Abstract
The main immunocompetent cells in sarcoidal lesions are epithelioid cells and multi-nucleated giant cells (MGC), both of which are derived from monocyte-macrophage lineage cells. To understand further the relevance of monocytes in sarcoidosis, we examined in vitro MGC formation using monocytes from sarcoidosis patients, patients with other granulomatous diseases (OGD) and healthy control subjects. The supernatant of concanavalin A-stimulated peripheral blood mononuclear cells (conditioned medium) generated Langhans type-MGC and foreign body type-MGC from monocytes. Conditioned medium from any three groups had the same ability to form MGC from normal monocytes. On the other hand, MGC were more highly formed using monocytes from sarcoidosis patients than from other groups. When macrophages induced by treatment of monocytes with macrophage colony-stimulating factor (M-CSF) were used, the rate of MGC formation in sarcoidosis patients was about threefold or fourfold as much as that in OGD patients or healthy controls, respectively. Oxidized ATP inhibited MGC formation in all groups. The susceptibility of monocytes cultured in conditioned medium for 24 h to 2′- and 3′-o-(4-benzoyl-benzoyl)ATP-mediated cytolysis was significantly higher in sarcoidosis patients than other groups. These findings suggest that the ability of monocytes to form MGC through P2×7 receptors is enhanced in sarcoidosis patients.
Keywords: multi-nucleated giant cells, monocytes, purinergic receptors, sarcoidosis
Introduction
Sarcoidosis is a systemic granulomatous disease characterized by non-caseating granulomas consisting of activated T lymphocytes, epithelioid cells and multi-nucleated giant cells (MGC). Previous work has focused on immunological aspects of sarcoidosis patients and it is generally recognized that sarcoidosis is a disorder of the T lymphocyte-mediated inflammatory response to unknown antigenic stimuli [1]. Increasing evidence suggests that T lymphocytes in sarcoidal lesions are CD4-positive and release Th1 type cytokines, namely interferon-γ (IFN-γ) and interleukin-2 (IL-2) [2].
For the accumulation of T lymphocytes in sarcoidal lesions, chemoattractant factors released from monocyte-macrophage lineage cells are important. It has been demonstrated that RANTES [3] and IL-16 [4] are highly expressed in sarcoidal lesions. IFN-inducible protein 10 (IP-10) was expressed in alveolar macrophages in sarcoidosis and there was a significant correlation between the concentration of IP-10 and the number of CD4+ T lymphocytes in the bronchoalveolar lavage [5]. Alveolar macrophages from sarcoidosis patients also express a great variety of cytokines such as tumour necrosis factor-α (TNF-α), (IL-1β) and IL-6 [6]. IFN-γ, TNF-α and IL-12 transcripts were highly expressed in granulomatous lymph nodes from sarcoidosis patients [7]. These cytokines are involved in the development and maintenance of granulomatous inflammation, leading to the accumulation of Th1 cells at the site of ongoing inflammation. Furthermore, cutaneous and alveolar macrophages in sarcoidosis exhibit a high density of class II molecules and adhesion molecules such as intercellular adhesion molecule-1 (ICAM-1) [8], indicating that antigen-presenting function of macrophages is enhanced in sarcoidosis patients. Finally, epithelioid cells and MGC are the main component cells of the mature lesions and considered to be derived from monocyte-macrophage lineage cells. Thus, monocyte-macrophage lineage cells are key cells in the initiation, development and maintenance of sarcoidal granulomatous lesions.
MGC in tuberculous granuloma were first described by Langhans in 1868 [9] and are seen in various other types of granulomas, including sarcoidosis. Morphologically MGC are subclassified into Langhans type (LGC) and foreign body type giant cells (FGC). LGC show a circular peripheral arrangement of nuclei and FGC have the nuclei scattered in an irregular fashion throughout the cell. LGC are often seen in many infective granulomatous disorders such as tuberculosis and leprosy as well as in sarcoidosis, while FGC are characteristic in foreign body granulomas. Despite the understanding of their morphological features, the functional significance of monocyte-macrophage-derived LGC and FGC is not clearly understood.
MGC are also induced in vitro from human blood monocytes by various stimuli, including IL-3, IFN-γ and other cytokines and growth factors. Supernatants from lectin-stimulated lymphocytes also have the capacity to produce MGC [10,11]. We [12] have reported that monocytes are fused to form predominantly LGC by muramyl dipeptide (MDP), a minimal essential structure for adjuvant activity of bacterial cell walls, together with supernatants from concanavalin A-stimulated mononuclear cells.
