Abstract
The splicing isoform of HLA-G that is expressed in xenogeneic cells, and its effect on NK-mediated direct cytotoxicity was examined, using stable Chinese hamster ovary (CHO) cell or swine endothelial cell (SEC) transfectants. cDNAs of HLA-G (G1 and G3) and human β2-microglobulin were prepared and subcloned into the expression vector, pCXN. The transfected HLA-G1 was easily expressed on SEC, and co-transfection with human β2-microglobulin led to an enhanced level of HLA-G1 expression, as evidenced by flow cytometry. The expressed HLA-G1 significantly suppressed NK-mediated SEC cell lysis, which is an in vitro delayed-type rejection model of a xenograft. On the other hand, the swine leucocyte antigen (SLA) class I molecules could be up-regulated as the result of the transfection of human β2-microglobulin, but did not down-regulate human NK-mediated SEC lysis. The HLA-G3 was not expressed on CHO and SEC in contrast to HLA-G1, as the result of the transfection. The gene introduction of HLA-G3 in SEC showed no protective effect from human NK cells. However, indirect evidence demonstrated that HLA-G3 transfection resulted in HLA-E expression, but not itself, when transfected to the human cell line, 721.221, thus providing some insight into its natural function in human cells. The present findings suggest that the expression of HLA-G1 on the cell surface could serve as a new approach to overcoming NK-mediated immunity to xenografts.
Keywords: MHC, transplantation, NK cells, endothelial cells
Introduction
The worldwide shortage of available organs for human transplantation has led to a revived interest in xenotransplantation [1,2]. The swine represents an ideal source of such organs because of their plentiful supply and their many anatomical and physiological similarities to the human.
While trials directed at the prevention of hyperacute rejection have been carried out in many institutes [3], the inhibition of complement in models of xenotransplantation has resulted in a delayed form of rejection [4], which is characterized by endothelial cell activation and mononuclear cell infiltration by NK cells and monocytes. In addition, an increasing body of evidence suggests that NK cells play a critical role in swine to human xenotransplantation [5].
In previous studies, we reported that the genetic remodelling of the α-galactosyl epitope (Galα1,3Galβ1,4GlcNAc-R), and probably other glycoantigens as well, on a xenograft represents one possible approach for achieving the down-regulation of NK-mediated lysis [6,7].
However, another possible strategy for inhibiting NK cell activity in lysis [8] or migration [9] involves the expression of the human MHC class I antigen on the target cell. NK cells are capable of lysing cells in which the surface expression of MHC class I molecules is either absent or altered. Among others, the role of MHC class I molecules on the cell surface is to provide signals for the inhibition of NK cell-mediated cytotoxicity via their interaction with NK inhibitory receptors, such as CD94/NKG2A, KIRs and ILTs [10].
HLA-G is a non-classical major histocompatibility complex class I molecule, which is selectively expressed on cytotrophoblasts at the fetal–maternal interface, where it plays a role in materno-fetal tolerance. Thus, the fetus is protected from maternal NK cell cytolysis via the expression of HLA-G on the trophoblast [11]. In contrast to classic HLA class I molecules, which can be recognized by alloreactive T cells, HLA-G is characterized by a reduced polymorphism [12], and by the varied transcripts of alternative spliced mRNAs, resulting in at least six different HLA-G mRNAs which potentially encode six HLA-G isoforms, namely the HLA-G1, HLA-G2, HLA-G3 and HLA-G4 membrane-bound isoforms, as well as two soluble HLA-G5 and HLA-G6 forms [13].
The present study describes an investigation of the effect of HLA-G1 and G3 on NK-mediated direct cytotoxicity, using stable Chinese hamster ovarian tumour (CHO) cells or swine endothelial cell (SEC) transfectants expressing these molecules.
Materials and methods
Cell culture
The SEC line, MYP30 [14], was cultured in Dulbecco's modified Eagle's medium. CHO cells and a classical HLA class I-deficient B-lymphoblast cell line, 721.221, were obtained from the American Type Culture Collection (Bethesda, MD) and cultured in Ham's F12 and RPMI1640 media, respectively. These media were supplemented with 10% heat-inactivated fetal bovine serum (FBS), l-glutamine and kanamycin/amphotericin B (Gibco/BRL). Cultures were maintained in a 5% CO2/95% air atmosphere at 37°C.
Effector cells
Fresh whole blood, obtained from healthy volunteers, was diluted in 1:1 in PBS, overlaid on Lymphoprep (NYCOMED, Oslo, Norway) and then centrifuged at 800 g for 30 min. Peripheral blood mononuclear cells (PBMC) were sampled from the monolayer and washed twice in PBS.
The NK-like cell line, YT cells, kindly provided by Drs J. Yodoi and K. Teshigawara (University of Kyoto, Japan) [15], was maintained in RPMI-1640 medium which contained 10% heat-inactivated FBS, l-glutamine and kanamycin/amphotericin B.
