Abstract
Murine extrahepatic bile duct epithelial cells (MEBEC) were isolated from extrahepatic bile ducts of BALB/c mice and established in primary culture. The epithelial origin was confirmed by positive cytokeratin 19 staining for these cells and the presence of microvilli and tight junctions under electron microscopy. By immunofluorescent staining with monoclonal antibodies and flow-cytometric analysis, MEBEC in culture constitutively express low levels of intercellular adhesion molecule (ICAM)-1, class I and class II major histocompatibility (MHC) antigens. The expression of ICAM-1 was significantly increased by interferon gamma (INF-γ) or tumour necrosis factor alpha (TNF-α) stimulation. Class I and class II antigen expression were significantly enhanced by INF-γ and in vitro murine cytomegalovirus (MCMV) infection. MEBEC infected with MCMV revealed a progressive cytopathic effect. MEBEC activated by INF-γ or infected by MCMV induced a low but significant proliferation of allogeneic T cells and displayed a significant decrease in the absorbance at O.D. 550 nm in a microtitre tetrazolium assay after these treated cells were co-cultured with allogeneic T cells. These results suggest that following the up-regulation of surface MHC antigen and adhesion molecule expression with cytokines or MCMV, the MEBEC can function as antigen-presenting cells and initiate T-cell proliferation, which in turn trigger the recognition of MEBEC by effector T-cell-mediated cytotoxic responses. These findings may be implicated in the pathogenesis of virally induced, immune-mediated extrahepatic bile duct damage disorders.
Keywords: murine extrahepatic, biliary epithelial cell, cytomegalovirus proliferation cytotoxicity, MHC antigens
Introduction
In extrahepatic biliary atresia (EHBA) and primary sclerosing cholangitis (PSC), it is hypothesized that immunological mechanisms initiated by some insults may cause inflammation of extrahepatic bile ducts. Such an inflammatory process may lead to progressive narrowing and complete obliteration of the extrahepatic bile duct lumens, and ultimately liver cirrhosis and failure [1]. This hypothesis in EHBA has been supported by serial histological studies of surgical specimens of extrahepatic bile duct tissue from some infants diagnosed initially as neonatal hepatitis with patent biliary tree, as shown by cholangiography, and found later to have EHBA [2]. The histopathological features of EHBA are similar to that found in PSC in which immune-mediated injury to extrahepatic biliary epithelium initiated from viral infection has been proposed as a possible mechanism [3]. The strong association of HLA haplotype B8, DR3 with PSC as well as with other autoimmune diseases and an aberrant class II antigen expression on biliary epithelial cells in PSC suggest that this disease is immunologically mediated [4,5]. Understanding the pathogenesis in both EHBA and PSC is important in determining the therapeutic intervention before end-stage liver disease develops. However, studies on the pathogenesis of EHBA or PSC have been difficult due to the lack of a suitable model. Establishment of a murine extrahepatic biliary epithelial cell (MEBEC) culture may provide a good in vitro method to study the pathogenetic mechanism of both diseases.
A recent population-based epidemiological study of EHBA provides some evidence that this disease may be the result of significant environmental exposure (possibly viral) during the perinatal period [6]. In EHBA, reovirus type 3 has been suggested as a possible viral agent producing initial injury, but there have been different results and thus no definite conclusion [7–9]. A recent study suggested a possible relationship between group C rotavirus and EHBA [10]. An animal experimental model showed that infection with rhesus rotavirus or group A human rotavirus induces in newborn BALB/c mice, a cholestatic picture leading mainly to complete biliary obstruction [11]. Cytomegalovirus infection has been considered as a possible cause of biliary atresia [12] and as a risk factor in disappearing bile ducts in liver allograft rejection and a possible viral aetiology in primary biliary cirrhosis [13]. These studies suggested that viral infections may be capable of inducing either extra- or intrahepatic biliary epithelial injury.
To investigate immunological mechanisms theoretically capable of extrahepatic bile duct cell injury, we established a primary culture of MEBEC similar to that reported previously by Schreiber et al. [14]. We studied the expression of class I and class II major histocompatibility antigens (MHC) and intercellular adhesion molecules (ICAM)-I on cultured MEBEC, their regulation by cytokines or murine cytomegalovirus (MCMV) infection and the capacity of MEBEC to induce T-cell proliferation or induce T-cell-mediated cytotoxicity.
Materials and methods
Cell isolation and culture
In each experiment, 16 6-week-old male BALB/c mice were given 0·15 ml of rabbit serum by alternate day intraperitoneal injections for a total of seven injections to induce proliferation of extrahepatic bile duct epithelial cells [15] and were then sacrificed 24 h after the last injection. As described previously [14], murine common bile ducts were dissected, minced into fine pieces, digested with 0·0625% trypsin (Gibco, Gaithersburg, MD, USA) in the presence of 0·005% DNase for 20 min in a 37°C shaking water bath and further digested with 200 units/ml collagenase (Sigma, St Louis, MD, USA) for 1 h at 37°C in an incubator with humidified CO2. After repeated centrifugation (180 g for 5 min) and resuspension in HBSS until the last removed supernatant was clear, the resuspended cells were incubated for 45 minutes on plastic Petri dishes to remove fibroblasts for a total of three times. Finally, the non-adherent cells were mainly epithelial cells and were seeded in a six-well culture plate (Falcon) containing Dulbecco's Modified Eagle Medium (DMEM) (Gibco BRL) with 10% FBS (complete medium) at 37°C for 24 h in an incubator with 5% humidified CO2. It took approximately 6–18 h for the MEBEC to adhere.
