Abstract
Abnormal CD4/CD8 ratios and T-cell function have previously been shown in patients with B-chronic lymphocytic leukaemia (B-CLL). We have demonstrated that CD4+ T cells containing both serine esterase and perforin (PF) are increased in the blood of these patients. Using flow cytometry, we have shown that the CD4+ PF+ cells were CD57+ but lacked expression of CD28, suggesting a mature population. The same phenotype in CD8+ T cells is characteristic of mature cytotoxic T cells. However, in contrast to the CD8+ T cells, the CD4+ T cells were more frequently CD45RO positive than CD45RA positive, indicating prior antigen experience. In contrast, this population lacked expression of either CD69 or HLA-DR, arguing that they were not activated or that they are an abnormal population of T cells. Their constitutive cytokine levels showed them mainly to contain IL4 and not IFNγ, suggesting a Th2 phenotype. The role of the CD4+ PF+ T-cell population is at present uncertain. However, this potentially cytotoxic T-cell population could contribute both to enhancing survival of the B-CLL tumour cells through production of IL4, and to the immunodeficient state frequently seen in patients with this tumour, independent of drug treatment.
Keywords: B-CLL, perforin, serine esterase, cytotoxic CD4 cells, CD57
Introduction
B-cell chronic lymphocytic leukaemia (B-CLL) is the most common adult leukaemia in the Western world and is characterized by the accumulation of circulating sIgM+ CD5+ CD19+ CD40+ cells. In addition, higher absolute numbers of circulating T cells are frequently found [1–3], mainly due to an increase in CD8+ T cells which results in a low CD4/CD8 ratio [3].
To date, a number of studies have identified markers on CD4+ and CD8+ cells in B-CLL. There are more CD45RO expressing CD4+ and CD8+ T cells in patients with B-CLL than in normal individuals. Responses to mitogenic stimulation, helper T-cell function, mixed lymphocyte response and T-cell colony formation have also been shown to be reduced [4]. Such decreased reactivity could, in part, be due to the aberrant expression of co-stimulatory molecules. As in normal controls, CD28 expression in B-CLL has been found to be weaker on CD8+ than on CD4+ T cells [5]. Another co-stimulatory molecule CD40 ligand (CD154) has also been shown to be weakly expressed on T cells in B-CLL, which could lead to aberrant T–B cell interactions [6].
T-cell subsets in B-CLL patients often demonstrate clonal expansion [7], with oligoclonal CD4+ and CD8+ populations predominantly expressing CD57+ [8]. Expansion of CD57+ cells was prominent amongst circulating CD4+ T cells, and a high frequency of CD4+ CD57+ T cells has been associated with more advanced disease [8]. With regard to the production of cytokines, the expression of CD57/CD28 appears to determine different profiles of cytokine release in the supernatant fluids by resting and mitogen-activated CD4+ and CD8+ T cells in B-CLL patients: the T-cell population enriched for CD57+ CD28− cells showed a markedly higher Th1 cytokine secretion (IL-2, IFN-γ, TNF-α) after the stimulation with anti-CD3 monoclonal antibodies (MoAbs) compared with CD57− CD28+ cells [9].
Perforin (PF) is an important mediator of cytotoxicity by CD8+ T cells. Granules containing the perforin also contain granzymes, which are serine esterases (SE), and these enter the cell to induce apoptosis [10].
In this paper, we show that there is an increase in CD4+ T cells expressing perforin and serine esterase in the blood of B-CLL patients compared with age-matched controls, and that they have the phenotypic characteristics of cytotoxic CD8+ T cells, and constitutively express predominantly the Th2 cytokine IL4.
Patients and methods
Patients
Eighteen untreated B-CLL patients (four women, 14 men), aged 52–84 years (mean age 66·2) at different stages of the disease Rai 0,1,2, 3 and 4, attending the UCL Hospitals Trust clinics, were studied with consent and Ethical Committee approval. The control group consisted of 15 healthy age- and sex-matched volunteers. Patients were considered to be untreated if they had received no treatment within 3 months prior to the study. They also had no other associated significant illnesses. None of the patients had been treated with the purine analogues fludarabine and cladribine (known to have long-lasting immunomodulatory effects) within 5 months prior to the study. Table 1 shows stages of the disease and white blood cell (WBC) counts of the patients studied.
Table 1.
Stages of B-CLL and white blood cell (WBC) counts of the patients studied
| WBC × 109/L |
|||
|---|---|---|---|
| Stage | Number of patients | Range | Mean ± s.d. |
| 0 | 3 | 8–22 | 15·3 ± 7·1 |
| 1 | 4 | 17–65 | 38·3 ± 20·6 |
| 2 | 4 | 5–29 | 16·4 ± 12·3 |
| 3 | 3 | 3–54 | 30·1 ± 25·4 |
| 4 | 4 | 3–69 | 26·5 ± 27·4 |
Cell preparation
Peripheral blood from patients and healthy controls was collected into preservative-free heparin, diluted 1:1 with Hanks's balanced salt solution (Sigma) and separated on Ficoll-Hypaque gradients (density 1·077; Sigma). Peripheral blood mononuclear cells (PBMC) were then washed twice in Hanks's balanced salt solution and resuspended at 5 × 106 cells/ml in medium RPMI-1640 (Gibco) supplemented with 10% heat-inactivated fetal bovine serum (FBS, Gibco) and 1% of penicillin/streptomycin solution (Sigma).