Construction
The cDNAs of HLA-G (G1 and G3) and human β2-microglobulin (hβ2m) were molecular cloned from the cDNA mixture prepared from JEG-3 cell mRNA and a human liver cDNA library (Clontech), respectively, by the PCR technique: 5′ primer for G1 and G3, 5′-TTTCCCGGGATGGTGGTCATGGCGCCC -3′ 3′primer for G1 and G3, 5′-CCCCTCGAGAGCCT GAGAGTAGCTCCC-3′ 5′ primer for hβ2m, 5′-CCCCCCGGGATGTCTCGCTCCGTGGCC-3′; 3′ primer for hβ2m, 5′-CCCCTCGAGGCTTACATGTCTCGATCCCA-3′. HLA-G1 and G3 were subcloned into the pCXN vector, in which chicken β-actin promoter and human cytomegalovirus enhancer drove the transcription of the inserted cDNA and contained the neomycin-resistant gene [16]. The hβ2m was also subcloned into its derivatives with the drug-resistant gene, pCXBL (blasticidin S) and pCXPL (puromycin) vectors. A synthetic oligonucleotide that encodes for the FLAG epitope was added immediately after the signal sequence of HLA-G1 and G3 [17]. The cDNA of HLA-G1 with FLAG (FG1), and HLA-G3 with FLAG (FG3), were then established and subcloned into the pCXN vector. The plasmid was separately transformed into Escherichia coli DH5α and amplified using standard techniques.
Expression of cDNAs
The cDNAs (20 µg) were introduced into CHO cells by electroporation and MYP-30 by lipid-mediated DNA transfection with lipofectamine (Gibco/BRL). Transfected cells were maintained in a complete medium for several days in an atmosphere of humidified 5% CO2 at 37°C. The cells were then transferred to a selection medium, which contained 0·7 mg/ml G418 (Calbiochem), 10 µg/ml blasticidin S (Funakoshi, Japan) or 10 µg/ml puromycin (Sigma) for selection [14]. CHO and SEC transfectants were isolated by the limiting dilution method. The cell surface expressions of HLA-G1 and hβ2m on the stable transfectants were confirmed by FACS analysis, and HLA-G3 was checked by Northern blot analysis.
Flow cytometry
Transfected cells (1 × 106) were incubated with a mouse monoclonal antibody (MoAb) against HLA-class I, B9.12.1 (CosmoBio) and control IgG2a (Cappel ICN) (4 µg/ml), or PA2.6 (ATCC) and control IgG1 (Cappel ICN) (10 µg/ml), a mouse MoAb anti-hβ2m, BM-010 (NBL Japan) and control IgG1 (Cappel ICN) (10 µg/ml), or a mouse MoAb against the swine leucocyte antigen (SLA), 74–11–10 (VMRD) and control IgG2b (Cappel ICN) (20 µg/ml), for 30 min at 4°C, and subsequently incubated with an FITC-labelled rabbit anti-mouse IgG secondary antibody (Cappel ICN) (40 µg/ml) for 30 min at 4°C. The stained cells were analysed using a FACS Calibur flow cytometer (Becton Dickinson). Naive CHO cells and MYP-30 were used as controls. In addition, a mouse anti-FLAG MoAb, M2 (Sigma), and control IgG1 were used for the detection of the FLAG-tagged protein as a first antibody (10 µg/ml). In each assay, a high MoAb concentration was used to ensure the stability of the antibody reaction.
Immunoblotting
The protein content of transfectants and naive cell lysates was quantified by the BCA method (Pierce), and 40 µg aliquots were subjected to 10% SDS-PAGE under reducing conditions [18]. The separated proteins were then electrophoretically transferred to a nitrocellulose membrane (Schleicher & Schuell, Germany). The membrane was blocked with 5% skim milk in Tris-buffered saline/0·05% Tween 20 (TBST) for 1 h at 25°C and then incubated in 1% BSA/0·5% skim milk/TBST with the rat anti-HLA-G α1 domain antibody at a 1:10 dilution for 1 h at 25°C. This antibody was generated by immunizing a rat with a synthetic peptide corresponding to amino acids 61–83 of the α1 domain of HLA-G, EEETRNTKAHAQTDRMNLQ [19]. After washing, the blots were incubated with a horseradish peroxidase conjugated rabbit anti-rat IgG secondary antibody (Cappel ICN) at a 1:500 dilution and the signal was developed using an ECL detection system (Amersham, UK).
Northern blotting
Total RNA was isolated from the transfectants and naive cells with TRIZOL (Gibco/BRL), and separated by electrophoresis (25 µg/lane). The probe used for hybridization was the cDNA restriction fragment of the α1 domain of HLA-G, and the hybridization signal on the Northern blots was evaluated by an ECL detection system (Amersham).
Immunofluorescent staining of transfected cells
CHO and SEC cells were cultured in collagen type I coated chamber slides (Iwaki, Japan) for 2 days. The slides and suspension of 721.221 cells were washed with Dulbecco's PBS (D-PBS) and fixed with 2% paraformaldehyde/D-PBS/0·1% Triton X-100 for cytoplasm staining, or simply with 2% paraformaldehyde/D-PBS for cell-surface staining, for 30 min on ice. The fixed cells were incubated with anti-FLAG monoclonal antibody M2 (Sigma) at a 1:250 dilution (50% FBS/0·6% BSA/D-PBS) for 1 h on ice. After removal of the excess antibody, the slides were reacted with FITC-conjugated rabbit anti-mouse IgG (Cappel ICN) at a 1:250 dilution (50% FBS/0·6% BSA/D-PBS) for 1 h on ice. The slides were viewed with a Zeiss Axioplan 2 universal microscope (Carl Zeiss, Germany).
NK cell-mediated cytotoxicity assay
This was performed using the standard 51Cr release assay. The level of amelioration of NK cell-mediated lysis by the transfectant molecules on CHO or SEC was determined as an in vitro delayed-type rejection model of a discordant xenograft.
Parental or transfected cells were plated at a level of 2 × 104 cells per well in a flat-bottomed, gelatin-coated, 96-well tray 1 day prior to the assay. After 15 h, the plates were incubated with 100 µCi/ml Na251CrO4 for 2 h at 37°C, and then effectors, PBMC or YT cells, at the required E:T ratio. Each assay was performed in triplicate. After 4 h of incubation at 37°C, the released 51Cr was measured. The spontaneous release of 51Cr from the target cells was less than 15% of the maximum release of 51Cr, as determined by treatment with 1% Triton X-100. The results are expressed as percentage of specific lysis [14].
