Abstract
There are few studies assessing the pathogenicity of human monoclonal anti-DNA antibodies. The use of SCID mice avoids the problem of rejection of the human hybridoma cells thus allowing in vivo assessment of human immunoglobulins. Using electron microscopy we have shown that the human IgG anti-dsDNA monoclonal antibody, RH14, is nephritogenic in SCID mice, causing morphological changes in the kidney due to immunoglobulin deposition. The problem with using SCID mice is that they have an abnormal immune system; normally they are used at about 2 months of age, at which time they have virtually no functional T or B cells. It is known that older SCID mice become increasingly ‘leaky’, that is they develop some mature lymphocyte clones. Our aim was to assess if implanting anti-DNA antibodies into older ‘leaky’ SCID mice would result in pathology which was observable by light microscopy. Eight-month-old SCID mice were implanted with human hybridoma cells secreting either RH14 an anti-dsDNA IgG, CL24, an antiphospholipid antibody or an irrelevant human IgG control. As previously, RH14 deposited in the kidney and caused proteinuria but unexpectedly we also observed hyaline thrombi in the kidney glomeruli and peritubular capillaries. These thrombi occurred only in the case of RH14 implanted mice and were found to stain positively for human IgG and fibrin. However, apart from the interesting thrombi, we did not observe any greater pathological damage resulting from the anti-dsDNA antibody deposition than we had seen in the younger mice; indeed, the electron microscopic findings were more limited.
Keywords: anti-dsDNA, IgG human hybridoma, SCID mice, systemic lupus, erythematosus
Introduction
Systemic lupus erythematosus (SLE) is an autoimmune rheumatic disease of unknown aetiology, characterized by the presence of autoantibodies against a multiplicity of nuclear, cytoplasmic and membrane antigens (reviewed in [1]). Autoantibodies which bind double-stranded DNA (dsDNA) are present in approximately 70% of patients with SLE. Antibodies which bind dsDNA may cause tissue damage resulting in glomerulonephritis by one or more hypothetical mechanisms, either by forming complexes with DNA passively trapped in the glomeruli, by direct or cross-reactive binding to glomerular structures or antigens planted in the glomerular basement membrane (GBM), such as DNA, nucleosomes, heparan sulphate or laminin, or by penetration of renal tubular epithelium cells and binding to cytoplasmic or nuclear structures (reviewed in [2,3]).
It is not clear at present which features distinguish pathogenic and non-pathogenic dsDNA antibodies. High affinity anti-DNA antibodies of the IgG isotype are believed to be the major culprits in the pathogenesis of lupus nephritis, especially IgG1 and IgG3 antibodies, which have the ability to fix complement. DNA binding is often enhanced by the presence of cationic amino acids, probably arising by somatic mutation, in the complementarity determining regions. However, it has been shown in both patients and murine models that not all anti-DNA antibodies deposit in the kidney and are pathogenic; only 30–50% of SLE patients with such antibodies develop lupus nephritis [4]. Studies assessing the pathogenicity of human anti-DNA antibodies are limited due to a paucity of human IgG monoclonals and difficulties with their use in most murine strains. Using immunodeficient mice, which lack an intact immune system, overcomes the problem of rejection of the human hybridoma cells, making them useful for studying the pathogenicity of human dsDNA antibodies.
In severe combined immunodeficiency (SCID) mice aberrant V(D)J recombination means that antigen receptors (TCR and Ig) are not expressed and the lymphocytes do not mature. Approximately 15% (2–25%) of young adult SCID mice are ‘leaky’ in that they produce readily detectable numbers of mature T and B cells, with limited repertoire of Ag receptors. ‘Leakiness’ increases with age and can easily be detected by assaying serum for murine immunoglobulin [5].