Staining of CD4+ and CD8+ cells for serine esterase
Serine esterase (SE) was detected as previously described by Smith et al. [11]. Briefly, cytocentrifuge preparations were air dried and fixed in 1% paraformaldehyde for 30 s. The slides were then transferred into the substrate and chromogen solution, 2 × 10−4 m Na-benzyloxy carbonyl-l-lysine thiobenxyl ester (Sigma) and 0·16 mg/ml Fast Blue BB salt (Sigma) in 0·2 m Tris-HCl, pH 8·1 (Sigma). After 15 min of incubation at 37°C, the slides were washed in PBS (pH 7·2). The slides were then incubated for 30 min with anti-CD4 or anti-CD8 MoAbs, or isotype control antibodies, washed, and stained with rabbit anti-mouse polyclonal antibodies conjugated to alkaline phosphatase. The alkaline phosphatase anti-alkaline phosphatase (APAAP) complex was finally added with Fast red/Naphthol ASBI as chromogen/substrate solution (Sigma). The slides were then counterstained with Harris' Haematoxylin, rinsed in distilled water, dried, and mounted in Apathy's mountant. At least 200 cells were counted by light microscopy, and the percentages of CD4+ and CD8+ cells expressing were SE calculated. Cytoplasmic expression of SE was identified by blue-coloured granules, while surface expression of CD4 and CD8 was detected by red membrane staining.
Analysis of intracellular expression of perforin by immunofluorescence
The method used was a modification of that used by Rutella et al. [12]. Briefly, 106 PBMC in 200 µl RPMI-FBS medium were placed into each well of a 96-well round-bottomed microplate (Nunc), washed by centrifuging at 1500 rev min−1 for 5 min and resuspended in PBS supplemented with 2% bovine serum albumin (BSA). The cells were resuspended in 20 µl anti-CD4 or anti-CD8 MoAbs conjugated with CyC, or a mixture of 20 µl CyC-conjugated anti-CD16 MoAb and 20 µl PE-conjugated anti-CD56 MoAb. Monoclonal antibodies used in this study are listed in Table 2. Following incubation on ice for 45 min, the cells were washed twice in PBS-BSA and resuspended in 100 µl fixative (FixPerm kit, Caltag) at room temperature (RT) for 15 min. After further washing, the cell pellet was resuspended in 100 µl permeabilization solution (FixPerm kit, Caltag) for 15 min at RT and then incubated with FITC-conjugated anti-PF MoAb (Ancell) for 45 min on ice. The cells were then washed twice in PBS-BSA, fixed in 2% paraformaldehyde (PFA) in PBS and analysed by flow cytometry. Antibody isotype controls were used both for cell surface and intracellular staining.
Table 2.
Mouse monoclonal antibodies (MoAbs) used in the study
| Specificity of MoAbs | Clone | Isotype | Conjugate | Manufacturer |
|---|---|---|---|---|
| anti-CD4 | RPA-T4 | IgG1 | CyC | Pharmingen |
| anti-CD8 | RPA-T8 | IgG1 | CyC | Pharmingen |
| anti-CD16 | 3G8 | IgG1 | CyC | Pharmingen |
| anti-CD28 | CD28·2 | IgG1 | PE | Immunotech |
| anti-CD45RO | UCHL1 | IgG2a | PE | Immunotech |
| anti-CD45RA | ALB11 | IgG1 | PE | Immunotech |
| anti-CD56 | N901 | IgG1 | PE | Immunotech |
| anti-CD57 | NC1 | IgM | PE | Immunotech |
| anti-CD69 | FN50 | IgG1 | PE | Pharmingen |
| anti-CD154 | TRAP1 | IgG1 | PE | Pharmingen |
| anti-DR | Tü36 | IgG2b | PE | Pharmingen |
| anti-PF | delta G9 | IgG2b | FITC | Ancell |
| anti-IL4 | 30340·11–3 | IgG1 | PE | R&D |
| anti-IFNγ | 25723·11 | IgG2b | PE | R&D |
Phenotypic analysis of PF expressing CD4+ and CD8+ cells
PBMC were stained with CyC-conjugated anti-CD4 or anti-CD8 MoAbs and co-incubated with either of the following PE-conjugated MoAbs: anti-CD28, anti-CD45R0, anti-CD45RA, anti-CD57, anti-CD69, anti-CD154 (CD40L) or anti-HLA-DR. The staining for PF was then carried out as described above.
Analysis of the constitutive expression of cytokines
PBMC from 10 B-CLL patients and seven healthy controls were stained for CD4 and CD8 surface markers, fixed, permeabilized, and stained for intracellular PF as described above. For cytokine staining, the cells were further incubated with PE-conjugated anti IL4 or IFNγ MoAbs for 45 min on ice, washed twice and fixed with PFA.
FACScan analysis
Cells were analysed using a FACScan (Becton/Dickinson, Oxford, UK) by a lymphocyte gate based on FSC-SSC parameters and WindMDI software. At least 50 000 lymphocytes were routinely analysed for each sample. The data are expressed as the percentages of positive cells and in some cases, the mean fluorescence intensity (MFI).
Statistical analysis
The data were analysed using the non-parametric Mann–Whitney test and P-values were considered significant at 0·05 or below. Correlation between the groups was analysed using a correlation coefficient CORREL, 0x,y (Excell statistical analysis package).
Results
CD4+, CD8+ and NK cell populations in B‐CLL patients
The percentages of CD4+ cells in B-CLL patients were almost eightfold lower than in normal controls (P < 0·001), giving rise to the low (below 1 in the majority of the cases) CD4/CD8 ratio (Table 3). Due to the high number of circulating B-CLL cells, the percentages of CD8 and NK cells were also lower than control values.
Table 3.
