Abstract
A HUVEC cDNA library was screened with sera from two patients who had developed transplant-associated coronary artery disease (TxCAD) following cardiac transplantation. A total of six positive clones were isolated from a primary screen of 40 000 genes. Subsequent DNA sequence analysis identified these to be lysyl tRNA synthetase, ribosomal protein L7, ribosomal protein L9, β transducin and TANK. Another gene whose product could not be identified showed homology to a human cDNA clone (DKFZp566M063) derived from fetal kidney. Full-length constructs of selected genes were expressed as his-tag recombinant fusion proteins and used to screen a wider patient base by ELISA to determine prevalence and association with TxCAD. Of these ribosomal protein L7 showed the highest prevalence (55·6%) with TxCAD sera compared to 10% non-CAD.
Keywords: antibodies, cardiac, chronic rejection, transplantation
Introduction
Transplant-associated coronary artery disease (TxCAD) is the most common long-term complication following cardiac transplantation. The condition is characterized by the concentric narrowing of the major epicardial and intramyocardial coronary arteries and veins [1] due primarily to the secretion of extracellular matrix components by activated smooth muscle cells [2–4]. The lesion components are generally bland compared to the calcified typical atherosclerotic plaque and endothelium is usually intact, although may appear plump [1]. The assessment of TxCAD is normally undertaken by routine angiography and classified as positive if 25% or greater luminal thickening affects one or more epicardial arteries.
Subendothelial leucocytes, predominantly macrophages, may be seen (endothelialitis), although their number is usually sparse compared to that seen in acute rejection [5] where T cells predominate [6]. It has been proposed that the proliferation of the intima may be a consequence of immune-mediated endothelial activation and/or damage inducing the release of cytokines and growth factors causing smooth muscle cell proliferation and secretion of extracellular matrix components [7–12].
The appearance of antibody reactivities to donor HLA antigens following transplantation has been found to be associated with an increased risk of developing TxCAD [13–17]. The possibility that non-HLA antiendothelial cell antibodies (AECA) may contribute to the development of TxCAD by either activating the endothelium to release SMC growth factors or causing endothelial cell damage and subsequent release of growth factors has been addressed. These studies have identified the appearance of antibodies reactive to a variety of antigens present in endothelial cell lysates associated with the development of early TxCAD (< 5 years) and identified vimentin, HSP60, 75 kDa glucose-regulated protein and triose phosphate isomerase as candidate TxCAD specific antigens by 2D-SDS PAGE [18–20]. Currently an ELISA to detect antivimentin antibodies has been developed [21] which shows a correlation with the development of TxCAD [22]. Although these studies identified cytosolic antigens, a clear association between the development of antiendothelial cell surface antigens following transplantation and the development of TxCAD has also been observed [23].
The possible involvement of antibodies recognizing antigens other than classical MHC determinants in graft deterioration is hampered by a full description of which reactivities may occur postoperatively. Furthermore, the possibility that pathogenic antibodies present prior to transplantation may contribute to the failure of the host's own organ and accelerate the development of TxCAD in the allograft complicates the issue. Elucidation of their possible role in pathology requires the identification of the range of target antigens to which antibody formation may occur, enabling serological investigation to determine the prevalence and association of these candidates with TxCAD.
In order to describe the immunoreactive profile of AECA in chronic rejection more fully we have undertaken a limited screen of a HUVEC cDNA expression library in an attempt to elucidate which antibody specificities may be associated with TxCAD.
Materials and methods
A commercially available HUVEC cDNA library (Uni-ZAP XR, Stratagene) was used to screen for clones showing immunoreactivity with TxCAD sera. The library was generated from a pool of four HUVEC donors and RNA isolated at passage four.
Selection of sera for immunoscreening the expression library
Patient 1 was a 51-year-old male diagnosed with ischaemic heart disease prior to transplantation and suffered one rejection episode in the first year. He was mismatched for donor HLA at HLA-A (×1), HLA-B (×2), HLA-DR (×1). Serum was collected at 5 years post-transplant, at which time he had 50% coronary arterial stenosis and a vimentin titre of 1/1000. Patient 2 was a 23-year-old male diagnosed with cardiomyopathy prior to transplantation and suffered two rejection episodes in the first year. He was mismatched for donor HLA at HLA-A (×2), HLA-B (×2), HLA-DR (×1). Serum was collected at 2 years post-transplant, at which time he had 50% coronary arterial stenosis and a vimentin titre of 1/400.
Sera were diluted 1/200 in PBS/5% milk/0·1% Tween-20/1% BSA supplemented with 0·02% sodium azide. To reduce non-specific binding to Escherichia coli (XL1 blue MRF′) and phage vector, diluted sera were pre-adsorbed ×3 on non-recombinant lifts (see below) for 1 h. Pre-adsorbed sera was then stored at 4°C and used within 6 months.
Primary immunoscreening
For primary immunoscreening the library was plated out at 10 000 pfu/140 mm plate using XL1 blue MRF′ host cells according to the supplier's instructions. For detection of immunoreactive clones two lifts were taken from separate plates for each serum. Briefly, Duralon UV membranes (Stratagene) were soaked in methanol, rinsed with PBS and incubated with 10 mm IPTG for 20 minutes and air-dried. Membranes were then overlaid onto the plates and incubated for 3 h at 37°C, after which they were carefully removed after marking their orientation, washed with PBS to remove excess agar and blocked for 2 h in 5% milk/PBS.