Recently, P2×7 receptors have been implicated in the formation of MGC [13,14]. P2×7 belongs to the P2X receptor family and is a bifunctional receptor [14]. Whereas transient stimulation with extracellular ATP allows this receptor to behave as a typical cation-selective ion channel permeable to K+, Na+ and Ca2+, repetitive stimulation allows it to change to a non-selective pore that allows transmembrane fluxes of hydrophilic molecule [15] and prolonged activation of P2×7 leads unavoidably to cell death. Therefore, when exposed to extracellular ATP for long periods of time, cells with a high expression of P2×7 are irreversibly damaged and release lactate dehydrogenase (LDH). In a previous study oxidized ATP (oATP), which is a covalent inhibitor for P2×7 receptors, inhibited the formation of MGC [13]. However, expression of this receptor on the monocyte-macrophage lineage cells from sarcoidosis patients has not been examined.
In the present study, to understand further the monocyte functions in sarcoidosis patients, we examined the ability of monocytes of sarcoidosis patients to form MGC in vitro and functionally assessed the expression of P2×7 receptors in monocytes of sarcoidosis patients.
Materials and methods
Subjects
All the present sarcoidosis patients were examined in the departments of dermatology, internal medicine and ophthalmology at our hospital and showed intratracheal, ocular or neural lesions as well as skin lesions (Table 1). The patients' clinical course was followed at least for 2 years. A histological examination of the skin lesions was performed in all cases, which confirmed the presence of non-caseating granulomas composed principally of epithelioid cells with occasional MGC. Infectious conditions were eliminated and monitored by specific stains for certain microorganisms. A diagnosis of sarcoidosis was made based on more than one clinical finding of sarcoidosis, and more than one characteristic laboratory abnormality associated with sarcoidosis, including PPD reaction anergy, high serum levels of γ-globulin, angiotensin converting enzyme and lysozyme and an accumulation of gallium scintigraphy, in addition to the histological evidence of sarcoidal granulomas. Other systemic disorders, such as malignant lymphoma, tuberculosis and berylliosis, were excluded prior to making a diagnosis of sarcoidosis. The patients with OGD were as follows: four patients with tuberculosis, four patients with cutaneous foreign body granuloma and two patients with annular granuloma. Ten age-matched healthy individuals were examined as a control subject.
Table 1.
Clinical features of 10 sarcoidosis patients
| No. | Patient (age, years/sex) | Cutaneous lesions | Chest lesions | Eye | Other lesions | PPD reaction | ACE (7·7–29·4 U/l) |
|---|---|---|---|---|---|---|---|
| 1 | 54/F | Nodular/SI | NR | NR | Nerve | ND | 9·8 |
| 2 | 50/F | Plaque/SI | NR | Uveitis | NR | Positive | 14·4 |
| 3 | 63/F | Nodular/SI | BHL/lung | Uveitis | NR | Negative | 33·8 |
| 4 | 67/F | Plaque/SI | BHL | NR | NR | Negative | 33·0 |
| 5 | 26/M | SI | BHL | NR | NR | Negative | 18·3 |
| 6 | 47/F | Plaque/SI | BHL | NR | NR | ND | 30·3 |
| 7 | 71/M | Nodular | NR | Uveitis | Lymph node | ND | 12·7 |
| 8 | 66/M | SI | BHL/lung | NR | NR | Negative | 15·8 |
| 9 | 36/M | Nodular/subcutaneous | NR | NR | Nerve | ND | 19·8 |
| 10 | 55/F | SI | BHL/lung | NR | Lymph node | Negative | 24·4 |
SI: scar infiltrates; NR: normal; BHL: bilateral hilar lymphadenopathy; ND: not done; ACE: angiotensin converting enzyme.
Medium and cytokines
RPMI 1640 medium (Nikken Biochem, Kyoto, Japan) was supplemented with 100 µg/ml streptomycin and 100 U/ml penicillin (Gibco BRL, Grand Island, NY, USA). Recombinant human macrophage colony-stimulating factor (rhM-CSF) was obtained from Genzyme Corp. (Cambridge, MA, USA).