Percentages of CD4+, CD8+ and NK cells in the peripheral blood of B-CLL patients and age-matched controls
| Parameters | CD4+, % | CD8+, % | CD4/CD8 | CD16/CD56, % |
|---|---|---|---|---|
| B-CLL | 6·2 ± 5·0 | 15·8 ± 16·7 | 0·79 ± 0·51 | 2·6 ± 1·9 |
| patients | (0·5–19·8) | (0·8 –55·0) | (0·20 –1·91) | (0·4–5·1) |
| Controls | 45·9 ± 9·8 | 27·0 ± 7·6 | 1·79 ± 0·68 | 7·9 ± 6·2 |
| (27·5–62·3) | (16·5–45·0) | (0·64 –3·48) | (2·9–19·8) | |
| P | < 0·001 | < 0·05 | < 0·001 | < 0·05 |
The data represent mean ± standard deviation. PBMC were stained with anti-CD4 or anti-CD8 MoAb, and a mixture of anti-CD16 and anti-CD56 MoAbs as described in the Methods.
Increased percentages of CD4+ and CD8+ cells express SE in B‐CLL patients
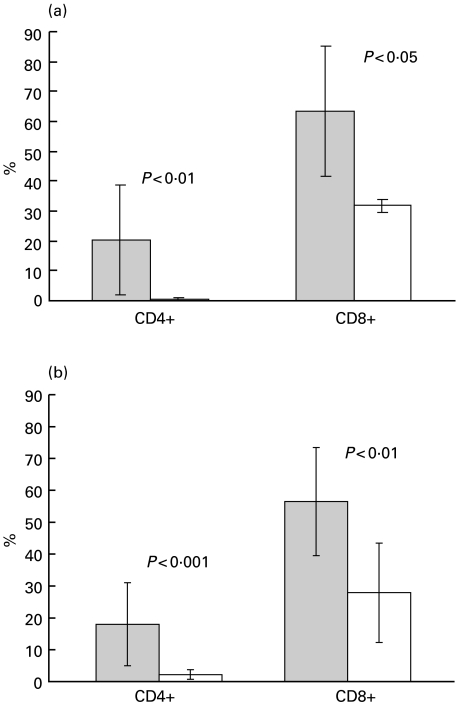
There was a significant increase in CD4+ cells expressing SE in B-CLL patients (P < 0·01; Fig. 1a). Although the number of CD4+ SE+ cells was neglible in healthy subjects, up to 50% of CD4+ SE+ cells (range 1·5–49·8%) were present in the patients. A significant increase in CD8+ SE+ cells (range 41·4–86·6%, P < 0·05) was also noted.
Fig. 1.
(a) CD4+ and CD8+ cells expressing serine esterase (SE) in B–CLL patients and controls. Cytocentrifuged PBMC from patients and controls were stained for SE and CD4+ or CD8+ as described in the Methods. At least 200 CD4+ and CD8+ cells were counted by light microscopy. The values represent mean ± standard deviation. Grey bars = patients: open bars = controls. (b) Perforin-containing CD4+ and CD8+ cells in B‐CLL patients and controls. PBMC stained with anti-CD4 or anti-CD8 MoAbs, fixed, permeabilized and stained with anti-PF MoAbs. Percentages of PF+ cells determined by flow cytometry as described in the Methods. All experiments were carried out in duplicate. The values represent mean ± standard deviation. Grey bars = patients; open bars = controls
Increased percentages of CD4+ and CD8+ cells contain perforin in B‐CLL patients
The B-CLL patients had higher percentages of circulating CD4+ PF+ cells (mean 18·0 ± 13·0%, range 1·4–52·9%) than those seen in 15 healthy controls (mean 2·1 ± 1·5%, range 0·2–4·9%, Fig. 1b: P < 0·001). Sixteen of the 18 patients had 20·9 ± 12·2% of CD4+ PF+ cells. The mean and range of the values were similar to those obtained for SE. All the PF+ CD4+ cells were CD3+ and appeared to be larger and more granular than most of the lymphocytes (data not shown). The two patients, who had levels of CD4+ PF+ cells in the normal range, were stage 0 and stage 1. In general, more PF+ CD4+ cells were detected at later stages of the disease (Rai 2–4: 20·9 ± 12·8%), compared with early stages (Rai 0/1: 13·3 ± 12·8%), although the difference between the stages was not statistically significant. In addition, absolute values of CD4+ PF+ cells, although variable (0·199 ± 0·195 × 109/L), were higher in patients than in the control range used by the CLL clinic at UCH (0·003–0·045 × 109/L).
A significant increase in PF-producing CD8+ cells (mean 56·5 ± 17·0%, range 26·7–82·3%, Fig. 1b) was also seen in the patients compared with healthy donors (mean 27·9 ± 15·6%, range 2·4–61·9%: P < 0·01). The levels of PF expression by CD16+ CD56+ (NK) cells were also higher at 94·6 ± 2·5% (range 91·5–98·2%) compared with 75·5 ± 14·7% (range 62·0–95·7%) in healthy controls (P < 0·05). Although there was a tendency for increased expression of PF by both CD4+ and CD8+ cells in B-CLL patients, the correlation coefficient between them was negligible (0·199). This suggests that expression of PF by CD4+ and CD8+ cells represented independent events. The correlation was much higher for the healthy controls (0·664). No appreciable correlation was found between the PF expression by CD4+ and CD16+/CD56+ cells. The percentages of CD8+ PF+ cells did not correlate with the stage of the disease (57·4 ± 13·4% and 55·0 ± 22·6%, respectively).