Pre-adsorbed sera was then added to the membranes and incubated at 37°C for 1 h at RT with gentle agitation. Following this, sera was retained and membranes washed four times with PBS/0·2% Tween-20 over 20 minutes. Bound antibody was detected using 1/1000 dilution of HRP conjugated rabbit antihuman IgA, IgG, IgM (Dako) in PBS/5% milk/0·1% Tween-20 for a further hour at RT with gentle agitation. Following a final four washes in PBS/0·2% Tween-20 over 20 minutes membranes were incubated with 4CN colour detection system (DuPont). Positive immunoreactive plaques were readily visible and easily distinguished from blue non-recombinants. Plaques corresponding to immunoreactive regions were cored from the original plate and resuspended in SM buffer containing 10 µl chloroform.
Secondary screening
Positive colonies identified from the primary immunoscreen were titred to between 100 and 500 pfu/140 mm plate and lifts taken as described above. A further three rounds of immunoscreening were sufficient to screen plaques to clonality.
Subcloning and identification of positive inserts
Cloned phage showing immunoreactivity was recovered as pBluescript by single-stranded rescue using the ExAssist (Stratagene) filamentous helper phage according to the supplier's instructions and used to transform SolR cells. Positive recombinants were then picked and grown overnight in 5 ml LB and plasmid DNA isolated using the Wizard SV miniprep system (Promega). DNA sequencing was conducted at the Molecular Biology Unit, King's College London.
Flanking vector sequences were identified and removed from the database query file and the remaining insert DNA submitted to a BLAST search using the GCG Wisconsin package at the HGMP resource centre, MRC.
Recombinant protein
Recombinant His-tag fusion proteins were constructed by PCR-based methods from total RNA isolated from primary HUVEC cultures at passage four using RNEasy (Quiagen). For selected candidate sequences PCR primers were designed to amplify the coding region, including the 5′ initiation codon and the 3′ termination codon, and included appropriate flanking restriction enzyme sites. The primers used were TANK (+) 5′-gctacgca tatggataaaaacattggc-3′ (–) 3′-aagttacctctctgaattcctaggattcga-5′; β-transducin (+) 5′-gacttgcatatgactgagcagatgaccct-3′ (–) 3′-actggtaaccgtgtgcgatcgtatacgacatg – 5′; RPL7 (+) 5′-gcactgca tatggagggtgtagaagagaagaag-3′ (–) 3′-gaataatcttcttacttgattgagctcc taggcgac-5′; RPL9 (+) 5′-gcatgacatatgaagactattctcagcaat-3′ (–) 3′-caagtcgtccgactacttattcctaggtagcgt – 5′. PCR products were then gel purified and cloned into pGEMT-Easy (Promega) and used to transform competent XL1 blue cells. Positive recombinants were selected on ampicillin plates and plasmid DNA isolated from 5 ml overnight cultures. The insert was then released by appropriate restriction digest, gel purified and ligated in frame to the pET15b vector (Novagen). The ligation mix was used to transform competent XL1 blue E. coli and where necessary orientation of the insert confirmed by asymmetric restriction digestion. Positive pET15b recombinants with the correct insert in the right orientation were then used to transform competent BL21(DE3) (Novagen) and selected on LB/ampicillin agar. Recombinant fusion protein was then expressed by IPTG induction (1 mm) for 2 h from 50 to 100 ml cultures at O.D.600 = 0·4–0·5. Following induction cells were pelleted and frozen overnight at − 40°C. The following day pellets were thawed on ice and resuspended in 20 mm Tris pH = 7·4 supplemented with 10 µg/ml lysozyme (Sigma)/0·1% Triton X-100 (Sigma) and incubated at RT for 10 minutes until viscous. DNA was degraded by the addition of 5 µg/ml DNAse I (Sigma) and a further 10-minute incubation at RT. Inclusion bodies were harvested as the insoluble fraction and washed ×3 in his-bind wash buffer (Novagen) supplemented with 0·1% Triton X-100. The final pellet was resuspended in his-bind buffer containing 6 m urea and incubated on ice for 1 h. Solubilized proteins were then harvested from the supernatant following centrifugation and incubated batchwise with 500 µl his-bind resin (Novagen) for 30 minutes at RT. The resin was then washed ×4 in low stringency wash buffer (Novagen) and bound fusion protein eluted in 1 m imidazol. Proteins were quantified using Bradford reagent (Sigma) and stored at − 20.
