoimmune hepatitis. Gastroenterology. 1995;108:1159–66. doi: 10.1016/0016-5085(95)90215-5. [DOI] [PubMed] [Google Scholar]
- 8.Bansi DS, Fleming KA, Chapman RW. Antineutrophil cytoplasmic antibodies in autoimmune hepatitis. Gastroenterology. 1995;109:2049–55. doi: 10.1016/0016-5085(95)90785-8. [DOI] [PubMed] [Google Scholar]
- 9.Zauli D, Ghetti S, Grassi A, et al. Anti-neutrophil cytoplasmic antibodies in type 1 and 2 autoimmune hepatitis. Hepatology. 1997;25:1105–7. doi: 10.1002/hep.510250510. [DOI] [PubMed] [Google Scholar]
- 10.Walmsley RS, Zhao MH, Hamilton MI, et al. Antineutrophil cytoplasm autoantibodies against bactericidal/permeability-increasing protein in inflammatory bowel disease. Gut. 1997;40:105–9. doi: 10.1136/gut.40.1.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Roozendaal C, Zhao MH, Horst G, et al. Catalase and α-enolase: two novel granulocyte autoantigens in inflammatory bowel disease (IBD) Clin Exp Immunol. 1998;112:10–6. doi: 10.1046/j.1365-2249.1998.00528.x. 10.1046/j.1365-2249.1998.00528.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Orth T, Kellner R, Diekmann O, Faust J, Meyer zum Büschenfelde KH, Mayet WJ. Identification and characterization of autoantibodies against catalase and α-enolase in patients with primary sclerosing cholangitis. Clin Exp Immunol. 1998;112:507–15. doi: 10.1046/j.1365-2249.1998.00583.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Halbwachs-Mecarelli L, Nusbaum P, Noel LH, et al. Antineutrophil cytoplasmic antibodies (ANCA) directed against cathepsin G in ulcerative colitis, Crohn's disease and primary sclerosing cholangitis. Clin Exp Immunol. 1992;90:79–94. doi: 10.1111/j.1365-2249.1992.tb05835.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Peen E, Almer S, Bodemar G, et al. Antilactoferrin antibodies and other types of ANCA in ulcerative colitis, primary sclerosing cholangitis and Crohn's disease. Gut. 1993;34:56–62. doi: 10.1136/gut.34.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Saxon A, Shanahan F, Landers C, Ganz T, Targan S. A distinct subset of antineutrophil cytoplasmic antibodies is associated with inflammatory bowel disease. J Allergy Clin Immunol. 1990;86:202–10. doi: 10.1016/s0091-6749(05)80067-3. [DOI] [PubMed] [Google Scholar]
- 16.Savige J, Gillis D, Benson E, et al. International consensus statement on testing and reporting of antineutrophil cytoplasmic antibodies (ANCA) Am J Clin Pathol. 1999;111:507–13. doi: 10.1093/ajcp/111.4.507. [DOI] [PubMed] [Google Scholar]
- 17.Rump JA, Schölmerich J, Gross V, et al. A new type of perinuclear anti-neutrophil cytoplasmic antibody (p-ANCA) in active ulcerative colitis but not in Crohn's disease. Immunobiology. 1990;181:406–13. doi: 10.1016/S0171-2985(11)80509-7. [DOI] [PubMed] [Google Scholar]
- 18.Terjung B, Herzog V, Worman HJ, et al. Atypical antineutrophil cytoplasmic antibodies with perinuclear fluorescence in chronic inflammatory bowel diseases and hepatobiliary disorders colocalize with nuclear lamina proteins. Hepatology. 1998;28:332–40. doi: 10.1002/hep.510280207. [DOI] [PubMed] [Google Scholar]
- 19.Terjung B, Spengler U, Sauerbruch T, Worman HJ. ‘Atypical p-ANCA’ in IBD and hepatobiliary disorders react with a 50 kD nuclear envelope protein of neutrophils and myeloid cell lines. Gastroenterology. 2000;119:310–22. doi: 10.1053/gast.2000.9366. [DOI] [PubMed] [Google Scholar]