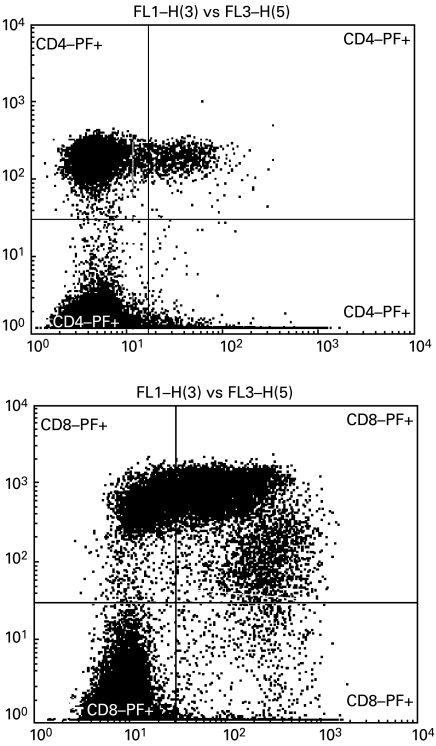
Typical FACScan profiles of the perforin-expressing populations of CD4+ and CD8 + cells are shown in Fig. 2. Note that consistent with the other studies [12], a single CD4+ PF+ population was detected, whilst PF-expressing CD8+ cells either have bright or dim surface expression of CD8. CD8+dim cells contained more perforin, which is reflected by the higher MFI, compared with both CD8+bright and CD4+ populations.
Fig. 2.
Typical profile of perforin-containing CD4 and CD8 cells from B–CLL patients. The cells were stained with anti-CD4 or anti-CD8 MoAbs (y axis) and anti-PF MoAb (x axis) as described in the Methods. Cells were gated on the lymphocyte population and quadrant gates were placed based on the isotype controls. Note the two populations of PF-expressing CD8+ cells with bright and dim surface expression of CD8. MFI PF+ CD4+ 41·8; MFI CD8+bright PF+ 78·5; MFI CD8+dim PF+ 288·4.
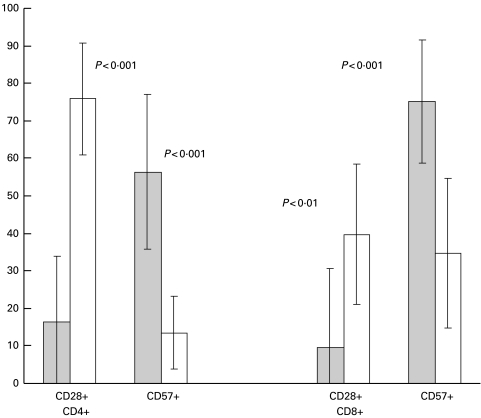
CD4+ PF+ cells mostly expressed CD57 but were negative for CD28
Both CD4+ and CD8+ perforin-containing cells were more frequently CD57+ (P < 0·001) and CD28− (P < 0·001) than the perforin-negative populations (Fig. 3). However, CD4+ PF− cells expressed more CD28 molecules/cell than the CD8+ PF− population (P < 0·01), as shown by the higher MFI (Table 4).
Fig. 3.
Expression of CD28 and CD57 by CD4+ and CD8+ cells stained for perforin. Cells were stained for surface markers, fixed, permeabilized and stained for intracellular PF as described in the Methods. The values represent mean ± standard deviation. PF+ cells, grey bars; PF− cells, open bars.
Table 4.
Mean fluorescence intensity (MFI) of the phenotypic markers studied on perforin-negative and perforin-positive CD4+ and CD8+ cells†
| CD4+ |
CD8+ |
|||
|---|---|---|---|---|
| Cell markers MFI | PF+ | PF− | PF+ | PF− |
| CD28 | 78 ± 48 | 110 ± 29* | 51 ± 30 | 66 ± 23* |
| CD57 | 110 ± 57 | 78 ± 32 | 158 ± 66‡ | 93 ± 43‡ |
| CD69 | 40 ± 17 | 47 ± 21 | 52 ± 55 | 53 ± 41 |
| HLA-DR | 65 ± 42 | 70 ± 34 | 62 ± 42 | 62 ± 57 |
| CD45RO | 117 ± 69 | 100 ± 31 | 78 ± 56 | 99 ± 61 |
| CD45RA | 53 ± 9§ | 68 ± 16¶ | 138 ± 102§ | 134 ± 75¶ |
| CD154 | 24 ± 12 | 22 ± 13 | 19 ± 16 | 26 ± 16 |
Cells were stained as described in the Methods.
Identical symbols are used for the comparison of each pair of the data.
Identical symbols are used for the comparison of each pair of the data.
P < 0·01;
P < 0·01;
P < 0·05.