ELISA for his-tagged recombinant fusion proteins
Solubilized fusion protein (∼0·5 mg/ml) in 6 m urea/elution buffer was rapidly diluted to 10 µg/ml in 0·5 m NaCl/20 mm Tris pH 8·8 and vortexed for 30 s; 100 µl of the diluted protein was added to each well of a maxisorb immunoplate (Nunc) and incubated for 6 h at RT. Plates were washed ×4 with 200 µl PBS/0·2% Tween-20 and blocked overnight at 4°C in PBS/2% BSA (Sigma). The following day sera were diluted 1/200 in PBS/0·1% Tween-20/1% BSA and 100 µl added to each well. Following 1 h incubation plates were washed ×4 with 200 µl PBS/0·2% Tween-20 and antibody detected with either HRP antihuman IgG, HRP antihuman IgM diluted 1/1000 or HRP rabbit antihuman IgA, IgG, IgM (all from Dako) diluted 1/1000. All secondary antibodies were diluted in PBS/0·1% Tween-20/1% BSA. After 1 h incubation at RT plates were washed ×4 with PBS/0·2% Tween-20 and antibody binding detected using OPD. O.D. was measured at 450 nm.
Selection of sera for ELISA
Initial screening was conducted by selecting nine serum samples from an archive of transplant patients dating back to 1987. For patients who subsequently developed TxCAD by angiogram within 5 years (≥ 25% luminal thickening in one or more vessels) sera were selected at or around the time of diagnosis where available. The mean age of recipients in the TxCAD group was 46·7 years (s.d. 12·0 years). The mean post-transplant elapsed time at which sera were selected was 24·6 months (s.d. 11·5 months) and sera tested on average 2 months following diagnosis of TxCAD (s.d. 9·2 months). In some experiments sequential and pretransplant serum samples were available.
For non-CAD cardiac recipients (no evidence of ≥ 25% luminal thickening in donor vessels after 5 years post-transplant), 10 serum samples were selected around 1 year post-transplant (mean 10 months, s.d. 3·5months). The mean age of the non-CAD patients was 43·5 years, s.d. 10·4.
Controls
To establish baseline normal range values 11 serum samples from healthy volunteers were included in all assays, diluted 1/200, and the positive cut-off threshold set at the mean of these samples + two standard deviations.
ELISA for anti-HUVEC antibodies
HUVEC at passage four were plated out at 2·5 × 104 cells/well on 96-well tissue culture plates (Nunc) in 200 µl of culture medium consisting of M199 with HEPES (Life Technologies), 2 mm l-glutamine, 20% FCS, ECGF (Roche Diagnostics), 50 µg/ml heparin (Sigma) and penicillin and streptomycin. Following overnight culture confluent HUVEC cultures were washed ×3 in 200 µl PBS and fixed with 0·1% glutaraldehyde in PBS for 10 minutes. Free aldehydes were blocked by the addition of 0·05 m Tris/HCl pH 7·5 for 20 minutes. After a further three washes in PBS 100 µl of sera was added diluted 1/200 in PBS/0·1% Tween-20/1% BSA for 45 minutes at RT. Wells were washed with 200 µl PBS/0·2% Tween-20 and 100 µl of the secondary HRP conjugated antihuman IgG or IgM antibody (Dako), diluted 1/1000 in PBS/0·1% Tween-20/1% BSA, added for a further 45 minutes at RT. After a final three washes in BS/0·2% Tween-20 OPD (Sigma) was added and the reaction stopped with 50 µl 1 m H2SO4. Plates were read at 450 nm.
Results
Primary immunoscreen
DNA sequencing of immunoreactive clones revealed no overlap or duplication and in fact produced a diverse list of candidate antigens which have not yet been described in TxCAD (Table 1). All the clones identified are cytoplasmic, show diverse expression and are not specific for endothelial cells.
Table 1.
Identity of immunoreactive clones
| Clone name[ref.] | Protein | MW (kDa):pI | Identity | Region of identity (bp) |
|---|---|---|---|---|
| M1 [29] | lysyl tRNA synthetase | 68·034: 5·94 | 94% | 1577–1997 (421) |
| M2 [30] | TANK | 47·799: 5·46 | 99% | 1342–1980 (640) |
| W1 [31] | β-transducin | 35·077: 7·60 | 99% | 80–449 (370) |
| W2 [32] | RPL7 | 29·266: 10·66 | 99% | 334–810 (475) |
| W3 | Unkown (AL110194) | – | 99% | 351–840 (490) |
| W5 [33] | RPL9 | 21·863: 9·96 | 98% | 93–706 (614) |
All the clones isolated consisted of truncated sequences including the coding region and a portion of untranslated region. BLAST homology searches indicated the identity of the clone with greater than 90%. Of note, all the truncated clones mapped to the 3′ region of expressed mRNA, with the exception of β-transducin, which mapped to the 5′ region. One clone, W3 (Table 1) approximately 700 bp matched an unknown cDNA (GenBank accession number AL110194) with 99% homology. The next significant match for this sequence was to a human genomic sequence (GenBank accession number AL031274) containing a high mobility group protein 1 (HMG1) pseudogene. Attempts to correlate the sequence of clone W3 with the human HMG1 sequence (GenBank accession number X12597) showed no significant similarities. The closest non-human sequence producing a significant match with clone W3 was pig HMG1(M21683), which gave 90% identity over 393 base pairs; however, this region of M21683 (bases 1786–2178) resides outside the coding sequence (coding sequence 9–656). At present we are investigating further the nature of clone W3. However, it should be noted that HMG1 has been reported to be a perinuclear antineutrophil cytoplasmic antibody (pANCA) autoantigen in autoimmuine hepatitis [24], rheumatoid arthritis, systemic lupus erythematosis (SLE), Sjogren's syndrome (SS), systemic sclerosis (SS) [25] and ulcerative colitis [26]. Furthermore, there is some evidence that HMG1 may be expressed on the surface of murine erythroleukaemia cells and that binding of anti-HMG1 to these cells can interfere with cell differentiation [27].