Previously our group have used SCID mice to show for the first time that a human IgG anti-dsDNA monoclonal antibody, RH14, was nephritogenic and that deposition of this antibody was sufficient to induce renal damage [6]. Hybridoma cells secreting RH14 were implanted into the peritoneum of the SCID mice, which subsequently developed proteinuria and fluorescence staining showed the presence of human IgG in the kidney. Electron microscopy of kidney sections from RH14 implanted SCID mice showed that the human immunoglobulins were deposited on the glomerular capillary basement membrane and in the mesangial matrix. The changes seen in the glomerular structures of these SCID mice, thickening of the basement membrane and ‘footpad’ fusion, resemble the pathological changes in patients with lupus nephritis. However, light microscopy of the RH14 implanted kidneys showed no evidence of leucocyte infiltration or fibrotic change. This might be due to lack of functional T and B cells in SCID mice, failure of RH14 to activate complement or to the short duration (4–5 weeks) of antibody exposure.
The SCID mice used in our previous experiments were approximately 2 months of age, at which time they possess virtually no functional T or B cells. It is known that by 10–14 months of age virtually all SCID mice become ‘leaky’, that is they possess some mature lymphocyte clones. Our aim was to assess whether implanting anti-DNA antibodies into these older ‘leaky’ SCID mice would result in pathology which was observable by light microscopy. Our underlying hypothesis was that the ‘leaky’ SCID mice have some mature lymphocyte clones allowing a limited immune response and hence greater pathology than seen in the 2-month-old SCID mice [6], but without the rejection of the hybridoma cells which would occur if using normal mice.
Materials and methods
Mice
Female Balb/C SCID mice were obtained from Harlan UK (Bicester, UK) at 8 months of age (ex-breeders). The mice were all housed in sterile conditions on vented racks.
Hybridoma cells
The human hybridoma cell line RH14 was produced from a patient with SLE as described previously [6] this antibody is IgG1 and in ELISA binds to dsDNA, ssDNA, histones, H1, H2 and nucleosomes. CL24, a human IgG3 human monoclonal antibody which binds to cardiolipin and β2 glycoprotein 1, was produced from a patient with secondary anti-phospholipid syndrome (a kind gift from Pojen Chen, UCLA, CA, USA) [7]. As an irrelevant human IgG control we used TW, a hybridoma cell line secreting human IgG of unknown specificity, which in our hands does not bind dsDNA (a kind gift from Thomas Winkler, Erlangen, Germany). CBF7 the non-secreting mouse–human heteromyeloma fusion partner cell was used as a negative control. The cells were cultured in RPMI 1640 medium containing 1% l-glutamine, 1% sodium pyruvate, 2% MEM non-essential amino-acids, 1% penicillin/streptomycin, 0·2% gentamycin (all from Gibco, UK) and 10% FCS (Sigma, UK).
Experimental schedule
The mice were acclimatized for 1 week and then tail-bled prior to the start of the experiment, in order to assess ‘leakiness’ by ELISA of murine IgM and IgG. The mice were primed with 500 µl i.p. of pristane (2,6,10,14-tetramethylpentadecane, Sigma) which activates macrophages to produce growth factors and create optimal environment for hybridoma cell growth. Ten days later the mice were implanted with hybridoma cells, i.p 1 × 106 cells in 500 µl of RPMI 1640. The 8-month-old SCID mice were implanted with RH14 (n = 5), CL24 (n = 3), TW (n = 5) and CBF7 (n = 5). Throughout the experiment proteinuria was assessed using Albustix (Bayer Diagnostics, Berks, UK), proteinuria is scored as negative or trace which is negligible (+) 0·3 g/l (+ +) 1·0 g/l (+ + +) 3·0 g/l and (+ + + +) more than 20 g/l. The mice were sacrificed when the ascites had developed to a degree which resulted in a 20% increase in body weight or after 2 months if ascites had not yet developed. On sacrifice, sera, ascites fluid and organs were collected for further analysis.