CD94/NKG2 blocking assay by anti-CD94 MoAb
Before the cytotoxicity assay was started, the effecter PBMC were incubated with 10 µg/ml anti-CD94 MoAb, HP-3B1 (Immunotech), or mouse IgG2a isotype control, at 25°C for 30 min. The treated PBMC were then added to each well, with MoAb present in the culture medium during the assay.
Results
Cell surface expression of HLA-G1 molecules
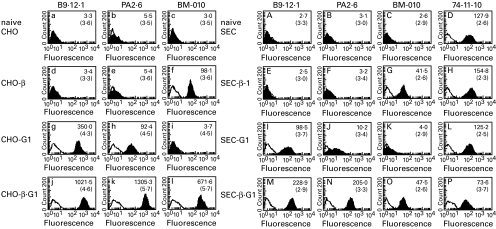
Stable CHO and SEC transfectants with HLA-G1 and/or hβ2m were stained with the following antibodies: B9.12.1 and PA2.6 (anti-HLA class I), BM-010 (anti-hβ2m) and 74–11–10 (anti-SLA), and the results are summarized in Fig. 1.
Fig. 1.
Flow cytometric profile of hβ2m and/or HLA-G1 transfected CHO and SEC. Typical flow cytometric histograms for the HLA-G1 transfectants are shown. Naive CHO (a-c) and SEC (A-D), and stable transfectants, were treated with several specific antibodies (closed histogram), B9.12.1 (a, d, g, j, A, E, I, M), PA2.6 (b, e, h, k, B, F, J, N), BM-010 (c, f, i, l, C, G, K, O) and 74–11–10 (D, H, L, P) and isotype control antibodies (open histogram). The mean shift value of specific antibodies and control antibodies (parenthesis) are indicated in each panel. HLA-G1 expression was up-regulated by co-transfection with hβ2m in CHO-β.G1 (j, k) and SEC-β.G1 (M, N). CHO-β, CHO transfectant with hβ2m; CHO-G1, CHO transfectant with HLA-G1; CHO-β.G1, CHO transfectant with hβ2m and HLA-G1; SEC-β-1, SEC transfectant with hβ2m; SEC-G1, SEC transfectant with HLA-G1; SEC-β.G1, SEC transfectant with hβ2m and HLA-G1.
The additional transfection of hβ2m to CHO-G1 or SEC-G1 enhanced the level of expression several fold (Fig. 1 j, k, M and N).
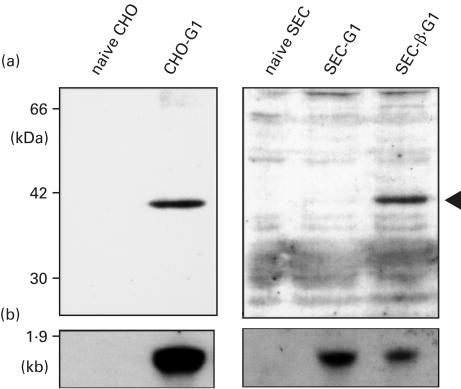
Western blot and Northern blot analysis of the HLA-G1 transfectants
Western blotting was performed in order to ascertain the molecular weight of the HLA-G1 molecule that was expressed in the transfectants, using the rat anti-HLA-G α1 domain serum (Fig. 2a). This analysis clearly revealed the presence of proteins with molecular masses of approximately 38 kDa. However, a faint signal was observed in the SEC transfectant with HLA-G1, SEC-G1, but in the case of the SEC transfectant with hβ2m and HLA-G1, SEC-β.G1, a moderate signal was detected.
Fig. 2.
Western and Northern blot analysis of HLA-G1 in transfectants. (a) Typical Western blots of transfectants with HLA-G1 are shown. A whole cell lysate was analysed by SDS-PAGE and Western blotting with rat polyclonal anti-HLA-G serum. (b) Typical Northern blots of the transfectants with HLA-G1 are shown. The probe used for hybridization was the cDNA restriction fragment of HLA-G α1 domain, and hybridization signals on the Northern blots were evaluated by an ECL detection system. CHO-G1, CHO transfectant with HLA-G1; SEC-G1, SEC transfectant with HLA-G1; SEC-β.G1, SEC transfectant with hβ2m and HLA-G1.
A Northern blotting was also performed in order to determine any alterations in mRNA production, using the HLA-G α1 domain probe (Fig. 2b). While protein production by HLA-G1 in SEC-G1 was weak, as evidenced by Western blotting, the mRNA signal was clear compared with those in the SEC-β.G1. The results also indicated the importance of hβ2m for the effective expression of HLA-G1 protein.