None of the genes identified in this screen have been described previously in TxCAD, and apart from RPL7 have not been described as antigens in any other disease. The identification of RPL7 is of particular interest as it has been described as an autoantigen in systemic lupus erythematosis (SLE) and scleroderma with a prevalence of 34% and 41%, respectively [28].
Comparison of the predicted MW and pI of these candidates (Table 1) with published data on TxCAD antigens identified by Western blotting shows no clear correlation, although a 46-kDa band in 17·6% of non-CAD sera was reported [19] which is in the molecular weight range for TANK. Comparison with 2D-SDS PAGE analysis shows a potential correlation of a 28-kDa immunoreactive protein found in TxCAD sera with RPL7 (predicted MW 29·2 kDa). In this study [20] the 28 kDa band was resolved into four spots, three of which were identified as triose-phosphate isomerase (predicted MW 26·5 kDa). It should, however, be noted that the isoelectric resolving range in these studies (pH 4–7) did not include proteins in the region of pI 10–11.
ELISA screening
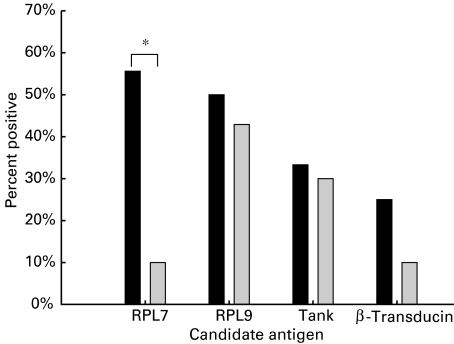
Recombinant his-tagged fusion proteins for TANK, β-transducin, RPL7 and RPL9 were constructed for further study using the pET15b vector (Novagen) and purified as inclusion bodies using nickel affinity chromatography. Prevalence of seropositivity among the CAD and non-CAD groups is shown in Fig. 1. The highest prevalence in the CAD group was to RPL7 (55·6%) and showed a significant association with the disease when compared to the non-CAD group (10%; P < 0·05 Fisher's exact test). In the non-CAD group the highest prevalence was to RPL9 (42·9%), although levels were similar to the CAD group (50·0%). Prevalence to the remaining antigens could be measured, although no clear distinction between CAD and non-CAD groups could be found. For RPL7 the majority of CAD sera (60%) were IgG positive with 20% showing either IgM or IgG/IgM reactivity (Table 2). In the non-CAD group only one serum (IgG and IgM) showed reactivity to RPL7. Notably, in the CAD group 100% of anti-RPL9 positive sera were IgM whereas 67% of the non-CAD group were IgM and 33% IgG. Binding of CAD sera to RPL7 showed a 2·010 ± 0·910 s.d. (IgG) and 1·897 ± 0·202 (IgM)-fold increase above the mean O.D. value for the normal range. Average O.D. values for normal control sera were between 0·246 and 0·625 (IgG) and 0·342–0·593 (IgM).Two patients who tested positive for RPL7 antibodies in this initial screen were selected to determine whether the antibody was present or absent prior to transplantation. In one patient RPL7 antibodies were absent prior to transplantation (see Fig. 2a). Notably, RPL7 antibodies were present in the other patient (data not shown), indicating clearly that in some cases these antibodies may be present prior to transplantation. Whether the presence of these antibodies correlates with pretransplant diagnosis is currently under investigation.
Fig. 1.
The percentage of patients in each group showing positive immunoreactivity to each of the candidate antigens is shown. Sera were judged as positive when the mean O.D. of duplicate wells exceeded the mean plus two standard deviations of a normal range of control sera (n = 11) run in parallel with the assay. A significant association of immuno-reactivity to RPL7 and the presence of CAD is indicated by the asterisk (Fisher's exact test P < 0·05). ▪, CAD (n = 9); ▪, non-CAD (n = 10). *P < Fisher's exact test.
Table 2.
Antibody class of positive sera to candidate antigens
| Antigen | ||||||||
|---|---|---|---|---|---|---|---|---|
| RPL7 | RPL9 | TANK | β-transducin | |||||
| Antibody class | CAD | Non-CAD | CAD | Non-CAD | CAD | Non-CAD | CAD | Non-CAD |
| IgM | 20% | – | 100% | 67% | – | 67% | – | – |
| IgG | 60% | – | – | 33% | 33% | – | 100% | 100% |
| IgM + IgG | 20% | 100% | – | – | 67% | 33% | – | – |
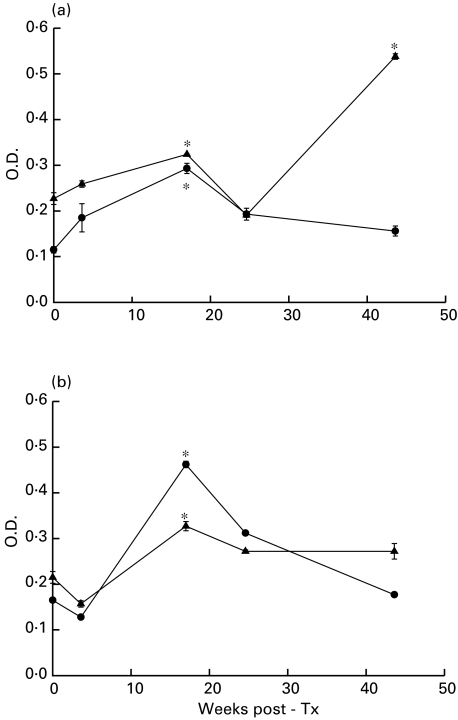
Fig. 2.