Human IgG ELISA
A standard solid-phase ELISA assay was used to measure the concentration of human IgG antibodies, produced by the implanted hybridoma cells, which were present in the sera and ascites fluid at termination of the experiment. Polystyrene 96-well plates (‘maxisorp’, Nunc, Roskilde, Denmark) were coated with 2·5 µg/ml of goat antihuman IgG (Sigma) diluted in PBS(pH 7·2). After incubation overnight at 4°C, plates were washed three times with PBS and blocked with 1% (w/v) BSA (Sigma) in PBS for 1 h at 37°C. Serum and ascites samples were titrated from 1: 20 to 1: 200 000 dilution in PBS containing 0·05%Tween 20 (Sigma), and were incubated on the plate for 1 h at 37°C. Following three washes, bound antibodies were detected by incubation for 1 h at 37°C with a 1: 1000 dilution of affinity purified goat antihuman IgG conjugated to alkaline phosphatase conjugate (Sigma) and following washing, developed with the substrate p-nitrophenol phosphate (Sigma). Optical density was measured at 405 nm with a reference filter 490 nm. The sample concentrations were calculated by reference to the linear portion of a standard curve of purified human IgG (Sigma) run on every plate.
Anti-dsDNA IgG ELISA
Nunc ‘maxisorp’ plates were half coated with 5 µg/ml of calf thymus DNA (Sigma) in citrate buffer (0·15 m sodium chloride and 0·015 m sodium citrate pH 8·0) and half with buffer alone for 2 h at 37°C. After washing in PBS the plates were blocked with 2% casein (Sigma) in PBS for 1 h at 37°C. Serum and ascites samples were titrated from 1: 100 in PBS/Tween20 and incubated on both DNA coated and uncoated sides of the plate for 1 h at 37°C. Bound antibodies were detected as described for the human IgG ELISA.
Murine IgM and IgG ELISA
Nunc ‘maxisorp’ plates were coated for 1 h at 37°C with 1 µg/ml of antimouse IgM or IgG (Sigma), respectively, following washing with PBS and blocking with 1% BSA in PBS for 1 h at 37°C, the serum samples were diluted to 1: 50 and 1: 500 in PBS/Tween20 and incubated on the plate for 1 h at 37°C. After washing with PBS/Tween20 bound antibodies were detected with goat antimouse IgM or IgG–alkaline phosphatase conjugate, respectively (Sigma) and developed as described above.
Haematoxylin and eosin histological stain
Formalin-fixed, paraffin-embedded kidney sections from the SCID mice were stained with haematoxylin and eosin. These were then examined by a histopathologist for morphological evidence of kidney disease.
Staining for human IgG
Formalin-fixed, paraffin-embedded kidney sections were dewaxed and endogenous peroxidase was blocked using 0·5% H202 in methanol for 10–15 minutes. The sections were washed in water and in order to expose the antigen after formalin fixation, then digested in 0·1% protease XX1V (Sigma) in distilled water adjusted to pH 7·8 with 0·1 m NaOH for 40 minutes at 37°C. The kidney sections were then blocked with 5% normal swine serum for 10 minutes and incubated in rabbit polyclonal antihuman IgG-HRP (Dako) and detected with 3,3′-diaminobenzidine (DAB).
Staining for murine IgG and IgM
Sections were dewaxed and digested with protease as described previously. The sections were then incubated with either goat F(ab′)2 antimouse IgG (H + l) or antimouse IgM (Southern Biotechnology, USA), at several dilutions in 3% BSA/PBS. Endogenous peroxidase was blocked with 1% H2O2 in PBS for 30 minutes and then the sections were incubated with peroxidase conjugated monoclonal antigoat IgG (clone GT-34, Sigma) diluted 1: 200 in 3% BSA/PBS; binding was visualized with DAB. The primary and secondary antibodies were chosen for their minimal cross-reactivity with human immunoglobulins.
Staining for fibrin
Formalin-fixed, paraffin-embedded kidney sections were dewaxed and stained for fibrin using two standard histological stains, MSB (martius yellow, brilliant crystal scarlet and soluble blue) and phosphotungstic acid haematoxylin (PTAH).
Electron microscopy
When the mice were sacrificed a small section of each kidney was fixed in 2% glutaraldehyde/PBS and these were then embedded and processed for electron microscopy.