Expression of activation markers by CD4+ PF+ and CD8+ PF+ cells
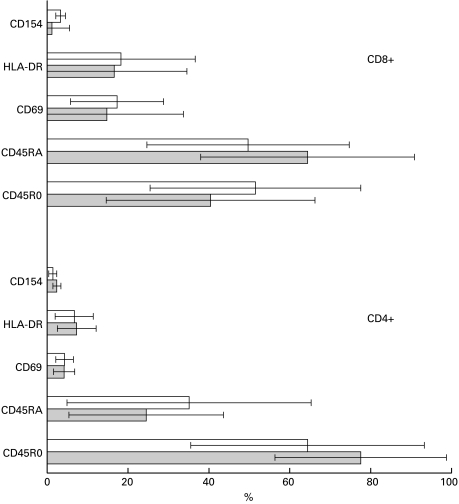
Expression of CD45RO indicates that the cell has previously experienced antigen. CD4+ cells were generally enriched for CD45RO expression in both the CD4+ PF+ (P < 0·001) and CD4+ PF− patient populations (P < 0·05: Fig. 4). Unlike the CD4+ cells, equal numbers of PF− CD8+ cells expressed CD45RO and CD45RA, but more PF+ CD8+ cells expressed CD45RA (P < 0·05). The MFI for CD45RA (Table 4) expressed by either PF+ or PF− CD8+ cells was significantly higher than that of the CD4+ cells (P < 0·05). Expression of both CD69 and HLA-DR on the overall CD4+ cell population was very low (Fig. 4), irrespective of perforin positivity, and was significantly higher on CD8+ cells (P < 0·05). Despite this, the MFI of CD4+ DR+ cells did not differ from the MFI of their CD8+ counterparts (Table 4). The expression of CD40L (CD154) on CD4+ and CD8+ cells of B-CLL patients was also very low.
Fig. 4.
Expression of activation and memory-related cell-surface markers by CD4+ and CD8+ cell populations stained for perforin. Patients' PBMC were stained for surface markers, fixed and permeabilized, stained with anti-PF MoAb as described in the Methods. The values represent mean ± standard deviation. PF+ cells, grey bars; PF− cells, open bars.
More CD4+ PF+ cells constitutively produce IL4 than IFNγ in patients
Similar percentages of CD4+ PF+ expressed IL4 in both patients and controls (Table 5). However, very few of these cells produced IFNγ in B-CLL patients (P < 0·05 compared with controls). Controls showed both IL4 and IFNγ production by CD4+ PF+ T cells, but few CD4+ PF− cells expressed either of these cytokines. Like the CD4+ cells, the CD8+ T cells (either PF+ or PF−) in B-CLL patients produced both IL4 and IFNγ. Neither PF+ nor PF− CD8+ cells in healthy controls contained detectable IL4 or IFNγ.
Table 5.
Constitutive expression of cytokines by PF+ and PF− CD4+ and CD8+ cells†
| Cells expressing cytokines, % | ||||
|---|---|---|---|---|
| IL4 | IFNγ | |||
| Cell populations | B-CLL | Controls | B-CLL | Controls |
| CD4+ PF+ | 20·8 ± 28·0 | 21·4 ± 20·2 | 0·7 ± 1·0* | 14·1 ± 6·6* |
| CD4+ PF− | 11·8 ± 20·6 | 0·9 ± 0·3 | 2·2 ± 4·0 | 0·5 ± 0·3 |
| CD8+ PF+ | 9·0 ± 21·3 | 0·7 ± 0·5 | 9·5 ± 10·2 | 1·7 ± 1·2 |
| CD8+ PF− | 12·6 ± 10·3‡ | 0·5 ± 0·6‡ | 11·0 ± 12·4 | 1·1 ± 0·9 |
PBMC were stained for the surface expression of CD4 or CD8, fixed, permeabilized and stained for intracellular expression of PF and IL4 or IFN γ as described in the Methods. The values represent mean ± standard deviation.
Identical symbols are used for the comparison of each pair of the data.
Identical symbols are used for the comparison of each pair of the data.
P < 0·05.
Discussion
In this study we show an increase in the percentages of CD4+ T cells expressing SE and PF in the blood of patients with B-CLL (Fig. 1a,b). The presence of perforin in CD4+ cells was confirmed using confocal microscopy (data not shown). Although increased, CD4+ PF + cell percentages did not correlate with the elevated numbers of either CD8+ PF+ cells or NK cells in individual patients, suggesting that these different populations are under independent control. The percentages of both PF+ CD8+ cells and PF+ CD16/56+ (NK cells) cells were also marginally increased in patients.
Expression of CD57 and CD28 by CD4+ PF+ T cells
CD57 was originally shown to be expressed mainly by NK cells [13] and is now thought to be associated with activation of T cells [14]. In this study we have shown that significantly more CD4+ PF+ than PF− cells in B-CLL patients express CD57 (Fig. 3). This was also the case for CD8+ cells. Our data are consistent with the findings of others in several haematological malignancies studied [9]. CD57 (HNK-1) is thought to be an important adhesion molecule involved in binding of CD4 and CD8+ T cells to both intercellular matrix (laminin) and other cells [15,16]. Previous studies have shown that cytotoxicity by CD8+ T cells in haemato-oncological patients [9], and during the response to human cytomegalovirus (HCMV) [17], correlates with expression of CD57 and lack of CD28. Indeed, studies of the properties of CD28− CD8+ cells have suggested that this phenotype may represent the terminal stages of their differentiation [18]. Thus, the similar phenotype of the CD4+ PF+ cells would argue that they are also mostly mature effector cells.
Expression of CD45RO, CD69, HLA–DR and CD154 by CD4+ PF+ T cells in B‐CLL
Low levels of CD69, HLA-DR and CD154 were expressed by CD4+ PF+ T cells, indicating a low level of activation of these cells. Our finding that significantly more CD4+ PF+ T cells expressed CD45RO than CD45RA (P < 0·001) suggests that the majority of them are ‘antigen experienced’. In contrast, more CD8+ PF+ than CD8+ PF− cells expressed CD45RA (P < 0·05: Fig. 4) and had a higher MFI (Table 4). The significance of this difference between CD4 and CD8 PF+-expressing cells is presently unclear.