Anti-RPL7 (a) and anti-HUVEC cell surface antibodies (b) were measured by ELISA using stored sequential sera from a patient who developed RPL7 antibodies following transplantation. A significant increase in both anti-RPL7 and anti-HUVEC antibodies is seen at week 17 during which time the patient suffered a rejection episode. At 44 weeks post-transplant levels of anti-HUVEC antibodies have receded to pretransplant levels although anti-RPL7 (IgG) are significantly elevated. This patient was diagnosed with CAD at week 56. The normal O.D. range for anti-RPL7 antibodies in this experiment was IgG 0·175 (s.d. 0·043) IgM 0·166 (s.d. 0·037) and for anti-HUVEC IgG 0·190 (s.d. 0·037) IgM 0·163 (s.d. 0·055). Asterisks indicate a significant increase in O.D. relative to pretransplant levels (P < 0·05 Student's t-test). ▴, IgG; •, IgM. P < 0·05 t-test.
Sequential sera and correlation with AECA
The presence of antibody to self-components may be only associated with disease if there is a correlation between antibody titre and disease activity. One patient with TxCAD and RPL7 antibodies was selected and sequential sera identified from the first year following transplantation. Anti-RPL7 and anti-HUVEC antibodies (IgG and IgM) were assayed in parallel (Fig. 2a). This patient was negative for RPL7 prior to transplantation, compared to normal sera. At 17 weeks following transplantation anti-RPL7 antibodies (both IgG and IgM) were significantly elevated compared to pretransplant levels (Fig. 2a). During this period the patient experienced a rejection episode which resolved with immunosuppression. At 24 weeks post-transplantation anti-RPL7 levels receded to those comparable with pretransplant. At 44 weeks post-transplant, levels of anti-RPL7 again increased significantly (IgG but not IgM) compared to pretransplant levels. At the 1-year angiography this patient was diagnosed with TxCAD (> 25% arterial stenosis).
Although fluctuations in anti-RPL7 antibodies apparently correlated with anti-HUVEC antibodies (IgG and IgM) at 17 weeks (Fig. 2b), this was not seen at 44 weeks. At 17 weeks a significant increase in anti-HUVEC antibodies (IgG and IgM) was seen, compared to pretransplant levels which also receded following the rejection episode. However, at 44 weeks when anti-RPL7 antibodies had risen again, levels of anti-HUVEC antibodies were still at levels comparable to pretransplant.
In summary, significant increases in IgG and IgM anti-RPL7 antibodies appeared in this patient following transplantation, which correlated with the onset and remission of a single rejection episode. Following this period the patient developed increased IgG anti-RPL7 antibody levels which preceded the diagnosis of TxCAD. These fluctuations in anti-RPL7 antibodies do not correlate with anti-HUVEC antibodies.
Discussion
This study set out to evaluate the applicability of expression cloning for the identification of candidate antigens associated with TxCAD. This method offers several advantages over proteomic analysis in that candidate immunoreactive antigens can be identified from clonal precursors and screened rapidly to homogeneity for identification by DNA sequencing and database homology searches. The disadvantages of the technique are that elimination of antigens which are common to both TxCAD and non-CAD transplant recipients is problematic and can only be performed reliably following production of recombinant antigen and serological investigations by ELISA. Furthermore, expression in a prokaryotic system will exclude antigen/antibody interactions dependent on eukaryotic specific glycosylation. None the less, we were successful in identifying six immunoreactive clones of which one, RPL7, showed a significant association with TxCAD. The identification of this antigen as a significant autoantigen in SLE and scleroderma [28] raises the possibility that TxCAD may share immunological aspects of autoimmune mixed connective tissue disease. In the study by Neu et al. attempts to correlate anti-RPL7 titre with clinical features of SLE identified a potential negative correlation with vasculitis and lung fibrosis. However, the inclusion of cardiovascular involvement, a known risk association with SLE which may represent a fivefold increased risk [29], was not included in this study.
At least 75 ribosomal proteins have been identified so far and it has been estimated that in total they constitute approximately 10% of the cell mass [30]. Consequently it has been mooted that the appearance of antibodies to these proteins reflects the abundance of antigen available for immune activation. The majority of the ribosomal proteins show a cytoplasmic localization; RPL7, however, is one of the few ribosomal proteins to also be found in the nucleus. Furthermore, in this study we also identified RPL9 as a candidate antigen but, although prevalent in both CAD and non-CAD groups, did not show the same degree of specificity for TxCAD as RPL7. A similar discordance between antibodies to RPL7 and the classical lupus ribosomal P protein has been noted in SLE [28]. These findings would suggest that distinct mechanisms other than mass action effects may operate in the sensitization to RPL7. In support of this, studies on apoptotic keratinocytes have identified the presence of fragmented endoplasmic reticulum and ribosomes in a population of cell surface blebs [31]. This demonstrates that cytosolic/nuclear antigens may be reorganized during apoptosis and potentially influence the manner in which the immune system detects and processes these antigens. Recently a macrophage receptor specific for negatively charged phospholipids exposed on the outer leaflet of apoptotic cells has been cloned [32]. It is likely that macrophages will detect these expressed on the outer membrane of blebs, which are rich in endoplasmic reticulum and ribosomes. Whether these bleb structures are processed in a particular antigenic form remains to be elucidated.