Results
‘Leaky’ SCID mice
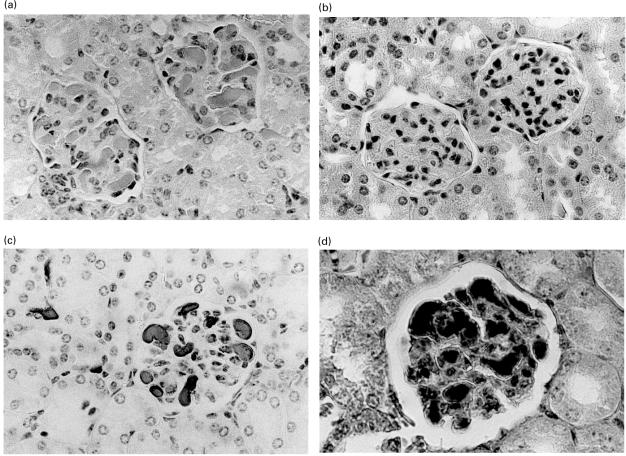
The 8-month-old SCID mice were confirmed to be ‘leaky’ since 17/18 mice had high levels of murine IgM and 3/18 mice had high levels of murine IgG (Table 1). The concentration of human IgG was measured both in the ascites fluid and in the sera on termination of the experiment. The human IgG present in sera or ascites fluid of individual mice varied widely, from over 5 mg/ml to under 2 µg/ml but significant levels of antibody were shown to be present after implantation with either RH14, CL24 or TW (Table 1). RH14 caused proteinuria in the SCID mice, which was greatest in mice with high levels of human IgG in the serum and ascites fluid (Table 1). The level of proteinuria + + − + + + (1·0–3·0 g/l) was higher than that found in the control groups, but was not as marked as that found in a murine model of lupus such as the MRL lpr/lpr mouse, which commonly have between 3·0 and 20 g/l. Haematoxylin and eosin staining of the kidneys showed that four of five mice implanted with RH14 had hyaline thrombi in the glomeruli and in some peritubular capillaries (Fig. 1a); these thrombi were positive when stained for human IgG (Fig. 1c) and fibrin (Fig. 1d). These thrombi were most numerous in the mouse with the highest levels of RH14, being present in all glomeruli of the sections which were stained. Mice which were implanted with CL24 (Fig. 1b), TW and CBF7 all had normal kidney morphology and had no deposition of human IgG. All liver, spleen and skin sections from RH14-treated SCID mice showed normal morphology and were negative for human IgG. As a further control we also tested the antibodies in parallel in 2-month-old SCID mice; unfortunately, in these mice the hybridomas failed to secrete antibody, preventing a direct comparison of pathological effects in the same experiment. Electron microscopic examination of the kidneys revealed that RH14 deposition resulted in a lesser degree of pathological change in the 8-month-old SCID mice than we reported previously in 2-month-old SCID mice [6], although the hyaline thrombi with fibrin could clearly be seen. In the 8-month-old SCID mice implanted with RH14 there was no effacement of the foot processes or thickening of the basement membrane, but there was occasional ischaemic-type wrinkling in paramesangial area, electron-dense fibrils, possibly fibrin within the mesangium and in one of the observed loops a degree of interposition of the GBM was noted.
Table 1.