The medium was renewed 24 h after seeding and cultures were maintained with medium changes every other day. Cells grown for 3–7 days were fixed for either ultrastructural or immunohistochemical studies or passaged by digestion with Dispase II (Boehringer, Mannheim, Germany). The viability of cells was assessed by trypan blue exclusion. For determination of doubling time, MEBEC in primary culture were plated in 24-well culture plates (5 × 105 cells/well). Cells were released by trypsin-EDTA and cell number was counted at 1, 2, 4, 6, 8 and 10 days using a Coulter-counter.
Characterization of MEBEC cultures
Immunocytochemical detection of antigens specific for biliary epithelial cells was performed as described previously [16] with slight modification. Briefly, acetone-fixed MEBEC were incubated with 0·3% H2O2 to remove endogenous peroxidase activity, then incubated with anticytokeratin 19 (CK-19, Amersham, Oakville, Ontario, Canada) or anticytokeratin AE1/AE3 (Biogenex, USA) followed by rabbit anti-mouse IgG-biotin and then streptavidin–peroxidase conjugate, and finally revealed with 3,3′-diaminobenzidine tetrahydrochloride. Control slides were prepared with the use of antivimentin monoclonal antibody (MoAb) (Serotec, Raleigh, NC, USA) in place of anti-CK19. Alternatively, cells cultured on chamberslides were incubated with anti-CK-19 followed by fluorescein isothiocyanate (FITC)-conjugated F(ab′)2 fragment of rabbit antimouse IgG (DAKO) and observed by immunofluorescence microscope. Additionally, cells released from culture plates were stained with CK-19, linked with FITC-conjugated anti-mouse IgG fragment and subjected to flow cytometric analysis for the purity of cultured MEBEC. The negative control consisted of FITC-conjugated isotype mouse IgG1 followed by an identical second-layer labelling as above. Anti-CD45 (Serotec), anti-CD34 (Serotec) and antivimentin (ICN Biochemicals, Inc. Costa Mesa, CA, USA) were also used to exclude leucocyte, endothelial cell or fibroblast contamination. Electron microscopic examination of MEBEC was performed by using a previously described method [16].
Virus and virus titration
The Smith strain of murine cytomegalovirus (MCMV) was purchased from the American Type Culture Collection (Rockville, MD) and passaged in mouse embryonic fibroblasts (MEF) cultured in Eagle's minimal essential medium (MEM). Virus pools (3 × 106 pfu/ml) were prepared from the supernatants of infected cells after clarification by centrifugation and stored in liquid nitrogen until use. The inoculation for mock-infected controls was prepared from supernatants of uninfected MEF culture. MCMV infection of MEF produced a cytopathic effect (CPE). The virus titre in the supernatant was determined by plaque assay in confluent monolayers of MEF in 24-well culture plates.
Cytokine stimulation of MEBEC
Freshly isolated MEBEC were cultured (5 × 105 cells/well) in complete medium for 3 days and then grew in complete medium alone or in medium supplemented with murine recombinant INF-γ (100 U/ml; Boehringer), murine recombinant tumour necrosis factor-alpha (TNF-α) (100 U/ml, Boehringer) for 1, 3 and 5 days and examined for the expression of surface immune determinants. Three days of cytokine treatment produced optimal expression of these surface markers and was used in the subsequent experiments.
Infection of MEBEC by MCMV
Freshly harvested MEBEC cultured in six-well culture plates (5·0 × 105 cells/well) for 3 days were infected with 3·0 × 105 pfu of MCMV at a MOI of 2 pfu per well. Mock-infected cells were exposed to a virus-free supernatant from non-infected MEF culture. After incubation in complete medium containing MCMV for additional 1, 3 or 5 days, the cells were used for assay of surface immune marker expression or T-cell proliferation to MEBEC. Three days of MCMV infection was finally chosen for subsequent experiments for the better expression. For observation of CPE of MEBEC, cultured cells were observed for 7–14 days after infection.
Infection of mice by MCMV
Six-week-old BALB/c mice were intraperitoneally inoculated with 1 × 106 pfu MCMV. Virus-free supernatant from non-infected MEF was used as a control for mock infection. The mice were sacrificed 2 weeks after MCMV inoculation, either for isolation or spleen removal for preparation of MCMV-primed T lymphocytes.
PCR amplification and detection of MCMV DNA in MEBEC
Cells released from culture plates were extracted for cellular DNA, treated with RNase (20 μg/ml) and extracted again. The extracted DNA was subjected to PCR using two oligonucleotide primers selected from the MCMV immediate-early gene 1 from published sequence data (primer A[1701–1730] and primer B[2400–2371] [antisense]) [17]. MCMV-infected MEF were used as positive controls. To avoid contamination of cellular DNA by extracellular MCMV particles, MCMV-infected MEBEC were washed thoroughly with DMEM and the last wash of DMEM was included in the DNA extraction and PCR procedure. The PCR was performed in a DNA thermal cycler 480 (Perkin-Elmer Cetus, Norwalk, CT, USA), the product was electrophoresed on 1% agarose gel and the expected band was visualized after ethidium bromide staining.
Flow cytometric analysis of cell surface antigen (ICAM-1, class I and class II antigens, B7 molecules and MCMV antigens)
MEBEC cells, 2× 105 (resting, cytokine stimulated or MCMV infected MEBEC), were suspended in 100 μl PBS and incubated with 20 μl FITC-labelled H-2Kd MoAb (class II, Pharmingen, San Diego, CA, USA), I-Ad MoAb (class I, Pharmingen) or anti-mouse CD54 (ICAM-1) MoAb (Pharmingen) in the dark at 4°C for 30 min. After centrifugation, the pelleted cells were resuspended in 0·5 ml FACScan buffer and analysed by flow cytometry (FACScan, Becton Dickinson, Mountain View, CA, USA). For each analysis, 10 000 epithelial cells were evaluated and FITC-conjugated irrelevant isotype mouse IgG1 was used as negative control antibody. Cell numbers were calculated by the software CellQuest (Becton Dickinson) which allowed a comparison of the reactivity with specific MoAb.