- 20.Billing P, Tahir S, Calfin B, et al. Nuclear localization of the antigen detected by ulcerative colitis-associated perinuclear antineutrophil cytoplasmic antibodies. Am J Pathol. 1995;147:979–87. [PMC free article] [PubMed] [Google Scholar]
- 21.Sobajima J, Ozaki S, Uesugi H, et al. High mobility group (HMG) non-histone chromosomal proteins HMG1 and HMG2 are significant target antigens of perinuclear antineutrophil cytoplasmic antibodies in autoimmune hepatitis. Gut. 1999;44:867–73. doi: 10.1136/gut.44.6.867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mallolas J, Esteve M, Rius E, Cabré E, Gassull MA. Antineutrophil antibodies associated with ulcerative colitis interact with the antigen(s) during the process of apoptosis. Gut. 2000;47:74–8. doi: 10.1136/gut.47.1.74. 10.1136/gut.47.1.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Eggena M, Cohavy O, Parseghian MP, et al. Identification of histone H1 as a cognate antigen of the ulcerative colitis-associated marker antibody p-ANCA. J Autoimmun. 2000;14:83–97. doi: 10.1006/jaut.1999.0340. [DOI] [PubMed] [Google Scholar]
- 24.Martin L, Herr JC, Wanamaker W, Kornguth S. Demonstration of specific antineuronal nuclear antibodies in sera of patients with myasthenia gravis. Indirect and direct immunofluorescence. Neurology. 1974;24:680–3. doi: 10.1212/wnl.24.7.680. [DOI] [PubMed] [Google Scholar]
- 25.Wiik A. Delineation of a standard procedure for indirect immunofluorescence detection of ANCA. APMIS. 1989;97(Suppl. 6):12–3. [PubMed] [Google Scholar]
- 26.Cance WG, Chaudhary N, Worman HJ, Blobel G, Cordon-Cando C. Expression of the nuclear lamins in normal and neoplastic human tissues. J Exp Clin Cancer Res. 1992;11:233–46. [Google Scholar]
- 27.Spickett GP, Broomhead V. Formalin fixation and patterns of antineutrophil cytoplasmic antibodies. J Clin Pathol. 1995;48:89–90. doi: 10.1136/jcp.48.1.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fricker M, Hollinshead M, White N, Vaux D. Interphase nuclei of many mammalian cell types contain deep, dynamic, tubular membrane-bound invaginations of the nuclear envelope. J Cell Biol. 1997;136:531–44. doi: 10.1083/jcb.136.3.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lock RJ. Detection of autoantibodies to neutrophil cytoplasmic antigens. J Clin Pathol. 1994;47:4–8. doi: 10.1136/jcp.47.1.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mulder AHL, Broekroelofs J, Horst G, Limburg PC, Nelis GF, Kallenberg CGM. Anti-neutrophil cytoplasmic antibodies (ANCA) in inflammatory bowel diseases: characterization and clinical correlates. Clin Exp Immunol. 1994;95:490–7. doi: 10.1111/j.1365-2249.1994.tb07024.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cambridge G, Rampton DS, Stevens TRJ, McCarthy DA, Kamm M, Leaker B. Antineutrophil antibodies in inflammatory bowel disease: prevalence and diagnostic role. Gut. 1992;33:668–74. doi: 10.1136/gut.33.5.668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chowdhury SM, Broomhead V, Spickett GP, Wilkinson R. Pitfalls of formalin fixation for determination of antineutrophil cytoplasmic antibodies. J Clin Pathol. 1999;52:475–7. doi: 10.1136/jcp.52.6.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lee SS, Lawton JWM, Chak W. Distinction between antinuclear antibody and p-ANCA. J Clin Pathol. 1991;44:962–3. doi: 10.1136/jcp.44.11.962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bird AG. Is there life in the formalin fixed neutrophil for ANCA testing? No! J Clin Pathol. 1999;52:403–4. doi: 10.1136/jcp.52.6.403. [DOI] [PMC free article] [PubMed] [Google Scholar]