‘Leaky’ 8-month-old SCID mice implanted with hybridoma cells producing human monoclonal antibodies
| Mouse IgM |
Mouse IgG |
Histology |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Optical density values at 410 nm |
Human IgG μg/ml |
Proteinuria (terminal values shown) |
Thrombi |
Hu IgG |
Fibrin |
|||||||
| Hybridoma | Ascites (day) | day –11 | terminal | day –11 | terminal | ascites | serum | plus score | mean arbitrary score | H&E | HRP | MSB |
| RH14 | 38 | 1·0 | 1·0 | 0·0 | 0·0 | 280 | 1254 | ++/++ + | 3·4 | + | + | + |
| 38 | 1·2 | 1·1 | 0·6 | 0·2 | 2240 | 5760 | ++/++ + | + + | + + | + + | ||
| 42 | 1·1 | 1·0 | 0·0 | 0·1 | 1103 | 23·8 | + + | – | – | – | ||
| 35 | 1·1 | 0·5 | 0·0 | 0·0 | 292 | 352 | ++/++ + | + | + | + | ||
| 34 | 0·5 | 0·6 | 0·0 | 0·0 | 464 | 1952 | ++/++ + | + | + | + | ||
| CL24 | 25 | 0·6 | 0·5 | 0·0 | 0·0 | 2916 | 1074 | + | 1·8 | – | – | – |
| 46 | 0·3 | 0·2 | 0·1 | 0·1 | 110 | 55 | trace/+ | – | – | – | ||
| 46 | 0·3 | 0·2 | 0·1 | 0·4 | 3100 | 1090 | + | – | – | – | ||
| TW | 37 | 0·7 | 0·6 | 1·4 | 1·3 | 31 | 50 | +/++ | 2·5 | – | – | – |
| 37 | 0·4 | 0·8 | 0·0 | 0·1 | 11 | 19 | + + | – | – | – | ||
| 37 | 0·7 | 0·3 | 0·0 | 0·1 | 2310 | 1660 | + | – | – | – | ||
| 37 | 0·6 | 0·3 | 0·0 | 0·1 | 2 | 4·5 | + | – | – | – | ||
| 33 | 0·4 | 0·4 | 0·0 | 0·0 | 270 | 243 | + + | – | – | – | ||
| CBF7 | 20 | 0·1 | 0·0 | 0·0 | 0·0 | 0 | 0 | + | 2 | – | – | – |
| 20 | 0·6 | 0·3 | 0·0 | 0·0 | 0 | 0 | + | – | – | – | ||
| 20 | 0·4 | 0·5 | 0·1 | 0·1 | 0 | 0 | + | – | – | – | ||
| 20 | 0·9 | 1·0 | 0·0 | 0·0 | 0 | 0 | + | – | – | – | ||
| 20 | 0·8 | 0·7 | 0·9 | 0·5 | 0 | 0 | + | – | – | – | ||
Proteinuria plus score: + = 0·3, + + = 1·0, + + + = 3·0 g/l of protein. Proteinuria arbitrary score: negative = 0, trace = 1, + = 2, + + = 3 and + + + = 4. Histology: (+) represents staining present in many glomeruli (+ +) staining present in all glomeruli in the kidney section.
Fig. 1.
Representative haematoxylin and eosin staining of paraffin wax sections from the kidneys of SCID mice implanted with (a) hybridoma cells secreting RH14, showing hyaline thrombi in the glomeruli, (b) control human hybridoma secreting CL24, showing normal kidney morphology. Paraffin wax sections from the kidney of a SCID mouse implanted with RH14 showing the hyaline thrombi stained with antihuman IgG and DAB (c) and PTAH staining of fibrin deposited in the thrombi (d). The magnification of all figures is × 400.
Discussion
In the older ‘leaky’ SCID mice, the primary conclusion is that as in the younger SCID mice, RH14 binds to the kidney and causes proteinuria. The binding of RH14 is probably enhanced by its ability to bind nucleosomes and histones as well as single- and double-stranded DNA. However, interestingly in these older SCID mice it appears that RH14 binding in the kidney also caused the development of hyaline thrombi. These thrombi occurred at greatest frequency in the kidney of the mouse which had the highest level of RH14. However, the kidneys of these mice showed no evidence of greater pathological changes, reminiscent of those seen in patients with lupus, than did the younger SCID mice.
It would seem that the formation of the hyaline thrombi is dependent on the binding specificity of RH14, since mice implanted with CL24 or TW, some of which had human IgG of over 2 mg/ml in their ascites fluid, showed no hyaline thrombi or deposition of antibody. In 2-month-old SCID mice, RH14 was shown to bind specifically in the kidney glomeruli [6] but this binding did not result in the formation of hyaline thrombi. However, the marked pathological changes due to deposition of RH14, which were seen at the electron microscopic level in the kidney glomeruli of the 2-month-old SCID mice [6], were not present in these older mice. In this experiment, some mice had human IgG levels present in the sera and ascites (Table 1) which were 10 times greater than those observed in the previous experiment, where human IgG levels were between 320 and 390 µg/ml [6], but in the current experiment the thrombi were also present in the mouse which had only 352 µg/ml of RH14 in the sera, which is equivalent to the levels achieved in the previous experiment in which no thrombi occurred.