Cytokine profile of CD4+ T cells in B‐CLL patients
As an approach to understanding the functional activity of the CD4+ PF+ cells, we examined the constitutive expression of the cytokines IFNγ and IL4 which, on their own, represent the activity of Th1 and Th2 cell populations, respectively [19]. In controls, the constitutive production of both IL4 and IFNγ was less than that seen in the B-CLL patients' T cells and was mostly limited to the CD4+ PF+ rather than PF− population (Table 5). This was not seen in the control CD8 population. However, within the CD4+ T cell population from B-CLL patients, the CD4+ PF+ constitutively contained IL4 but not IFNγ, whereas the PF− cells contained both cytokines. This suggests that the CD4+ PF+ cells were mainly Th2-like in the patients, but not in the controls. To our knowledge, there is no other information to date on the constitutive production of IL4 and IFNγ by perforin-expressing CD4+ T cells in B-CLL. Since we did not double-stain for both cytokines, we do not know how many were Th1, Th2 or Th0. Work by other investigators has shown that the presence of CD57 and absence of CD28 in B-CLL does appear to influence cytokine production [9], at least following stimulation with anti CD3. They showed that CD57+ CD28− T cells displayed a markedly higher Th1 cytokine secretion (IL-2, IFN-γ, TNF-α) compared with CD57− CD28+ cells. However, these authors measured cytokines in the supernatant fluids of CD57+-enriched cultures (CD4+ and CD8+) after their stimulation and not directly in single CD57+ cells. The predominant production of IL4 by the CD4+ PF+ T cells shown in our study is similar to that seen in both NK cells and CD3+ CD8+ CD28− large granular lymphocytes (LGL), which are considerably expanded in CLL, produce IL-4 at the clonal level and belong to the Th2 population [20]. In fact, other investigators have shown that CD4+ CD57+ effector T cells activated by anti-CD3 and anti-CD28 produced Th2 (IL4) but not Th1 cytokines [21]. In this study we have focused on the constitutive ex vivo cytokine composition of the cells. We cannot exclude the possibility that the cytokine production pattern could be different after stimulation.
Origin and possible function(s) of CD4+ PF+ cells in B‐CLL patients
Populations of CD4+ CD57+ T cells in B‐CLL patients have been identified as oligoclonal [8], but expression of PF was not evaluated in that study. It is also not uncommon for these oligoclonal populations of CD4+ and CD8+ cells to develop ‘normally’ in later life, although the nature of these cells and their relationship to those seen in B‐CLL is not clear. The identity of the antigen(s) driving the CD4+ T cells is unknown, although B‐CLL patients not infrequently develop viral infections that are not all due to immunodeficiency resulting from treatment [3]. In our studies there was a tendency for a relative number of CD4+ PF+ cells to increase with the stage of the disease. Since, in normal individuals, CD4+ CD57+ cells (many of which were PF+ in our study) are more frequent than CD4+ CD57− in lymphoid tissue germinal centres (GC) [22,23], a trivial explanation for their increase in the circulation in B‐CLL patients could be either that they are exported from the GC, or change their trafficking properties. Increases in percentages of cytotoxic CD4+ T cells have been seen following mycobacterial [24] and EBV infection [25], where they have been shown to play a role in protection against these organisms. In addition, they have also been found in HIV infection [26]. The large increase in CD4+ PF+ cells seen in CLL and their functional significance is at present unclear. Evidence that the CD4+ CD57+ cells in B‐CLL patients are potentially functional as cytotoxic cells comes from recent studies in which a CD4+ T-cell line derived from a patient with B‐CLL showed cytotoxicity to the autologous tumour cells [27]. CD8+ T cells activated with CD3xCD19 bispecific antibodies and bivalent CD28 antibodies were shown to be able to lyse autologous B‐CLL cells [28]. In preliminary experiments, we have shown apoptosis of B‐CLL tumour cells as determined by Annexin V binding by freshly isolated CD4+ T cells through CD3xCD19 bispecific antibodies. Interestingly, there are also now reports of the cytotoxicity-related molecule TiA by CD4+ as well as CD8 + cells in non-Hodgkin lymphomas and other lymphomas ([29] and A. Dogan, personal communication), suggesting that they may have a role in other lymphoid malignancies.
Recent data from knockout mice experiments have suggested that perforin plays a role in immunoregulation, and cells bearing it might use it to suppress immune responses [30]. Since CD4+ PF+ T cells have now been documented in several autoimmune disorders [31,32], and with a CD4+ CD57+ CD28− phenotype, it is possible that they might play a role in immunoregulation in B‐CLL. In this regard, patients with B‐CLL frequently develop immunodeficiency, as measured by decreases in serum immunoglobulin, which does not appear to be related to immunosuppressive drug treatment. Cognate interaction between the CD4+ PF+ CD57+ cells and the normal B cells could lead to apoptosis of the B cells rather than help, resulting in the hypogammaglobulinaemia seen in patients particularly at later stages in the disease.
Since the CD4+ PF+ cells constitutively produce IL4 in the B‐CLL patients, these cells, together with the Th2-like CD8+ and NK cells in B‐CLL patients [20], could provide an anti-apoptotic environment for the tumour cells [33]. Inability to kill the tumour cells by the CD4+ T cells could, in turn, lead to an uncontrolled increase in their number, as suggested by Hahn and Erb [30].
Finally, it might be possible to harness the cytotoxic potential of the CD4+ PF+ cells to kill the autologous tumour cells directly, which, although insensitive to receptor cross-linking [34] and Fas/FasL-mediated apoptosis [35], are susceptible to killing by allogeneic CD8+ T cells [36].
Future experiments will be directed towards defining the function of the elevated CD4+ PF+ cells in B‐CLL in terms of immunoregulation and optimizing their cytotoxicity for autologous B‐CLL tumour cells.