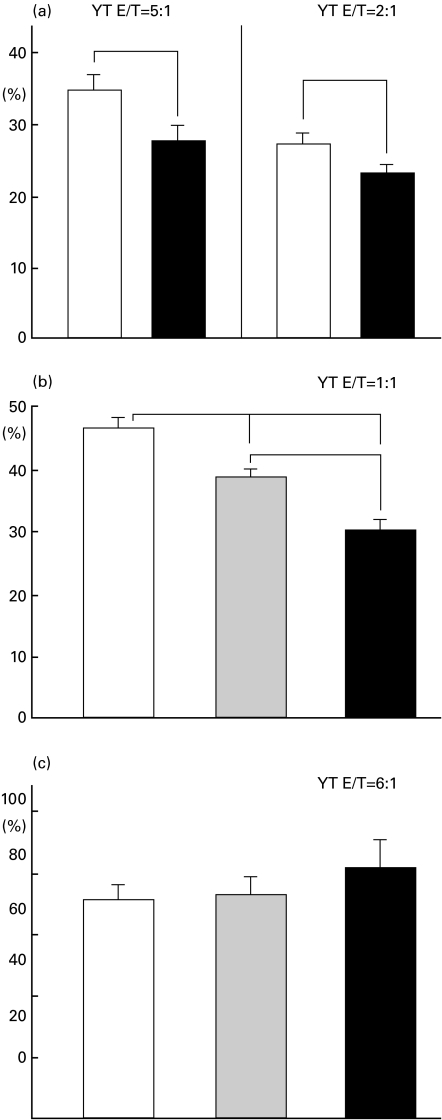
NK cell cytotoxicity assay of transfected CHO and SEC with HLA-G1
The direct NK cell-mediated lysis against CHO or SEC transfectants was assessed. The amelioration of NK cell-mediated lysis by the transfected HLA-G1 was then calculated using standard 51Cr release assay data.
The down-regulation of NK cell-mediated direct killing by the HLA-G1 molecule was observed in these transfectants. HLA-G1 significantly suppressed CHO and SEC lysis by YT cells by about 20% and 35%, respectively (Fig. 3a,b). On the other hand, the up-regulated SLA on SEC by hβ2 m transfection (Fig. 1H) did not lead to the down-regulation of NK-mediated SEC lysis (Fig. 3c). In addition to the data obtained with the YT NK cell line, the killing of SEC mediated by PBMC from several different donors was similarly inhibited by the expression of HLA-G1 compared with the killing control SEC. However, significance could not be reached due to low absolute killing values (data not shown).
Fig. 3.
NK cell-mediated cytotoxicity assay. Amelioration of NK cell-mediated lysis by HLA-G1 molecule on CHO or SEC was tested as an in vitro delayed-type rejection model of a discordant xenograft. (a) Naive CHO and transfected cells were incubated with YT cells at the required E:T ratio. The results are expressed as a percentage of specific lysis. (□) Naïve CHO; (▪) CHO-G1. (b) The naive SEC and transfected cells were incubated with YT cells at the E:T ratio; 1:1. The results are expressed as a percentage of specific lysis. (□) Naïve SEC; (▪) SEC-G1; (▪) SEC-β-G1. (c) The naive SEC and two independent transfected cells with hβ2m were incubated with YT cells at the E:T ratio 6:1. The results are expressed as a percentage of specific lysis. (□) Naïve SEC; (▪) SEC-β-1; (▪) SEC-β-2.Values are presented as mean ± s.e.m. (n = 5–8). indicates a significant difference (P < 0·05). CHO-G1, CHO transfectant with HLA-G1; SEC-G1, SEC transfectant with HLA-G1; SEC-β.G1, SEC transfectant with hβ2m and HLA-G1; SEC-β, SEC transfectant with hβ2m.
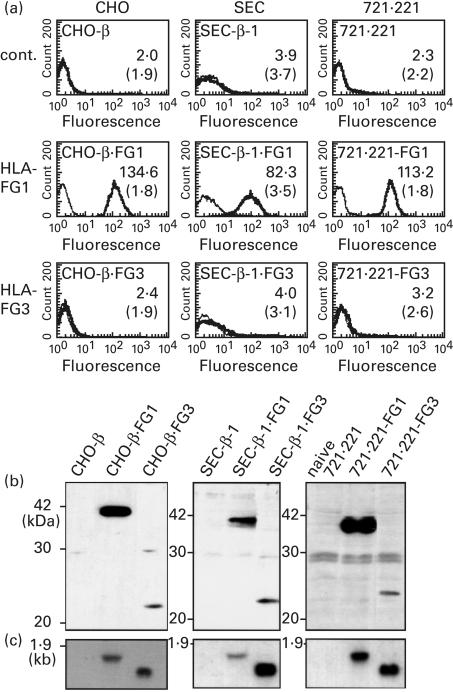
Flow cytometry, Western and Northern blot analysis of FLAG-tagged HLA-G3 transfectants
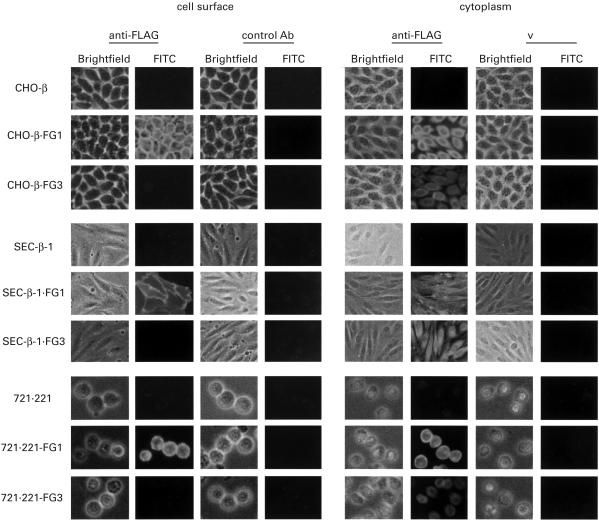
HLA-G3 was transfected into CHO cells and the SEC transfectants with or without hβ2m, and several stable tansfectants that contained HLA-G3 were established. Western and Northern blotting were performed using rat anti-HLA-G1 α1 domain serum or the HLA-G α1 domain DNA probe, respectively. While mRNA production could readily be confirmed, the protein corresponding to HLA-G3 was not identified in all transfectants (data not shown). To investigate the possibility of HLA-G3 expression by xenogenic and allogenic cells further, FLAG-tagged HLA-G1 (FG1) and G3 (FG3) were constructed. The cDNAs of FG1 and FG3 were transfected into the CHO or SEC transfectants with hβ2m (CHO-β or SEC-β-1), and into 721.221 as well. A number of clones with FG1 or FG3 were established and analysed by FACS, Western and Northern blotting using mouse anti-FLAG MoAb or the HLA-G α1 domain nucleotide probe (Fig. 4). Additionally, immunohistochemical staining of the cell surface and cytoplasm were performed in order to confirm FG3 expression (Fig. 5). The FG3 molecule was clearly expressed in the cytoplasm, whereas it was not detected on the cell surface (Figs 4 and 5). In addition, we observed no significant difference in expression levels between HLA-G1 and FG1 transfectants by FACS analysis (data not shown).