Anti-CD80 (B7–1) (Pharmingen) and anti-CD86 (B7–2) (Pharmingen) were used to assess the expression of the B-7 molecules on MEBEC. Because a high homology of viral DNA nucleotide sequences existed between murine CMV and human CMV and there was no commercially available MoAb against murine CMV antigens, we used anti-human CMV early antigen MoAb and anti-human CMV late antigen MoAb (Chemicon International, Inc., CA, USA) to assess the proportion of cells that are actually infected and express MCMV antigens.
Isolation and preparation of primed or unprimed murine T cells
The spleens were removed from MCMV-infected or non-infected BALB/c mice and minced. After centrifugation and lysis of RBC, the leucocyte pellet was subjected to Mouse T cell Enrich Column (R&D Systems Inc, Minneapolis, MN, USA) to obtain murine purified T cells. More than 85% of the isolated cells were identified as T cells by αβ TCR MoAb (Pharmingen) staining followed by flow cytometric analysis.
Proliferation assays
Freshly isolated MEBEC cultured in 96-well flat-bottomed plates (1 × 105 cells/well) for 3 days were then incubated with complete medium containing murine TNF-α (100 U/ml), INF-γ (100 U/ml), or MCMV (1·5 × 105 pfu/well) for another 3 days. Purified T cells from untreated or MCMV-infected mice were then added to each well and incubated with untreated or treated MEBEC in a cell ratio (MEBEC : T cells) of 1 : 1 and 1 : 4 with a total volume of each well finally adjusted to 200 μl by the addition of complete medium alone. After culture for 3 days, each well was pulsed with 1 μCi of [3H]-thymidine (NEN, Boston, MA, USA) during the final 18 h of incubation. The cells were harvested onto glass fibre paper (Matrix 9600, Packard) and the radioactivities were counted on a scintillation counter (Matrix 9600, Packard). Results were calculated from the mean cpm in triplicate wells from each of the two separate experiments. Test wells with cpm > 3× background control (T cells co-cultured with resting MEBEC) were considered as positive.
For each experiment, positive controls included a mixture of BALB/c T cells and C57BL/6 mice T cells, and BALB/c T cells stimulated with concanavalin A at a dose of 10 μg/ml. Negative controls were measured in wells containing T cells alone.
Cellular cytotoxicity assays
To determine the cytotoxic effect of murine T cells on MEBEC, a MTT-based colourimetric assay (Cell Proliferation Kit I, Boehringer Mannheim GmbH, Germany) was used. The assay is useful for the quantification of viable cells because the yellow tetrazolium salt MTT is cleaved to formazan dye only by metabolic active cells. Briefly, freshly isolated MEBEC were seeded onto 96-well, flat-bottomed culture plates (1 × 105 cells/well) and cultured for 3 days. Cells in each well were then incubated with 100 μl complete medium containing murine TNF-α (100 U/ml), INF-γ (100 U/ml) or MCMV (1·5 × 104 pfu) for another 3 days. Then purified T cells from MCMV-infected mice at a concentration of 5 × 105 cells/100 μl/well was added and incubated for 3 days. Each well then was reacted with MTT labelling reagent followed by solubilization solution (10% SDS in 0·01 m HCL) and allowed to stand overnight at 37°C. The absorbance of the samples was measured by an ELISA reader at O.D. 550 nm.
Cytotoxicity of MEBEC was determined by reduction of absorbance in MTT assay. Since the absorbance revealed strongly correlates to the viable cell numbers, the magnitude of T cell-mediated cytotoxicity was expressed as ratio (%) of residual viable cells = ODt/ODu, in which ODt was the O.D. value of residual viable epithelial cells from treated MEBEC co-cultured with allogeneic murine T cells; ODu was the O.D. value of residual viable epithelial cells from untreated MEBEC co-cultured with allogeneic murine T cells and were used as background control (100%). Thus, a lower ratio of residual viable epithelial cells indicated a higher T cell-mediated cytotoxicity to MEBEC. Considering the cytotoxic effect of MCMV itself on MEBEC, MCMV-infected MEBEC alone without addition of T cells was also used for comparison in MTT assay.
Statistics
All results are expressed as mean ± s.e.m. Statistical comparison was performed by using Student's t-test.
Results
Characterization of MEBEC
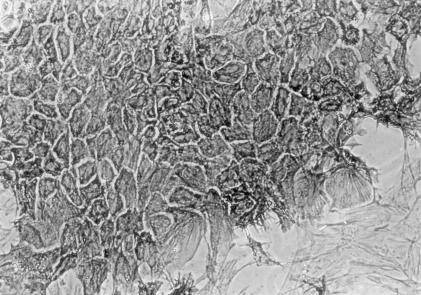
MEBEC cultures were routinely observed by phase contrast microscopy. Primary cell clusters usually attached to the culture plate within 24 h of plating and proliferated outward. Cells usually became confluent monolayers with an uniform epithelial morphology 6–7 days after plating. The confluent cells were polygon-shaped and contained a round- or oval-shaped, pale nucleus with one or more nucleoli and dense granules. Cells showed contact inhibition after confluency. Positive staining for cytokeratin 19 (Fig. 1) and cytokeratin AE-1/AE-3 confirmed that these were biliary epithelial cells. Electron microscopic examination showed that these cultured MEBEC exhibited microvilli and an apical tight intercellular junction.