Several groups have examined the potential role of the related ribosomal protein P0 antigen in the pathogenesis of SLE. Affinity purified antiribosomal P0 antibodies have been reported to bind the surface of HUVEC [33–38] and plasma membranes of hepatoma cells [39], although this latter study did not differentiate between the cell surface membrane and endoplasmic reticulum. Furthermore, antiribosomal P0 antibodies have been reported to penetrate live hepatocytes and cause inhibition of protein synthesis [40], although it should be noted that the levels of antibodies used in these studies (5–10 mg/ml) would suggest that the effect is likely to be mediated by passive mechanisms rather than specific antigen/antibody interactions. The results reported in this study do not support a role for cell surface expression of RPL7, as AECA staining did not correlate with RPL7 titre in patient sera.
The prevalence of the remaining antigens identified in this study indicates the presence of antibody titres in excess of that seen in the normal population in cardiac transplant recipients irrespective of whether they develop TxCAD. The appearance of common antibodies to other proteins has been reported previously in CAD and non-CAD groups and suggests that these specificities occur as a consequence of transplantation, due presumably to the release of cytoplasmic antigens in the context of trauma induced inflammation during and after surgery. The lack of a correlation of these other antigens with TxCAD would suggest, therefore, that sensitization to RPL7 may either predispose a proportion of those recipients with pre-existing antibodies, or those who develop them following surgery, to the disease. Currently there is controversial evidence concerning the potential role of these antibodies directly in the pathogenesis of the disease. Alternative considerations regarding the association of these antibodies are likely to reflect distinct cellular and immunological pathomechanisms specific to this disease process.
In animal models sensitization to cardiac myosin accelerates rejection in an organ-specific manner [41]. Although RPL7 shows a diverse tissue expression the association of anti-RPL7 with vascular disease in SLE and scleroderma may be consistent with the vascular anomalies observed in TxCAD. Clonal expansion of RPL7 specific B cells may potentiate the capture of antigen released from damaged endothelium via B cell surface Ig. In line with this is the known association of acute rejection episodes with TxCAD [17]. The relative lack of RPL7 antibodies in the non-CAD group may therefore reflect less endothelial cell damage.
A further consideration in the context of allotransplantation is the possibility that RPL7 may act as a minor histocompatibility antigen. There are currently five sequences for this gene in the database which by comparison show single nucleotide substitutions. These are sufficient to cause amino acid changes which may enable them to be perceived as non-self if they are present in the donor organ. We are currently investigating by SSCP whether polymorphisms can be identified for this gene.
Acknowledgments
This work was supported by a British Heart Foundation programme grant RG/96003, and the Welcome Trust.
References
- 1.Billingham ME. Histopathology of graft coronary disease. J Heart Lung Transplantation. 1992;11:S38–S44. [PubMed] [Google Scholar]
- 2.Lou H, Zhao Y, Delafontaine P, et al. Estrogen effects on insulin-like growth factor-I (IGF-I)-induced cell proliferation and IGF-I expression in native and allograft vessels. Circulation. 1997;96:927–33. doi: 10.1161/01.cir.96.3.927. [DOI] [PubMed] [Google Scholar]
- 3.Sasaguri S, Eishi Y, Tsukada T, et al. Role of smooth-muscle cells and macrophages in cardiac allograft arteriosclerosis in rabbits. J Heart Transplant. 1990;9:18–24. [PubMed] [Google Scholar]
- 4.Shi Y, O'Brien JE, Jr, Mannion JD, et al. Remodeling of autologous saphenous vein grafts. The role of perivascular myofibroblasts. Circulation. 1997;95:2684–93. doi: 10.1161/01.cir.95.12.2684. [DOI] [PubMed] [Google Scholar]
- 5.Hayry P, Renkonen R, Leszczynski D, et al. Local events in graft rejection. Transplant Proc. 1989;21:3716–20. [PubMed] [Google Scholar]
- 6.Akimoto H, McDonald TO, Weyhrich JT, Thomas R, Rothnie CL, Allen MD. Antibody to CD18 reduces neutrophil and T lymphocyte infiltration and vascular cell adhesion molecule-1 expression in cardiac rejection. Transplantation. 1996;61:1610–7. doi: 10.1097/00007890-199606150-00011. [DOI] [PubMed] [Google Scholar]