Acknowledgments
The authors would like to thank Dr Arne Akbar for helpful discussions. TK was a recipient of the Welcome Trust vacation scholarship. This work was in past, funded by INTAS.
References
- 1.Kay NE, Perri RT. Immunobiology of malignant B cells and immunoregulatory cells in B chronic lymphocytic leukemia. Clin Lab Med. 1988;8:163–77. [PubMed] [Google Scholar]
- 2.Zaknoen SL, Kay NE. Immunoregulatory cell dysfunction in chronic B cell leukemias. Blood. 1990;4:165–74. doi: 10.1016/0268-960x(90)90044-s. [DOI] [PubMed] [Google Scholar]
- 3.Bartik MM, Welker D, Kay NF. Impairments in immune cell function in B cell chronic lymphocytic leukemia. Sem Oncol. 1998;25:27–33. [PubMed] [Google Scholar]
- 4.Prieto A, Garcia-Suerez J, Reyes E, et al. Diminished DNA synthesis in T cells from B chronic lymphocytic leukemia after phytohemagglutinin, anti-CD3, and phorbol myristate acetate mitogenic signals. Exp Haematol. 1993;21:1563–9. [PubMed] [Google Scholar]
- 5.Rossi E, Matutes E, Morilla R, Owusu-Ankomah K, Heffernan AM, Catovsky D. Zeta chain and CD28 are poorly expressed on T lymphocytes from chronic lymphocytic leukemia. Leukemia. 1996;10:494–7. [PubMed] [Google Scholar]
- 6.Cantwell M, Hua T, Pappas J, Kipps TJ. Acquired CD40-ligand deficiency in chronic lymphocytic leukemia. Nature Med. 1997;3:984–9. doi: 10.1038/nm0997-984. [DOI] [PubMed] [Google Scholar]
- 7.Wen T, Mellstedt H, Jondal M. Presence of clonal T cell populations in chronic B lymphocytic leukemia and smoldering myeloma. J Exp Med. 1990;171:659–66. doi: 10.1084/jem.171.3.659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Serrano D, Monteiro J, Allen SL, et al. Clonal expansion within the CD4 (+) CD57 (+) and CD8 (+) CD57 (+) T cell subsets in chronic lymphocytic leukemia. J Immunol. 1997;158:1482–9. [PubMed] [Google Scholar]
- 9.Van den Hove LE, Van Gool SW, Van den Berghe P, Boogaerts MA, Ceuppens JL. CD57+/CD28– T cells in untreated hemato-oncological patients are expanded and display a Th1-type cytokine secretion profile, ex vivo cytolytic activity and enhanced tendency to apoptosis. Leukemia. 1998a;12:1573–82. doi: 10.1038/sj.leu.2401146. [DOI] [PubMed] [Google Scholar]
- 10.Trapani JA, Davis J, Sutton VR, Smyth MJ. Proapoptotic functions of cytotoxic lymphocyte granule constituents in vitro and in vivo. Curr Op Immunol. 2000;12:323–9. doi: 10.1016/s0952-7915(00)00094-7. [DOI] [PubMed] [Google Scholar]
- 11.Smith MD, Worman C, Yuksel F, et al. T gamma delta-cell subsets in cord and adult blood. Scand J Immunol. 1990;32:491–5. doi: 10.1111/j.1365-3083.1990.tb03189.x. [DOI] [PubMed] [Google Scholar]
- 12.Rutella S, Rumi C, Lucia MB, et al. Flow cytometric detection of perforin in normal human lymphocyte subpopulations defined by expression of activation/differentiation antigens. Immunol Lett. 1998;60:51–5. doi: 10.1016/s0165-2478(97)00132-6. [DOI] [PubMed] [Google Scholar]
- 13.Hannet I, Erkeller-Yuksel F, Lydyard P, Denys V, DeBruyere M. Development and maturational changes in human lymphocyte subpopulations. Immunol Today. 1992;13:215–8. doi: 10.1016/0167-5699(92)90157-3. [DOI] [PubMed] [Google Scholar]
- 14.Mollet L, Sadat-Sowti B, Duntze J, et al. CD8hi+CD57+ T lymphocytes are enriched in antigen-specific T cells capable of down-modulating cytotoxic activity. Int Immunol. 1998;10:311–23. doi: 10.1093/intimm/10.3.311. [DOI] [PubMed] [Google Scholar]
- 15.Hall H, Deutzmann R, Timpl R, et al. HNK-1 carbohydrate-mediated cell adhesion to laminin-1 is different from heparin-mediated and sulfatide-mediated cell adhesion. Eur J Biochem. 1997;246:233–42. doi: 10.1111/j.1432-1033.1997.t01-1-00233.x. [DOI] [PubMed] [Google Scholar]
- 16.Schmidt JT, Schachner M. Role for cell adhesion and glycosyl (HNK-1 and oligomannoside) recognition in the sharpening of the regenerating retinotectal projection in goldfish. J Neurobiol. 1998;37:659–71. doi: 10.1002/(sici)1097-4695(199812)37:4<659::aid-neu13>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 17.Weekes MP, Wills MR, Mynard K, et al. Large clonal expansions of human virus-specific memory cytotoxic T lymphocytes within the CD57+CD28–CD8+ T cell population. Immunology. 1999;98:443–9. doi: 10.1046/j.1365-2567.1999.00901.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Borthwick NJ, Lowdell M, Salmon M, Akbar AN. Loss of CD28 expression on CD8+ T cells is induced by IL-2 receptor gamma chain signalling cytokines and type 1 IFN, and increases susceptibility to activation-induced apoptosis. Int Immunol. 2000;12:1005–13. doi: 10.1093/intimm/12.7.1005. 10.1093/intimm/12.7.1005. [DOI] [PubMed] [Google Scholar]