Fig. 1.
Seven-day-old confluent murine extrahepatic biliary epithelial cells were immunostained with an antibody against cytokeratin 19. Note some fibroblasts at right lower corner were negative for CK-19 (×200).
According to flow cytometric analysis, over 90% of the cells stained positively for CK-19. Contaminated, fibroblast-like cells were always less than 5% of total cell populations in each analysis of MEBEC preparation. Cells were positive for CK-19 throughout the primary culture periods. In addition, there was no significant contamination by cells expressing CD45 or CD34. Doubling time calculated from the slope of the line for a plot of cell number versus time in mid-log phase of primary culture was 42·7 h.
Viral inoculation, cytopathic effect and MCMV antigen expression in MCMV-infected MEBEC
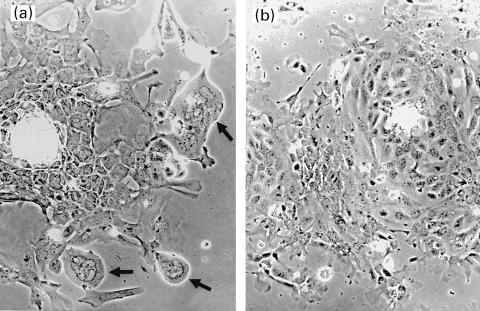
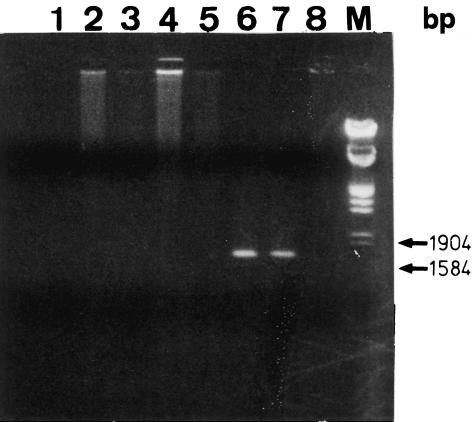
Morphological changes characteristic of MCMV infection were observed by phase contrast microscopy. MEBEC in culture with MCMV demonstrated progressive cellular rounding and smooth contours and formed large multi-nucleated giant cells (Fig. 2a). These changes were initiated mainly from the periphery of confluent cells. By day 7 of culture, approximately 30% of cells manifested CPE. Mock-infected MEBEC did not have altered morphology over the same period (Fig. 2b). After co-culture with MCMV, MEBEC were washed thoroughly with DMEM and DNA was extracted and subjected to amplification of the sequence of MCMV major immediate-early gene by PCR. MCMV DNA was not detectable in the last wash with DMEM, but was detectable in the MEBEC, confirming the inoculation of MCMV into the MEBEC (Fig. 3). Following MCMV infection, the percentage of MEBEC that expressed immediate early antigen was 29·0% (control [mock-infected MEBEC] 0·8%) at 6 h but decreased to 7·1% (control 0·1%) by 24 h; that which expressed the late antigen was 0·4% (control 0·5%) at 24 h and increased to 31·1% (control 0·1%) by 72 h. Because anti-human CMV monoclonal antibodies were used for assessment of murine CMV antigen expression, the results that 31% of infected MEBEC expressing late antigen might have been underestimated.
Fig. 2.
Appearance of murine extrahepatic biliary epithelial cells infected by murine cytomegalovirus in vitro and grown for 4 days. Note the large, multi-nucleated, giant cells with smooth contours (arrow, 2a) (×100). Mock-infected MEBEC did not have similar changes over the same period (2b) (×100).
Fig. 3.
Detection of the major immediate-early gene sequence of murine cytomegalovirus by PCR in various samples. Products run on 1·0% agarose gel. Extrahepatic bile duct tissue (lane 1) and liver tissue (lane 2) removed at 14 days after intraperitoneal injection of MCMV; lane 3: pure water; lane 4: last wash of DMEM used for washing MCMV-infected biliary epithelial cells; lane 5: uninfected murine embryonic fibroblasts; lane 6: cultured biliary epithelial cells infected with MCMV in vitro; lane 7: MCMV-infected murine embryonic fibroblasts; lane 8: uninfected cultured biliary epithelial cells; M: ladder of DNA size markers; bp: base pairs.
Expression of class 1 and class II antigen, ICAM-1 and B7 molecules on MEBEC
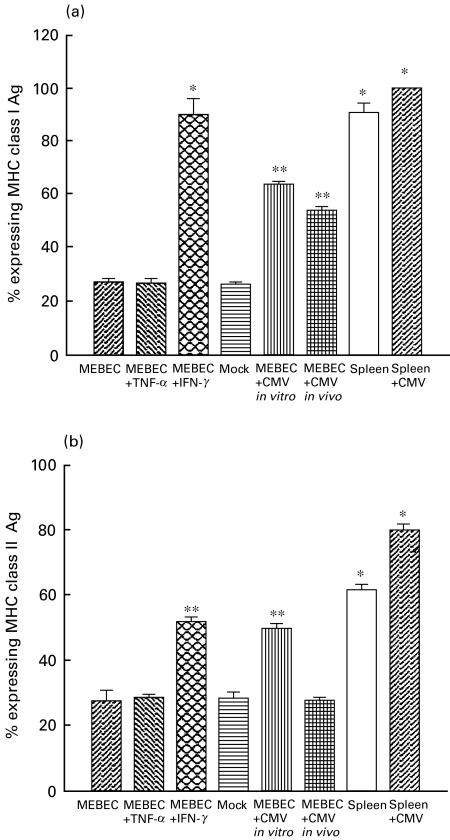
As shown in Figs 4 and 5, MEBEC constitutively expressed ICAM-1, class I and class II MHC antigens at a low level. ICAM-1 level was significantly increased by IFN-γ and TNF-α, but not altered by MCMV infection. INF-γ or MCMV strongly provoked class I antigen expression. INF-γ stimulation or MCMV infection significantly increased the expression of class II antigen. The percentage expression of CD80 on cultured MEBEC, TNF-α stimulated MEBEC, INF-γ stimulated MEBEC and MCMV-infected MEBEC were similarly low (20·8%, 20·0%, 21·2% and 18·3%, respectively), while the expression of CD86 on the above cells were slightly higher (41·1%, 45·7%, 54·6% and 23·9%, respectively).