Fig. 4.
Flow cytometric profiles, Western and Northern blotting of the FG1 and FG3 transfected cells. (a) Typical flow cytometric histograms for the FG1 and FG3 transfectants are shown. The control cells and stable transfectants with FG1 or FG3 were treated with anti-FLAG antibody (thick line) or isotype antibody (thin line). The mean shift values of each cell for anti-FLAG antibody and isotype antibody (inside parethesis) are indicated in each panel. (b) Western blotting of the transfectants. A whole cell lysate was separated by SDS-PAGE and Western blotting with anti-FLAG MoAb is shown. (c) Northern blottings of the transfectants. The probe used for the hybridization was the cDNA restriction fragment of HLA-G α1 domain.
Fig. 5.
Cell surface and cytoplasmic immunohistochemical staining of FG1 and FG3 transfectants. The parental cells and transfectants were stained with anti-FLAG MoAb and isotype control antibody. Bright-field represents the image of each specimen. CHO-β.FG1, CHO transfectant with hβ2m and FG1; CHO-β.FG3, CHO transfectant with hβ2m and FG3; SEC-β.FG1, SEC transfectant with hβ2m and FG1; SEC-β.FG3, SEC transfectant with hβ2m and FG3; 721.221-FG1, 721.221 transfectant with FG1; 721/221-FG3, 721/221 transfectant with FG3.
The suppressive effect of HLA-G3 was examined using SEC transfectants
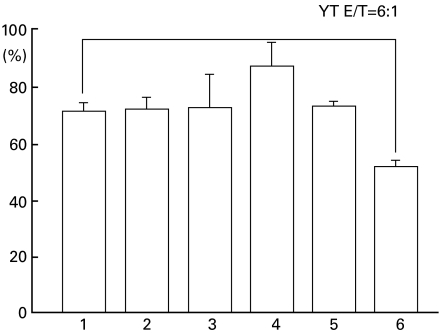
To ascertain if the limited level of HLA-G3 is capable of down-regulating NK cell-mediated cell lysis, a standard 51Cr release assay was performed. While the inhibition of NK-mediated SEC lysis was clearly evident in the HLA-G1 and FG1 transfectants, HLA-G3 and FG3 molecules had no apparent suppressive effect on SEC lysis (Figs 3 and 6).
Fig. 6.
NK cell-mediated cytotoxicity assay using HLA-G3 transfectants. The naive SEC or transfected cells were incubated with YT cell at the E:T ratio 6:1. The results are expressed as a percentage of specific lysis (n = 3). Naive SEC (lane 1); SEC-β-1, SEC with hβ2m (lane 2); SEC-G3, SEC with HLA-G3 (lane 3); SEC-β-1.G3, SEC with hβ2m + HLA-G3 (lane 4); SEC-β-1.FG3, SEC with hβ2m + FG3 (lane 5); SEC-β-1.FG1, SEC with hβ2m + FG1 (lane 6). Values were presented as mean ± s.e.m. Indicates significant difference (P < 0·05).
Additional studies on the function of HLA-G3 in human cells
HLA-G3 function in human cells was also studied. Stable 721.221 transfectants with HLA-G3 were established. Northern blot analysis and NK cell-mediated cytotoxicity assay were performed (Fig. 7a,b). Contrary to the data for the SEC transfectants shown in Fig. 6, the cytotoxicity assay indicated that 721.221 with the HLA-G3 plasmid clearly down-regulated the NK cell-mediated direct cell lysis.
Fig. 7.