Some but not all human anti-dsDNA antibodies produced by hybridoma cells bind to the kidney when implanted into SCID mice and furthermore individual antibodies exhibit different localization of binding [6,8]. This variability in antibody pathogenicity or kidney localization has also been demonstrated for murine monoclonal anti-DNA antibodies [9–11] with some anti-DNA antibodies exhibiting mesangial deposition, others resulting in cell-proliferative lesions associated with macrophage infiltrates or in subendothelial hyaline deposits resembling wire-loops [11,12]. Some of the hyaline deposits found by these authors appear very similar to the hyaline thrombi which we found, although we did not observe wire-loop lesions in our mice. However, the interesting observation in our experiment was that the same human monoclonal antibody, RH14, showed a different localization of binding in two sets of experiments with the only apparent difference being the ages of the SCID mice, in this case 2 months [6] and 8 months of age. This poses the question of whether the thrombi we found in the older ‘leaky’ SCID mice are due to the additional presence of murine immunoglobulins, changing the type of glomerular lesion caused by RH14. These thrombi were not observed in the SCID mice implanted with CL24 or TW which also had murine IgM or IgG antibodies present. Might the thrombi result from complexes of human and murine immunoglobulin but only bind to the kidney when the human immunoglobulin can bind specifically, as in the case of RH14? Possible support for this hypothesis is provided by the work of Ito et al. [12], which showed the remodelling of glomerular lesions in SCID mice, caused by the injection of a murine monoclonal anti-DNA antibody, by non-nephritogenic bystander IgM antibodies.
In order to address this question we carried out two further experiments. Large immunoglobulin complexes could cause thrombi formation, so we attempted to look for complexes of murine and human antibodies by incubating the sera in ELISA plates coated with antihuman IgG and subsequently detecting any complexes using antimurine IgM and IgG conjugated with alkaline phosphatase. Using this method we determined that there were no detectable complexes of human and murine immunoglobulins present in the sera of the SCID mice. We also looked directly at the thrombi for the presence of murine IgM and IgG by immunohistochemistry in kidney sections. The vast majority of the thrombi that we observed were negative when stained for murine IgM or IgG and therefore these antibodies do not appear to be involved in the initial clot formation. There were occasional thrombi with very limited staining; however, the murine antibodies probably adhered to the already formed thrombi.
Thus if the thrombi are not caused by the deposition of large complexes of human and murine immunoglobulins, could they be due to deposition of complexes of RH14 and nucleosomes? Nucleosomes are believed to facilitate the binding of anti-DNA antibodies to heparan sulphate on the glomerular basement membrane via their cationic histone component [13]. The older SCID mice could have higher levels of apoptosis and therefore more nucleosomes than the younger mice resulting in immune complexes and subsequent thrombi formation. Alternatively, there is evidence to suggest that apoptosis is the most prevalent mode of cell death in hybridoma cell cultures [14]; this would generate nucleosomes which could form complexes with anti-DNA antibodies. It is possible that the RH14 culture used in this experiment may have contained higher levels of such nucleosomes than those used in the previous experiment [6], thus allowing the formation of large immune complexes which could result in thrombi instead of simple antibody deposition. Whatever the mechanism involved, it is interesting that a particular human anti-DNA antibody can exhibit a different localization of binding in the glomeruli of ‘leaky’ SCID mice as compared with the 2-month-old SCID mice used in the majority of experiments.
Acknowledgments
We wish to thank Mr Keith Miller for carrying out the human IgG staining, Dr Giorgio Landon for the electron microscopy and Dr Meryl Griffiths for evaluating the histological slides. We also want to thank Pojen Chen (UCLA) for supplying the CL24 clone. This work was funded by a programme grant from the Arthritis Research Campaign.