- 7.Weis M, von Scheidt W. Cardiac allograft vasculopathy: a review. Circulation. 1997;96:2069–77. doi: 10.1161/01.cir.96.6.2069. [DOI] [PubMed] [Google Scholar]
- 8.Ross R, Glomset JA. The pathogenesis of atherosclerosis. N Engl J Med. 1976;295:369–77. doi: 10.1056/NEJM197608122950707. [DOI] [PubMed] [Google Scholar]
- 9.Ross R, Glomset JA. The pathogenesis of atherosclerosis. N Engl J Med. 1976;295:420–5. doi: 10.1056/NEJM197608192950805. [DOI] [PubMed] [Google Scholar]
- 10.Duquesnoy RJ, Demetris AJ. Immunopathology of cardiac transplant rejection. Curr Opin Cardiol. 1995;10:193–206. doi: 10.1097/00001573-199503000-00014. [DOI] [PubMed] [Google Scholar]
- 11.Rabinovitch M, Molossi S, Clausell N. Cytokine-mediated fibronectin production and transendothelial migration of lymphocytes in the mechanism of cardiac allograft vascular disease: efficacy of novel therapeutic approaches. J Heart Lung Transplant. 1995;14:S116–23. [PubMed] [Google Scholar]
- 12.Geraghty JG, Stoltenberg RL, Sollinger HW, Hullett DA. Vascular smooth muscle cells and neointimal hyperplasia in chronic transplant rejection. Transplantation. 1996;62:502–9. doi: 10.1097/00007890-199608270-00013. [DOI] [PubMed] [Google Scholar]
- 13.Reed EF, Hong B, Ho E, Harris PE, Weinberger J, Suciu-Foca N. Monitoring of soluble HLA alloantigens and anti-HLA antibodies identifies heart allograft recipients at risk of transplant-associated coronary artery disease. Transplantation. 1996;61:566–72. doi: 10.1097/00007890-199602270-00009. [DOI] [PubMed] [Google Scholar]
- 14.Barr ML, Cohen DJ, Benvenisty AI, et al. Effect of anti-HLA antibodies on the long-term survival of heart and kidney allografts. Transplant Proc. 1993;25:262–4. [PubMed] [Google Scholar]
- 15.Suciu-Foca N, Reed E, Marboe C, et al. The role of anti-HLA antibodies in heart transplantation. Transplantation. 1991;51:716–24. doi: 10.1097/00007890-199103000-00033. [DOI] [PubMed] [Google Scholar]
- 16.Smith JD, Rose ML, Pomerance A, Burke M, Yacoub MH. Reduction of cellular rejection and increase in longer-term survival after heart transplantation after HLA-DR matching. Lancet. 1995;346:1318–22. doi: 10.1016/s0140-6736(95)92341-1. [DOI] [PubMed] [Google Scholar]
- 17.Hornick P, Smith J, Pomerance A, et al. Influence of acute rejection episodes, HLA matching, and donor/recipient phenotype on the development of ‘early’ transplant-associated coronary artery disease. Circulation. 1997;96:II–148–53. [PubMed] [Google Scholar]
- 18.Dunn MJ, Crisp SJ, Rose ML, Taylor PM, Yacoub MH. Anti-endothelial antibodies and coronary artery disease after cardiac transplantation. Lancet. 1992;339:1566–70. doi: 10.1016/0140-6736(92)91832-s. [DOI] [PubMed] [Google Scholar]
- 19.Crisp SJ, Dunn MJ, Rose ML, Barbir M, Yacoub MH. Antiendothelial antibodies after heart transplantation: the accelerating factor in transplant-associated coronary artery disease? J Heart Lung Transplant. 1994;13:81–91. [PubMed] [Google Scholar]
- 20.Wheeler CH, Collins A, Dunn MJ, Crisp SJ, Yacoub MH, Rose ML. Characterization of endothelial antigens associated with transplant-associated coronary artery disease. J Heart Lung Transplant. 1995;14:S188–97. [PubMed] [Google Scholar]
- 21.Jurcevic S, Dunn MJ, Crisp S, et al. A new enzyme-linked immunosorbent assay to measure anti-endothelial antibodies after cardiac transplantation demonstrates greater inhibition of antibody formation by tacrolimus compared with cyclosporine. Transplantation. 1998;65:1197–202. doi: 10.1097/00007890-199805150-00010. [DOI] [PubMed] [Google Scholar]
- 22.Jurcevic S, Ainsworth ME, Pomerance A, et al. Antivimentin antibodies are an independent predictor of transplant-associated coronary artery disease after cardiac transplantation. Transplantation. 2001;71:886–92. doi: 10.1097/00007890-200104150-00011. [DOI] [PubMed] [Google Scholar]
- 23.Ferry BL, Welsh KI, Dunn MJ, et al. Anti-cell surface endothelial antibodies in sera from cardiac and kidney transplant recipients: association with chronic rejection. Transpl Immunol. 1997;5:17–24. doi: 10.1016/s0966-3274(97)80021-4. [DOI] [PubMed] [Google Scholar]
- 24.Sobajima J, Ozaki S, Uesugi H, et al. High mobility group (HMG) non-histone chromosomal proteins HMG1 and HMG2 are significant target antigens of perinuclear anti-neutrophil cytoplasmic antibodies in autoimmune hepatitis. Gut. 1999;44:867–73. doi: 10.1136/gut.44.6.867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Uesugi H, Ozaki S, Sobajima J, et al. Prevalence and characterization of novel pANCA, antibodies to the high mobility group non-histone chromosomal proteins HMG1 and HMG2, in systemic rheumatic diseases. J Rheumatol. 1998;25:703–9. [PubMed] [Google Scholar]