Fig. 4.
Expression of class I antigen (a) and class II antigen (b) by cultured biliary epithelial cells with different culture characteristics by using flow cytometric analysis. MEBEC: cells cultured in growth medium (GM) only; MEBEC + TNF-α: GM supplemented with 100 U/ml TNF-α; MEBEC + INF-γ: GM supplemented with 100 U/ml INF-γ; mock: GM supplemented with supernatant from non-infected mouse embryonic fibroblast cultures; MEBEC + CMV in vitro: cultured biliary epithelial cells in each well exposed to 1·5 × 105 pfu murine cytomegalovirus in GM for 3 days; MEBEC + CMV in vivo: cultured biliary epithelial cells isolated from mice inoculated with 1 × 106 pfu murine cytomegalovirus; spleen: splenocytes from untreated BALB/c mice; spleen + CMV: splenocytes from BALB/c mice inoculated with 1 × 106 pfu murine cytomegalovirus 2 weeks before sacrifice. *P < 0·05; **P < 0·01, when compared with control (cultured in GM only). Results were the average of six separate experiments, error bars represent standard error.
Fig. 5.
Expression of ICAM-1 by cultured biliary epithelial cells with different culture characteristics by using flow cytometric analysis: GM: cells cultured in growth medium only; TNF-α: growth medium supplemented with 100 U/ml TNF-α; INF-γ: growth medium supplemented with 100 U/ml INF-γ; CMV: cultured cells in each well incubated with 1·5 × 105 pfu murine cytomegalovirus for 3 days in growth medium. *P < 0·05; **P < 0·01, when compared with control (GM only). Results were the average of six replicates, error bars represent standard error.
Proliferative response of T cells to cultured MEBEC
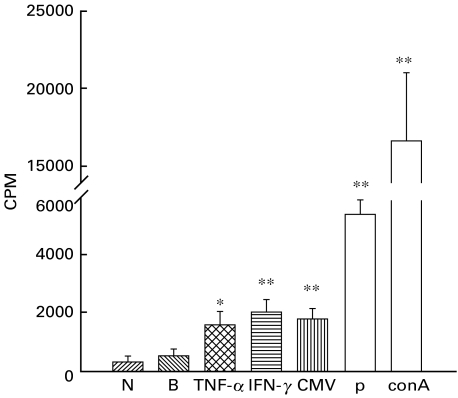
Figure 6 shows that, compared to T cell culture alone (negative control, 316 ± 84 cpm) or T cells co-cultured with resting MEBEC (background control, 541 ± 83 cpm), a significant proliferation (> 3× of background control) was observed when INF-γ activated MEBEC or MCMV-infected MEBEC were co-cultured with MCMV-primed allogeneic T cells (2011 ± 169 cpm, and 1784± 138 cpm, respectively), while TNF-α activated MEBEC did not induce a significant lymphoproliferation (1578 ± 179 cpm). Both congenic mixed lymphocyte cultures (5408 ± 218 cpm) and ConA stimulated T lymphocytes (16723 ± 1942 cpm) revealed a significant proliferative response. In another separate experiment, MEBEC culture isolated from uninfected mice also did not provoke a significant proliferation of allogeneic virally primed T cells from MCMV-infected mice (stimulation index 1·12, compared to the cpm of MEBEC from uninfected mice co-cultured with allogeneic T cells from mice untreated by rabbit serum injection).
Fig. 6.
Proliferative response of allogeneic murine T cells from MCMV-infected mice to resting or treated biliary epithelial cells at cell ratio (biliary epithelial cells: T cells = 1 : 4). N: negative control (T cells alone); B: background control (T cells incubated with untreated biliary epithelial cells); INF-γ: T cells incubated with INF-γ‐stimulated biliary epithelial cells; CMV: T cells incubated with murine cytomegalovirus-infected biliary epithelial cells (cultured cells in each well of 6-well culture plates incubated with 1·5 × 105 pfu murine cytomegalovirus for 3 days in growth medium); P: positive control (mixed BALB/c T cells and C57BL/6 T cells); ConA: BALB/c T cells stimulated with concanavalin A at 10 μg/ml. *P < 0·05; **P < 0·01, when compared with negative control (T cells alone). Each value represents the mean of six replicates; error bars represent SEM.