FACS, Northern blotting and NK cell-mediated cytotoxicity assay of 721.221 transfectants with HLA-G3. (a) Northern blotting of the transfectants is shown. The probe used for the hybridization was the cDNA restriction fragment of HLA-G α1 domain. (b) NK cell-mediated cytotoxicity assay. The parental 721.221 cell or transfected cells were incubated with PBMC at the E:T ratio 30:1. The results are expressed as a percentage of specific lysis. Values are presented as mean ± s.e.m. (n = 6). (□) Naive 721.221; (▪) 721.221-G3-1; (▪) 721.221-G3-2; (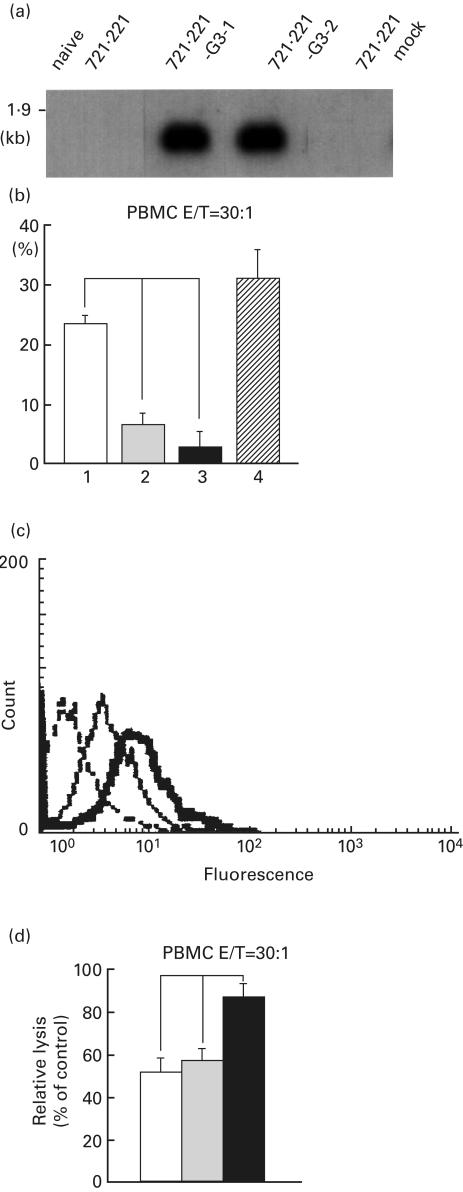 ) 721.221-mock. (c) FACS profiles of 721.221 transfectants with HLA-G3. Naive cell (dotted line) and 721.221 transfectants, 721.221-G3-2, were treated with anti-HLA class I MoAb, B9–12–1 (thick line), or control isotype antibody (thin line). (d) Blocking assay by anti-CD94 MoAb. To determine whether the down-regulatory effect is related to the CD94/NKG2 receptor, NK cell-mediated cytotoxicity assay was performed using HLA-G3 transfected 721.221 cells, 721.221 – G3–2, and mock transfectant, 721.221-mock, as the control. An IgG2a isotype MoAb was also used as a control MoAb. Relative lysis of 721.221 – G3–2 with or without anti-CD94 MoAb is shown compared with the mock transfectant cell line. Values are presented as mean ± s.e.m/ (n = 6). Indicates significant difference (P < 0·05). (□) No antibody; (░) control antibody; (▪) anti-CD94 antibody.
) 721.221-mock. (c) FACS profiles of 721.221 transfectants with HLA-G3. Naive cell (dotted line) and 721.221 transfectants, 721.221-G3-2, were treated with anti-HLA class I MoAb, B9–12–1 (thick line), or control isotype antibody (thin line). (d) Blocking assay by anti-CD94 MoAb. To determine whether the down-regulatory effect is related to the CD94/NKG2 receptor, NK cell-mediated cytotoxicity assay was performed using HLA-G3 transfected 721.221 cells, 721.221 – G3–2, and mock transfectant, 721.221-mock, as the control. An IgG2a isotype MoAb was also used as a control MoAb. Relative lysis of 721.221 – G3–2 with or without anti-CD94 MoAb is shown compared with the mock transfectant cell line. Values are presented as mean ± s.e.m/ (n = 6). Indicates significant difference (P < 0·05). (□) No antibody; (░) control antibody; (▪) anti-CD94 antibody.
The 721.221, which inherently lacks the HLA-A, B, C and G gene, only transcribes HLA-E mRNA, but no expression on the cell surface is observed because of the loss of the appropriate peptide required for binding. However, the HLA-G3 transfectants, 721.221–G3–2, clearly expressed the HLA class I molecule, presumably HLA-E (Fig. 7c).
Furthermore, a blocking analysis of the NK cell-mediated cell lysis was carried out using anti-CD94 antibody (Fig. 7d). The transfectants became susceptible to NK cells as a result of blocking antibody. The experimental data strongly suggest that the suppression of NK-mediated killing by transfection of the HLA-G3 plasmid is caused, not by HLA-G3 itself, but by the HLA-E that is expressed on 721.221 using the HLA-G3 leader peptide.
Discussion
It was previously reported that the introduction of the HLA-G genome to SEC resulted in a significant suppression of NK-mediated lysis, as evidenced by the transient transfection system [8]. However, a detailed examination of the expression of HLA-G1 and other alternative forms in connection with those of hβ2m on xenogeneic cells had not been completed. As a result, the present study was carried out involving the use of several stable transfectants, the focus of which was mainly on two points, one being the relation between HLA-G1 expression and swine or human β2 microglobulin. The other involved the possibility of HLA-G3 expression and its function in xenogenic cells compared with that for HLA-G1.
In this study, HLA-G1 was expressed at a relatively low level on the SEC surface by transfection of HLA-G1 alone, supporting the view that the expression of HLA-G1 involves binding to the β2 microglobulin molecule. Hamster and swine β2m molecules might have some interaction with HLA-G1, in terms of the expression of this molecule. Therefore, it is not surprising that the additional transfection of human β2m enhanced the expression level of the HLA-G1 on CHO cells and SEC. On the other hand, while SLA expression was enhanced by transfection with the hβ2m molecule, an additional transfection with HLA-G1 to SEC-β‐1 reduced SLA expression levels, suggesting that the affinity of hβ2m for SLA is weaker than for HLA (Fig. 1H, P).
Kwiatkowski et al. reported on the increased levels of SLA class I molecules on SEC by the induction of TNF-α, reduced human NK cell-mediated lysis [20]. However, as had been previously shown by a number of researchers [6,7], human NK cells were effective in the direct killing of SEC, suggesting that SLA class I molecules are unable to efficiently down-regulate human NK-mediated cell lysis. Moreover, in this study, up-regulated SLA, as the result of the transfection of hβ2m, is completely unable to suppress human NK activity on SEC. This contradiction might be related to the allelic differences of SLA class I molecules for each cell line.