- 19.Romagnani P, Annunziato F, Piccinni M-P, Maggi E, Romagnani S. Cytokines and chemokines in T lymphopoiesis and T cell effector function. Immunol Today. 2000;21:416–8. doi: 10.1016/s0167-5699(00)01670-4. 10.1016/S0167-5699(00)01670-4. [DOI] [PubMed] [Google Scholar]
- 20.De Totero D, Reato G, Mauro F, et al. IL4 production and increased CD30 expression by a unique CD8+ T cell subset in B-cell chronic lymphocytic leukemia. Br J Haematol. 1999;104:589–99. doi: 10.1046/j.1365-2141.1999.01219.x. [DOI] [PubMed] [Google Scholar]
- 21.Brinkmann V, Kristofic C. Massive production of Th2 cytokines by human CD4+ effector T cells transiently expressing the natural killer cell marker CD57/HNK1. Immunology. 1997;91:541–7. doi: 10.1046/j.1365-2567.1997.00298.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bouzahzah F, Bosseloir A, Heinen E, Simar LJAD. Human germinal center CD4+CD57+ T cells act differently on B cells than do classical T-helper cells. Dev Immunol. 1995;4:189–97. doi: 10.1155/1995/76790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hufert FT, van Lunzen J, Janossy G, et al. Germinal centre CD4+ T cells are an important site of HIV replication in vivo. AIDS. 1997;11:849–57. doi: 10.1097/00002030-199707000-00003. [DOI] [PubMed] [Google Scholar]
- 24.Conradt P, Hess J, Kaufmann SH. Cytolytic T-cell responses to human dendritic cells and macrophages infected with Mycobacterium bovis BCG and recombinant BCG secreting listeriolysin. Microbe-Inf. 1999;1:753–64. doi: 10.1016/s1286-4579(99)80077-x. [DOI] [PubMed] [Google Scholar]
- 25.Munz C, Bickham KL, Subklewe M, et al. Human CD4+ T lymphocytes consistently respond to the latent Epstein-Barr virus nuclear antigen EBNA1. J Exp Med. 2000;191:1649–60. doi: 10.1084/jem.191.10.1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ehret R, Heikelein M, Siciliano RF, Jassoy C. Human immunodeficiency virus glycoprotein-specific CD4+ cytotoxic T lymphocytes are involved in two types of cytotoxicity: antigen-specific and cell-cell fusion-related cell lysis. AIDS Res Hum Retroviruses. 1997;13:1017–21. doi: 10.1089/aid.1997.13.1017. [DOI] [PubMed] [Google Scholar]
- 27.Chu P, Deforce D, Rassenti LZ, Kipps T. Characterization of CD4 cytotoxic T cells specific for autologous chronic lymphocytic leukemia B cells. Blood. 2000;(Suppl.):157a. [Google Scholar]
- 28.Bohlen H, Hopff T, Manzke O, et al. Lysis of malignant B cells from patients with B-chronic lymphocytic leukaemia by autologous T cells activated with CD3xCD19 bispecific antibodies in combination with bivalent CD28 antibodies. Blood. 1993;82:1803–12. [PubMed] [Google Scholar]
- 29.Perambakam S, Naresh K, Nerurkar A, Nadkarni J. Intra-tumoral cytolytic cells. Pattern of distribution in B-cell non Hodgkins lymphoma. Path Oncol Res. 2000;6:114–7. doi: 10.1007/BF03032360. [DOI] [PubMed] [Google Scholar]
- 30.Hahn S, Erb P. The immunomodulatory role of CD4-positive cytotoxic T-ymphocytes in health and disease. Int Rev Immunol. 1999;18:449–64. doi: 10.3109/08830189909088493. [DOI] [PubMed] [Google Scholar]
- 31.Xanthou G, Tapinos NI, Polihronis M, et al. CD4 cytotoxic and dendritic cells in the immunopathologic lesion of Sjogren's syndrome. Clin Exp Immunol. 1999;118:154–63. doi: 10.1046/j.1365-2249.1999.01037.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shustov A, Luzina I, Nguyen P, et al. Role of perforin in controlling B cell hyperactivity and humoral autoimmunity. J Clin Invest. 2000;106:R39–R47. doi: 10.1172/JCI8876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pu QQ, Bezdowa WR. Interleukin-4 prevents spontaneous in vitro apoptosis in chronic lymphatic leukaemia but sensitizes B-CLL cells to melphalan cytotoxicity. Br J Haematol. 1997;98:413–7. doi: 10.1046/j.1365-2141.1997.2113028.x. [DOI] [PubMed] [Google Scholar]
- 34.Porakishvili N, Kulikova N, Elliott S, et al. Apoptosis of leukaemic cells in patients with B chronic Lymphocytic Leukaemia (B-CLL) induced by the ligation of CD5. Blood. 1999;94(Suppl. 1):313b. [Google Scholar]
- 35.Lewinsohn DM, Bement TT, et al. Human purified protein derivative-specific CD4+ T cells use both CD95-dependent and CD95-independent cytolytic mechanisms. J Immunol. 1998;160:2374–9. [PubMed] [Google Scholar]
- 36.Chu P, Wierda WG, Kipps TJ. CD40 activation does not protect chronic lymphocytic leukemia B cells from apoptosis induced by cytotoxic T lymphocyte. Blood. 2000;95:3853–8. [PubMed] [Google Scholar]