Cytotoxicity assay using T cells from MCMV-infected mice as effectors and MEBEC as targets
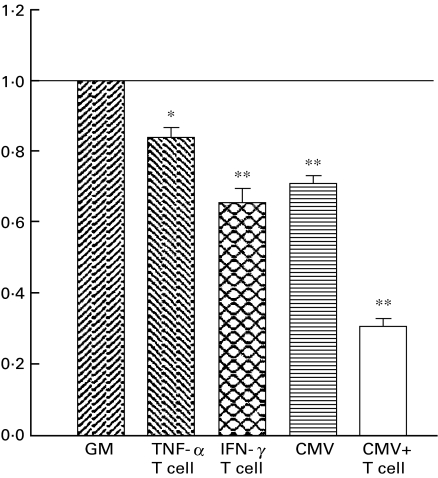
Cytotoxic effect mediated by T cells was measured as absorbance at O.D. 550 nm in MTT assay. When the absorbance of each measurement of residual viable epithelial cells from T cells in culture with untreated MEBEC [0·5543 ± 0·00631 (mean ± s.e.m.), N = 6, range 0·4180–0·6680] was regarded as background (1·00), the average absorbance of residual viable MEBEC in different experimental conditions were the following: T cells in culture with TNF-α stimulated MEBEC was 0·84 ± 0·03 (P < 0·05); T cells in culture with INF-γ stimulated MEBEC was 0·65 ± 0·04 (P < 0·01); T cells in culture with MCMV-infected MEBEC was 0·31 ± 0·03 (P < 0·01); and MCMV-infected MEBEC alone was 0·71 ± 0·02 (P < 0·01). Thus MCMV infection did significantly increase the susceptibility of MEBEC to T cell mediated cytotoxic injury (Fig. 7).
Fig. 7.
Cytotoxic activity of murine cytomegalovirus-primed murine T lymphocyte on cultured biliary epithelial cells with different culture characteristics. GM: growth medium (control); TNF-α: GM supplemented with TNF-α (100 U/ml); INF-γ: GM supplemented with INF-γ(100 U/ml); CMV: cultured cells exposed to 1·5 × 104 pfu murine cytomegalovirus/well (96-well culture plate) for 3 days in GM without further coculture of T cells. CMV: cultured biliary epithelial cells exposed to 1·5 × 104 pfu murine cytomegalovirus/well in GM for 3 days, with further addition of T cells. *P < 0·05; **P < 0·01, when compared with control. Each value (expressed as the ratio of absorbance of residual viable cells in specific condition over that of control) were the average of six replicates, error bars represent s.e.
Discussion
The biliary epithelial origin of our primary cultures of MEBEC was documented by positive staining for cytokeratin-19 and the presence of microvilli and tight junctions. Only a few previous studies have characterized tissue cultures from either human or mouse extrahepatic biliary duct structures [18–20]. A major advantage of our method is that we can harvest sufficient cells within a limited time for various experiments. The use of serum injection pretreatment to increase the cell yield inevitably raises doubts as to the extent to which the cells elicited are representative of the native state. This approach is certainly, however, more ‘physiological’ than alternative approaches which have required biliary epithelial cell immortalization.
Our resting cultured MEBEC constitutively expressed low levels of class I, class II and ICAM-1. The basal expression of class I and ICAM-1 was greatly enhanced by INF-γ and that of class II was moderately enhanced. This is different from immortalized murine intrahepatic biliary epithelial cells in which the basal expression of class I antigen is very high and class II antigen very low, and INF-γ induces a significantly increased class II but slightly increased class I antigen [21]. The different basal expression and different susceptibility to cytokine induction between extra- and intrahepatic biliary epithelial cells may imply why certain hepatobiliary diseases affect biliary tree at different anatomical levels.
In the normal human liver, intrahepatic biliary epithelial cells can express HLA class I but only weakly express HLA class II and ICAM-1 [22]. In putative immune-mediated bile duct damage disorders such as PBC, PSC or following allograft rejection, ICAM-1 and HLA class II expression are up-regulated [23,24]. An expression of HLA-DR antigens as well as a strong expression of ICAM-1 on bile duct epithelium in patients with biliary atresia has also been reported [25,26]. In an experimental transplantation model, a rejection injury to murine extrahepatic bile ducts is characterized by fibrosclerosing lesion in that the level of MHC antigen expression in bile duct epithelium influences the susceptibility to this sclerosing lesion [27]. These and our studies suggest that cell surface immune determinant expression modified by cytokines secreted in inflammatory microenvironment may regulate the interaction between lymphocytes and biliary epithelial cells which mediates extrahepatic biliary epithelial injury.
The role of CMV in immune-mediated biliary epithelial cell injury is unclear. In transplant patients, CMV DNA was persistently detectable in hepatocytes but was undetectable in bile duct cells of those with vanishing bile duct syndrome [28]. Newborn mice inoculated with reovirus type 3 often showed an inflammatory reaction of bile ducts and also extrahepatic biliary tissue free of virus [29]. In our viral model, MCMV was detectable by PCR in cultured MEBEC infected with MCMV in vitro and MHC antigen expression was higher in infected MEBEC than uninfected MEBEC.
Because MEBEC and T cells were from the same strain in the proliferation experiment, data reported here is indeed a mixed lymphocye reaction reflecting the different MHC expression levels. Also, one could argue that proliferation of MEBEC may be induced by intraperitoneal inoculation of normal rabbit serum resulting in increased rate of cell division. However, using ‘resting untreated MEBEC incubated with T cells’ as control, we found that INF-γ activated or MCMV infected MEBEC did stimulate a significant lymphoproliferative response (> 3 × cpm of control). It is possible that these treated MEBEC may produce neoantigen on the cell surface, increase the expression of MHC class II molecules or other adhesion molecules, and thus can enhance T cell-epithelial cell communication triggering T cell proliferation [30]. A recent study showed a lack of co-stimulatory molecules resulting in the absence of immunogenicity in cytokine-treated human intrahepatic bile duct epithelial cells [31]. However, we found a detectable B7 molecule expression on our MEBEC, which is required for an efficient antigen-specific helper T cell-APC binding that initiate the cell-mediated immune responses [32].