- 26.Sobajima J, Ozaki S, Uesugi H, et al. Prevalence and characterization of perinuclear anti-neutrophil cytoplasmic antibodies (P-ANCA) directed against HMG1 and HMG2 in ulcerative colitis (UC) Clin Exp Immunol. 1998;111:402–7. doi: 10.1046/j.1365-2249.1998.00491.x. 10.1046/j.1365-2249.1998.00491.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Passalacqua M, Zicca A, Sparatore B, Patrone M, Melloni E, Pontremoli S. Secretion and binding of HMG1 protein to the external surface of the membrane are required for murine erythroleukemia cell differentiation. FEBS Lett. 1997;400:275–9. doi: 10.1016/s0014-5793(96)01402-0. 10.1016/S0014-5793(96)01402-0. [DOI] [PubMed] [Google Scholar]
- 28.Neu E, von Mikecz AH, Hemmerich PH, et al. Autoantibodies against eukaryotic protein L7 in patients suffering from systemic lupus erythematosus and progressive systemic sclerosis: frequency and correlation with clinical, serological and genetic parameters. The SLE Study Group. Clin Exp Immunol. 1995;100:198–204. doi: 10.1111/j.1365-2249.1995.tb03653.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bruce IN, Gladman DD, Urowitz MB. Premature atherosclerosis in systemic lupus erythematosus. Rheum Dis Clin North Am. 2000;26:257–78. doi: 10.1016/s0889-857x(05)70138-1. [DOI] [PubMed] [Google Scholar]
- 30.Kenmochi N, Kawaguchi T, Rozen S, et al. A map of 75 human ribosomal protein genes. Genome Res. 1998;8:509–23. doi: 10.1101/gr.8.5.509. [DOI] [PubMed] [Google Scholar]
- 31.Casciola-Rosen LA, Anhalt G, Rosen A. Autoantigens targeted in systemic lupus erythematosus are clustered in two populations of surface structures on apoptotic keratinocytes. J Exp Med. 1994;179:1317–30. doi: 10.1084/jem.179.4.1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fadok VA, Bratton DL, Rose DM, Pearson A, Ezekewitz RA, Henson PM. A receptor for phosphatidylserine-specific clearance of apoptotic cells. Nature. 2000;405:85–90. doi: 10.1038/35011084. [DOI] [PubMed] [Google Scholar]
- 33.Yoshio T, Masuyama J, Kano S. Antiribosomal P0 protein antibodies react with the surface of human umbilical vein endothelial cells. J Rheumatol. 1996;23:1311–2. [PubMed] [Google Scholar]
- 34.Shiba K, Stello T, Motegi H, Noda T, Musier Forsyth K, Schimmel P. Human lysyl-tRNA synthetase accepts nucleotide 73 variants and rescues escherichia coli double-defective mutant. J Biol Chem. 1997;272:22809–16. doi: 10.1074/jbc.272.36.22809. [DOI] [PubMed] [Google Scholar]
- 35.Kaye KM, Devergne O, Harada JN, et al. Tumor necrosis factor receptor associated factor 2 is a mediator of NF-kappa B activation by latent infection membrane protein 1, the Epstein–Barr virus transforming protein. Proc Natl Acad Sci USA. 1996;93:11085–90. doi: 10.1073/pnas.93.20.11085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Guillemot F, Billault A, Auffray C. Physical linkage of a guanine nucleotide-binding protein-related gene to the chicken major histocompatibility complex. Proc Natl Acad Sci USA. 1989;86:4594–8. doi: 10.1073/pnas.86.12.4594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Seshadri T, Uzman JA, Oshima J, Campisi J. Identification of a transcript that is down-regulated in senescent human fibroblasts. Cloning, sequence analysis, and regulation of the human L7 ribosomal protein gene. J Biol Chem. 1993;268:18474–80. [PubMed] [Google Scholar]
- 38.Mazuruk K, Schoen TJ, Chader GJ, Iwata T, Rodriguez IR. Structural organization and chromosomal localization of the human ribosomal protein L9 gene. Biochim Biophys Acta. 1996;1305:151–62. doi: 10.1016/0167-4781(95)00201-4. [DOI] [PubMed] [Google Scholar]
- 39.Koren E, Reichlin MW, Koscec M, Fugate RD, Reichlin M. Autoantibodies to the ribosomal P proteins react with a plasma membrane-related target on human cells. J Clin Invest. 1992;89:1236–41. doi: 10.1172/JCI115707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Koscec M, Koren E, Wolfson-Reichlin M, et al. Autoantibodies to ribosomal P proteins penetrate into live hepatocytes and cause cellular dysfunction in culture. J Immunol. 1997;159:2033–41. [PubMed] [Google Scholar]
- 41.Fedoseyeva EV, Zhang F, Orr PL, Levin D, Buncke HJ, Benichou G. De novo autoimmunity to cardiac myosin after heart transplantation and its contribution to the rejection process. J Immunol. 1999;162:6836–42. [PubMed] [Google Scholar]