The function of the HLA-G1 molecule on NK cell-mediated cell lysis was examined in addition to the expression analysis. HLA-G1 significantly down-regulated NK-mediated CHO and SEC lysis. In our experiments, 35% suppression was observed in the SEC transfectants against YT cell line. Judging from these data, there is little doubt that NK-mediated SEC direct lysis is reduced by HLA-G1. With respect to the ability of the human NK cell to recognize HLA-G1 molecule, the expression of HLA-G1 on SEC is effective in down-regulating human NK cell activities.
The expression profiles and function analysis of HLA-G3 were examined. HLA-G3 is devoid of the α2 and α3 domains. Therefore, the α1 domain was directly conjugated to the transmembrane sequence, which results from the splicing out of the exon 3 and exon 4 sequences during mRNA maturation [12]. HLA-G3 mRNA is present at relatively high levels in the placenta, as well as HLA-G1 and G2 [12]. In this study, attempts were made to produce highly-expressed cell lines with HLA-G3 by transfection of pCXN-HLA-G3, a construct similar to pCXN-HLA-G1. Unfortunately, HLA-G3 was not expressed on the cell surface.
It was initially thought that HLA-G3 would be much easier to express on the cell surface than HLA-G1 because of the short length of its cDNA. However, the stable clones, which clearly expressed mRNA, indicated that the HLA-G3 protein expression rate was much lower than those of HLA-G1. In general, the folding of an MHC class I molecule depends on its association first with β2m and then a peptide, otherwise the MHC class I molecules are unstable and are eventually translocated back into the cytosol of the cell where they undergo degradation [21]. Therefore, the inadequate binding to β2m and the peptide might cause the unsuccessful expression of HLA-G3 in this study.
Since the discovery of the HLA-G molecule [22], a number of studies have reported that HLA-G, including G1 and HLA-G2, inhibits NK-mediated cytotoxicity [23,24]. However, in 1998 a significant and very interesting paper appeared which reported that HLA-E binds preferentially to a peptide, which is derived from amino acid residues 3–11 of the signal sequence of most HLA-A, -B, -C and -G molecules, and that the surface expression of HLA-E was sufficient to protect target cells from lysis by CD94/NKG2A(+) NK cell clones [25]. As a result of this, the presence of the HLA-E molecule must be considered in HLA-G experiments when human cells are used, since HLA-E might be able to down-regulate NK cell function. Although a number of these reports have been disproved, this issue is not applicable to our study, in which xenogeneic cells were used.
Apart from the field of xenotransplantation, some experiments have been carried out to better understand the role and physical relevance of the HLA-G3 molecule using the human lymphoblast cell line, 721.221, which contains no HLA-class I gene except for HLA-E. The transfection of an HLA-G3 plasmid resulted in a substantial down-regulation of NK-mediated cell lysis to 721.221 cells, presumably via the expression of the inherent HLA-E molecules. This phenomenon might explain the high levels of HLA-G3 mRNA expression in placenta [12].
Future studies will focus on the relation between HLA-G and HLA-E expression, and functional differences between the two in xenogeneic cells, using SEC transfectants with these molecules, with the object of completely suppressing human NK activity against xenografts.
In conclusion, transfected HLA-G1 was readily expressed on SEC, and co-transfection with hβ2-microglobulin enhanced the level of HLA-G1 expression. The expressed HLA-G1 significantly reduced NK-mediated SEC cell lysis. It is noteworthy that the HLA-G3 molecule could not be expressed on xenogenic and human cells. HLA-G3 might have a different meaning and role from HLA-G1 in avoiding NK-mediated killing. The expression of HLA-G1 on the cell surface serves as a new approach to overcoming NK-mediated immunity to xenografts.
Acknowledgments
We thank Dr Milton S. Feather for editing the manuscript. This work was supported by Grant-in-Aids for Scientific Research Japan, and Program for Promotion of Basic Research Activities for Innovative Biosciences from the Ministry of Agriculture, Forestry and Fisheries.