Cytotoxic T cell effector cell mechanisms would require direct contact between biliary epithelial cells and cytotoxic lymphocytes. From our results, MCMV-primed T-cell-mediated cytolysis could be observed in INF-γ treated or MCMV-infected MEBEC with enhanced class I MHC antigen expression, or even in TNF-α activated MEBEC with increased ICAM-1 expression. Although one could argue that INF-γ, TNF-γ or MCMV itself would induce cytotoxicity of MEBEC, we could demonstrate in MTT assays the net cytotoxic effect induced by primed T cells on MCMV-infected MEBEC. This indicates that MCMV-infected MEBEC may express both viral specific antigens and class I antigen at their cell surface and can function sufficiently as target cells for effector T cell-mediated cytotoxic responses. The possibility of autoimmune cytolysis that virally primed T lymphocytes may recognize epitopes cross reactive between CMV and biliary epithelial cells was not confirmed in this murine model because no significant proliferation or cytotoxicity was found in allogeneic MCMV-primed T cells co-cultured with untreated MEBEC.
In contrast to previous studies, we demonstrated that MCMV is able to infect cultured MEBEC resulting in an up-regulated expression of MHC class I and class II antigens, as well as ICAM-1 and B7 molecules. These virally infected MEBEC can function as antigen-presenting cells initiating T cell proliferation and as target cells during T cell-mediated cytotoxic response. Further assessment of biliary epithelial surface immune determinant expression in vivo in mice, the effect of other viruses or cytokines on MEBEC and the determination of cytokine or virally induced bile duct epithelial antigens recognized by T cells may extend our understanding on the possible contribution of viral infections to the pathogenesis of extrahepatic cholangiopathy such as biliary atresia and help in developing a significant treatment for immune-mediated biliary epithelial cell injury diseases in the future.
Acknowledgments
We thank sincerely Dr Richard A. Schreiber, Department of Paediatric Gastroenterology, Montreal Children's Hospital, Montreal, Quebec, Canada and Dr Ronald E. Kleinman, Department of Paediatric Gastroenterology and Nutrition, Massachusetts General Hospital, Boston, Massachusetts, USA, for their kind instruction and help in the establishment of murine extrahepatic bile duct epithelial cell cultures. We also thank Chi-Ju Wang MS for his excellent technical help during the study and Ms Chang Ying-Ying and Mr Chang Yun-Chun for their kind help in preparing electron micrographs. This study was supported by grants NSC 85–2331-B002–314, NSC 86–2314-B002–043 and NSC 88–2314-B002–132 from National Science Council, Taiwan, ROC.
References
- 1.Mowat AP. Liver disorders in childhood. London: Butterworths; 1987. pp. 72–88. [Google Scholar]
- 2.Landing BH. Considerations of the pathogenesis of neonatal hepatitis, biliary atresia and choledochocyst – the concept of infantile obstructive cholangiopathy. Prog Pediatr Surg. 1984;6:113–39. [PubMed] [Google Scholar]
- 3.Desmet VJ. Cholangiopathies: past, present, and future. Semin Liver Dis. 1987;7:67–76. doi: 10.1055/s-2008-1040566. [DOI] [PubMed] [Google Scholar]
- 4.Strober W, James SP. The immune pathogenesis of gastrointestinal and hepatobiliary diseases. JAMA. 1987;258:2962–9. [PubMed] [Google Scholar]
- 5.Chapman RWG, Jewell DP. Primary sclerosing cholangitis- an immunologically mediated disease? West J Med. 1985;143:193–5. [PMC free article] [PubMed] [Google Scholar]
- 6.Yoon PW, Bresee JS, Olney RS, James LM, Khoury MJ. Epidemiology of biliary atresia: a population-based study. Pediatrics. 1997;99:376–82. doi: 10.1542/peds.99.3.376. [DOI] [PubMed] [Google Scholar]
- 7.Glaser JH, Morecki R. Reovirus type 3 and neonatal cholestasis. Semin Liver Dis. 1987;7:100–7. doi: 10.1055/s-2008-1040569. [DOI] [PubMed] [Google Scholar]
- 8.Steele MI, Marshall CM, Lloyd RE, Randolph ME. Reovirus 3 not detected by reverse transcriptase-mediated polymerase chain reaction analysis of preserved tissue from infants with cholestatic liver disease. Hepatology. 1995;21:697–702. [PubMed] [Google Scholar]
- 9.Tyler KL, Sokol RJ, Oberhaus SM, et al. Detection of reovirus RNA in hepatobiliary tissues from patients with extrahepatic biliary atresia and choledochocysts. Hepatology. 1998;27:1475–82. doi: 10.1002/hep.510270603. [DOI] [PubMed] [Google Scholar]
- 10.Riepenhoff-Talty M, Gouvea V, Evans MJ, et al. Detection of group C rotavirus in infants with extrahepatic biliary atresia. J Infect Dis. 1996;174:8–15. doi: 10.1093/infdis/174.1.8. [DOI] [PubMed] [Google Scholar]
- 11.Petersen C, Biermanns D, Kuske M, Schakel K, Meyer-Junghanel L, Mildenberger H. New aspects in a murine model for extrahepatic biliary atresia. J Pediatr Surg. 1997;32:1190–5. doi: 10.1016/s0022-3468(97)90680-1. [DOI] [PubMed] [Google Scholar]