References
- 1.Mason LJ, Isenberg DA. Immunopathogenesis of systemic lupus erythematosus. Baillière's Clin Rheumatol. 1998;12:385–403. doi: 10.1016/s0950-3579(98)80026-5. [DOI] [PubMed] [Google Scholar]
- 2.Hahn BH. Antibodies to DNA. N Engl J Med. 1998;338:1359–68. doi: 10.1056/NEJM199805073381906. [DOI] [PubMed] [Google Scholar]
- 3.Isenberg DA, Ravirajan CT, Rahman A, Kalsi J. The role of antibodies to DNA in systemic lupus erythematosus: a review and introduction to an international workshop on DNA antibodies held in London, May 1996. Lupus. 1997;6:290–304. doi: 10.1177/096120339700600316. [DOI] [PubMed] [Google Scholar]
- 4.Ter Borg EJ, Horst G, Hummel EJ, Limburg PC, Kallenberg CG. Measurement of increases in anti-double-stranded DNA antibody levels as a predictor of disease exacerbation in systemic lupus erythematosus: a long term prospective study. Arthritis Rheum. 1990;33:634–43. doi: 10.1002/art.1780330505. [DOI] [PubMed] [Google Scholar]
- 5.Vladutiu AO. The severe combined immunodeficient (SCID) mouse as a model for the study of autoimmune diseases. Clin Exp Immunol. 1993;93:1–8. doi: 10.1111/j.1365-2249.1993.tb06488.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ravirajan CT, Rahman MA, Papadaki L, et al. Genetic, structural and functional properties of an IgG DNA-binding monoclonal antibody from a lupus patient with nephritis. Eur J Immunol. 1998;28:339–50. doi: 10.1002/(SICI)1521-4141(199801)28:01<339::AID-IMMU339>3.0.CO;2-C. 10.1002/1521-4141(199801)28:01`339::AID-IMMU339b3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 7.Zhu M, Olee T, Le DT, et al. Characterisation of IgG monoclonal anti-cardiolipin/anti-β2GP1 antibodies from two patients with antiphospholipid syndrome reveals three species of antibodies. Br J Haematol. 1999;105:102–9. [PubMed] [Google Scholar]
- 8.Ehrenstein MR, Katz DR, Griffiths MH, et al. Human IgG anti-DNA antibodies deposit in kidneys and induce proteinuria in SCID mice. Kidney Int. 1995;48:705–11. doi: 10.1038/ki.1995.341. [DOI] [PubMed] [Google Scholar]
- 9.Vlahakos DV, Foster MH, Adams S, et al. Anti-DNA antibodies form immune deposits at distinct glomerular and vascular sites. Kidney Int. 1992;41:1690–700. doi: 10.1038/ki.1992.242. [DOI] [PubMed] [Google Scholar]
- 10.Itoh J, Nose M, Takahashi S, et al. Induction of different types of glomerulonephritis by monoclonal antibodies derived from an MRL/lpr lupus mouse. Am J Pathol. 1993;143:1436–43. [PMC free article] [PubMed] [Google Scholar]
- 11.Mostoslavsky G, Fischel R, Yachimovich N, et al. Lupus anti-DNA autoantibodies cross-react with a glomerular structural protein: a case for tissue injury by molecular mimicry. Eur J Immunol. 2001;31:1221–7. doi: 10.1002/1521-4141(200104)31:4<1221::aid-immu1221>3.0.co;2-p. 10.1002/1521-4141(200104)31:4`1221::AID-IMMU1221b3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 12.Ito MR, Terasaki S, Kondo E, Shiwaku H, Fukuoka Y, Nose M. Experimental lupus nephritis in severe combined immunodeficient (SCID) mice: remodelling of the glomerular lesions by bystander IgM antibodies. Clin Exp Immunol. 2000;119:340–5. doi: 10.1046/j.1365-2249.2000.01133.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Amoura Z, Koutouzov S, Piette J-C. The role of nucleosomes in lupus. Curr Opin Rheumatol. 2000;12:369–73. doi: 10.1097/00002281-200009000-00003. [DOI] [PubMed] [Google Scholar]
- 14.Franek F, Vomastek T, Dolnikova J. Fragmented DNA and apoptotic bodies document the programmed way of cell death in hybridoma cultures. Cytotechnology. 1992;9:117–23. doi: 10.1007/BF02521738. [DOI] [PubMed] [Google Scholar]