References
- 1.Lambrigts D, Sachs DH, Cooper DKC. Discordant organ xenotransplantation in primates. Transplantation. 1998;66:547–61. doi: 10.1097/00007890-199809150-00001. [DOI] [PubMed] [Google Scholar]
- 2.Miyagawa S, Hirose H, Shirakura R, et al. The mechanism of discordant xenograft rejection. Transplantation. 1988;46:825–30. doi: 10.1097/00007890-198812000-00007. [DOI] [PubMed] [Google Scholar]
- 3.Rosengard AM, Cary NR, Langford GA, Tucker AW, Wallwork J, White DJ. Tissue expression of human complement inhibitor, decay-accelerating factor, in transgenic pigs. A potential approach for preventing xenograft rejection. Transplantation. 1995;59:1325–33. [PubMed] [Google Scholar]
- 4.Platt JL. New directions for organ transplantation. Nature. 1998;392(Suppl.):11–7. doi: 10.1038/32023. [DOI] [PubMed] [Google Scholar]
- 5.Lin Y, Goebels J, Xia G, Ji P, Vandeputte M, Waer M. Induction of specific transplantation tolerance across xenogenic barriers in the T-independent immune compartment. Nature Med. 1998;4:173–80. doi: 10.1038/nm0298-173. [DOI] [PubMed] [Google Scholar]
- 6.Miyagawa S, Nakai R, Yamada M, et al. Regulation of natural killer cell-mediated swine endothelial cell lysis by the genetic remodeling of a glycoantigen. J Biochem. 1999;126:1067–73. doi: 10.1093/oxfordjournals.jbchem.a022551. [DOI] [PubMed] [Google Scholar]
- 7.Artrip JH, Kwiatkowski P, Michler RE, et al. Target cell susceptibility to lysis by human natural killer cells is augmented by alpha(1,3)-galactosyltransferase and reduced by alpha(1,2)-fucosyltransferase. J Biol Chem. 1999;274:10717–22. doi: 10.1074/jbc.274.16.10717. 10.1074/jbc.274.16.10717. [DOI] [PubMed] [Google Scholar]
- 8.Sasaki H, Xu XC, Smith DM, Howard T, Mohanakumar T. HLA-G expression protects porcine endothelial cells against natural killer cell-mediated xenogenic cytotoxicity. Transplantation. 1999;67:31–7. doi: 10.1097/00007890-199901150-00005. [DOI] [PubMed] [Google Scholar]
- 9.Dorling A, Monk NJ, Lechler RI. HLA-G inhibits the transendothelial migration of human NK cells. Eur J Immunol. 2000;30:586–93. doi: 10.1002/1521-4141(200002)30:2<586::AID-IMMU586>3.0.CO;2-Y. 10.1002/(SICI)1521-4141(200002)30:02<586::AID-IMMU586>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 10.López-Botet M, Bellón T. Natural killer cell activation and inhibition by receptors for MHC class I. Curr Opin Immunol. 1999;11:301–7. doi: 10.1016/s0952-7915(99)80048-x. [DOI] [PubMed] [Google Scholar]
- 11.Kovats S, Main EK, Librach C, Stubblebine M, Fisher SJ, DeMars R. A class I antigen, HLA-G, expressed in human trophoblasts. Science. 1990;248:220–3. doi: 10.1126/science.2326636. [DOI] [PubMed] [Google Scholar]
- 12.Ishitani A, Geraghty DE. Alternative splicing of HLA-G transcripts yields proteins with primary structures resembling both class I and class II antigens. Proc Natl Acad Sci USA. 1992;89:3947–51. doi: 10.1073/pnas.89.9.3947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rouas FN, Khalil DI, Riteau B, et al. The immunotolerance role of HLA-G. Cancer Biol. 1999;9:3–12. doi: 10.1006/scbi.1998.0103. [DOI] [PubMed] [Google Scholar]
- 14.Miyagawa S, Shirakura R, Iwata K, et al. Effects of transfected complement regulatory proteins, MCP, DAF, MCP/DAF hybrid, on complement-mediated swine endothelial cell lysis. Transplantation. 1994;58:834–40. [PubMed] [Google Scholar]
- 15.Yodoi J, Teshigawara K, Nikaido T, et al. TCGF (IL−2)-receptor inducing factor(s). I. Regulation of IL−2 receptor on natural killer-like cell line (YT cells) J Immunol. 1985;134:1623–30. [PubMed] [Google Scholar]
- 16.Niwa H, Yamamura K, Miyazaki J. Efficient selection for high-expression transfectants with a novel eukariotic vector. Gene. 1991;108:193–200. doi: 10.1016/0378-1119(91)90434-d. [DOI] [PubMed] [Google Scholar]
- 17.Matsunami K, Miyagawa S, Yamada M, Yoshitatsu M, Shirakura R. A surface-bound form of human C1-esterase-inhibitor improves xenograft rejection. Transplantation. 2000;69:749–55. doi: 10.1097/00007890-200003150-00013. [DOI] [PubMed] [Google Scholar]
- 18.Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–5. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 19.McMaster MT, Librach CL, Zhou Y, et al. Human placental HLA-G expression is restricted to differentiated cytotrophoblasts. J Immunol. 1995;154:3771–8. [PubMed] [Google Scholar]
- 20.Kwiatkowski P, Artrip JH, John R, et al. Induction of swine major histocompatibility complex class I molecules on porcine endothelium by tumor necrosis factor-alpha reduces lysis by human natural killer cells. Transplantation. 1999;67:211–8. doi: 10.1097/00007890-199901270-00005. [DOI] [PubMed] [Google Scholar]
- 21.York IA, Rock KL. Antigen processing and presentation by the class I major histocompatibility complex. Ann Rev Immunol. 1996;14:369–96. doi: 10.1146/annurev.immunol.14.1.369. [DOI] [PubMed] [Google Scholar]
- 22.Geraghty DE, Koller BH, Orr HT. A human major histocompatibility complex class I gene that encodes a protein with a shortened cytoplasmic segment. Proc Natl Acad Sci USA. 1987;84:9145–9. doi: 10.1073/pnas.84.24.9145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rouas FN, Marchal RE, Kirszenbaum M, Dausset J, Carosella ED. The alpha 1 domain of HLA-G1 and HLA-G2 inhibits cytotoxicity induced by natural killer cells: is HLA-G the public ligand for natural killer cell inhibitory receptors? Proc Natl Acad Sci USA. 1997;94:5249–54. doi: 10.1073/pnas.94.10.5249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pazmany L, Mandelboim O, Vales GM, Davis DM, Reyburn HT, Strominger JL. Protection from natural killer cell-mediated lysis by HLA-G expression on target cells. Science. 1996;274:792–5. doi: 10.1126/science.274.5288.792. 10.1126/science.274.5288.792. [DOI] [PubMed] [Google Scholar]
- 25.Braud VM, Allan DS, O'Callaghan CA, et al. HLA-E binds to natural killer cell receptors CD94/NKG2A, B and C. Nature. 1998;391:795–9. doi: 10.1038/35869. [DOI] [PubMed] [Google Scholar]