- 12.Oppenheimer EH, Esterly JR. Cytomegalovirus, a possible cause of biliary atresia. Am J Pathol. 1973;71:22. [Google Scholar]
- 13.Manez R, White LT, Kusne S, et al. Association between donor-recipient HLA-DR compatibility and cytomegalovirus hepatitis and chronic rejection in liver transplantation. Transplant Proc. 1993;25:908–9. [PMC free article] [PubMed] [Google Scholar]
- 14.Schreiber RA, Huang JQ, Kleiman RE. Isolation and culture of biliary epithelial cells from extrahepatic bile ducts. Gastroenterology. 1992;102:A3336. [Google Scholar]
- 15.Glaser JH, Morecki R, Fallon-Friedlander S, Horwitz MS. An extrahepatic bile duct growth factor: in vivo effect and preliminary characterization. Hepatology. 1987;7:272–6. doi: 10.1002/hep.1840070211. [DOI] [PubMed] [Google Scholar]
- 16.Ishii M, Vroman B, LaRusso NF. Isolation and morphologic characterization of bile duct epithelial cells from normal rat liver. Gastroenterology. 1989;97:1236–47. doi: 10.1016/0016-5085(89)91695-8. [DOI] [PubMed] [Google Scholar]
- 17.Keil GM, Ebeling-Keil A, Koszinowski UH. Sequence and structural organization of murine cytomegalovirus immediate early gene 1. J Virol. 1987;51:1901–8. doi: 10.1128/jvi.61.6.1901-1908.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schier C, Schier F, Voss B, Von Bassewitz DB, Pfautsch M. Characterization of human extrahepatic biliary duct epithelial cells in cultures. Exp Mol Pathol. 1988;48:301–10. doi: 10.1016/0014-4800(88)90066-4. [DOI] [PubMed] [Google Scholar]
- 19.Saidman SL, Duquesnoy RJ, Zeevi A, Fung JJ, Starzl TE, Demetris AJ. Recognition of major histocompatibility complex antigens on cultured human biliary epithelial cells by alloreactive lymphocytes. Hepatology. 1991;13:239–46. [PMC free article] [PubMed] [Google Scholar]
- 20.Katayanagi K, Kono V, Nakanuma Y. Isolation, culture and characterization of biliary epithelial cells from different anatomical level of the intrahepatic and extrahepatic biliary tree from a mouse. Liver. 1998;18:90–8. doi: 10.1111/j.1600-0676.1998.tb00133.x. [DOI] [PubMed] [Google Scholar]
- 21.Paradis K, Le Oanh NL, Russo P, St-Cyr M, Fournier H, Bu D. Characterization and response to interleukin 1 and tumor necrosis factor of immortalized murine biliary epithelial cells. Gastroenterology. 1995;109:1308–15. doi: 10.1016/0016-5085(95)90593-6. [DOI] [PubMed] [Google Scholar]
- 22.Vanden Oord JJ, Sciot R, Desmet VJ. Expression of MHC products by normal and abnormal bile duct epithelium. J Hepatol. 1986;3:310–7. doi: 10.1016/s0168-8278(86)80483-4. [DOI] [PubMed] [Google Scholar]
- 23.Chapman RW, Kelly PMA, Heryet A, Jewell Fleming KA. Expression of HLA-DR antigens on bile duct epithelium in primary sclerosing cholangitis. Gut. 1988;29:422–7. doi: 10.1136/gut.29.4.422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Adams DH, Hubscher SG, Shaw J, et al. Increased expression of intercellular adhesion molecule-1 on bile ducts in primary biliary cirrhosis and primary sclerosing cholangitis. Hepatology. 1991;14:426–31. [PubMed] [Google Scholar]
- 25.Muraji T, Hashimoto K, Ifuku H, et al. Increased expression of HLA-DR antigens on biliary epithelial cells in biliary atresia. J Jpn Soc Ped Surg. 1988;24:792–5. [Google Scholar]
- 26.Dillon P, Belchis D, Tracy T, Cilley R, Hafer L, Krummel T. Increased expression of intercellular adhesion molecules in biliary atresia. Am J Pathol. 1994;145:263–7. [PMC free article] [PubMed] [Google Scholar]
- 27.Schreiber RA, Kleiman RE, Barksdale EM, Maganaro TF, Donahoe PK. Rejection of murine congenic bile ducts: a model for immune mediated bile duct disease. Gastroenterology. 1992;102:924–30. doi: 10.1016/0016-5085(92)90178-2. [DOI] [PubMed] [Google Scholar]
- 28.Arnold JC, Portman BC, O'Grady JG, Naoumov NV, Alexander GJM, Williams R. Cytomegalovirus infection persists in the liver graft in the vanishing bile duct syndrome. Hepatology. 1992;16:285–92. doi: 10.1002/hep.1840160202. [DOI] [PubMed] [Google Scholar]
- 29.Parashar K, Tarlow MJ, McCrae MA. Experimental reovirus type 3-induced murine biliary tract disease. J Pediatr Surg. 1992;27:843–7. doi: 10.1016/0022-3468(92)90380-p. [DOI] [PubMed] [Google Scholar]
- 30.Schreiber RA, Kleinman RE. Genetics, immunology and biliary atresia: an opening or diversion? J Pediatr Gastroenterol Nutr. 1993;16:111–3. doi: 10.1097/00005176-199302000-00001. [DOI] [PubMed] [Google Scholar]
- 31.Allison JP. CD28–B7 interaction in T-cell activation. Curr Opin Immunol. 1994;6:414–9. doi: 10.1016/0952-7915(94)90120-1. [DOI] [PubMed] [Google Scholar]
- 32.Leon MP, Bassendine MF, Wilson JL, Alis S, Thick M, Kirby J. Immunogenicity of biliary epithelium: Investigation of antigen presentation to CD4+ T cells. Hepatology. 1996;24:561–7. doi: 10.1002/hep.510240317. [DOI] [PubMed] [Google Scholar]