Preparation of monocytes and macrophages
Peripheral blood mononuclear cells (PBMC) were harvested after obtaining informed consent, and isolated from the heparinized blood by density gradient centrifugation with Lymphoprep™ (Nycomed, Oslo, Norway). The cells were washed twice in phosphate-buffered saline (PBS) and resuspended in RPMI 1640 medium. Cells were added to 48-well tissue culture plates (Falcon 3078; Becton Dickinson Co., Lincoln Park, NJ, USA) at a density of 1 × 106 cells per well. After incubation for 1·5 h at 37°C, non-adherent cells were removed by repeated vigorous washing with warm RPMI 1640. The resulting cultures contained at least 90% monocytes, as shown by their morphology and phenotype, as analysed by cytofluorography on a FACScan (Becton Dickinson Immunocytometry System; Becton Dickinson, Mountain View, CA, USA). These monocytes were used in our studies. To prepare macrophages, monocytes were cultured by rhM-CSF (1000 U/ml) for 4 days [16]. They were evaluated by morphology and phagocytic ability using latex particles.
Generation of MGC
PBMC were cultured in RPMI 1640 supplemented with 10% fetal calf serum (FCS; Gibco BRL, Grand Island, NY, USA) and 16 µg/ml concanavalin A (ConA; Sigma, St Louis, MO, USA) at a density of 2 × 106 cells/ml for 72 h. The cell-free supernatant was used as conditioned medium and stored at − 40°C before use. MGC were induced by culturing monocytes in RPMI 1640 supplemented with a final concentration of 25% conditioned medium. Three days later, the medium was removed and cells were stained with Giemsa (Merck, Tokyo, Japan) in situ.
Determination of the fusion index
The fusion rate of monocytes was determined by examining the stained plate under a microscope using a ×20 objective lens with ×10 eyepieces and counting the number of nuclei within MGC (> 3 nuclei/cell) in a given area and the total number of nuclei in the same area. LGC show a circular peripheral arrangement of nuclei and FGC have the nuclei scattered in an irregular fashion throughout the cell. The fusion index (FI) was calculated according to the following formula: FI (%) = (number of nuclei within MGC)/(total number of nuclei counted) × 100. Between 300 and 500 nuclei from selected representative fields were counted for each experiment.
Measurement of cytolysis
Cytolysis was assessed by measuring the release of the cytosolic enzyme LDH. Adherent cells on 48-well tissue culture plates were cultured with conditioned medium for 24 h and incubated in serum-free RPMI medium with or without 300 µm 2′- and 3′-o-(4-benzoyl-benzoyl)ATP (BzATP: Sigma, St Louis, MO, USA) for 1 h. The LDH activity was determined spectrophotometrically by measuring the disappearance rate of reduced nicotinamide adenine dinucleotide (NADH), at 340 nm as the main wavelength, during the LDH-catalysed conversion of pyruvate to lactate according to the Wroblewski–La Due method [17]. The LDH activity is expressed as U/l at 37°C. Percentage of specific lysis was calculated as (BzATP – spontaneous)/(Tween 20 – spontaneous) × 100. Spontaneous lysis was generally less than 10%. Data are presented as average for triplicate samples.
Statistical analysis
Statistical significance of differences was determined by Student's t-test and a P-value of less than 0·05 was considered to be significant. Results are expressed as the mean ± s.d. of 10 patients or controls.
Results
MGC formation by conditioned medium from sarcoidosis patients
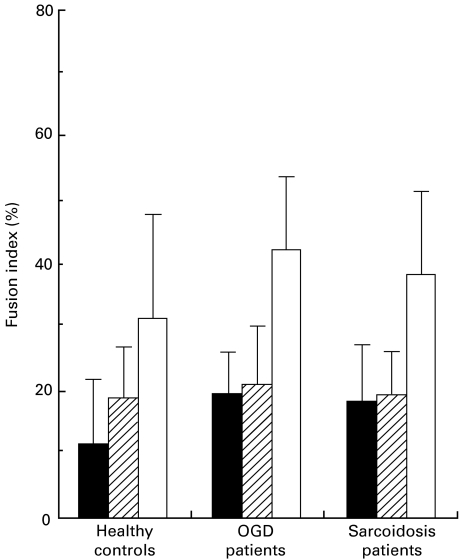
To compare the ability of conditioned medium to produce MGC between sarcoidosis patients and OGD patients or healthy subjects, monocytes of normal controls were cultured in 25% of conditioned medium from three groups (Fig. 1). We evaluated MGC formation at 3 days' culture in all experiments, because the maximal FI for LGC and FGC were reached at 3 days of culture in our previous study [12]. When monocytes were cultured with conditioned medium from healthy controls, the FI for FGC (18·7 ± 7·8%) was slightly higher than that for LGC (12·0 ± 9·6%). On the other hand, when conditioned medium from the patients with sarcoidosis or OGD was used, the FI for FGC (sarcoidosis: 19·6 ± 6·5%, OGD: 21·4 ± 7·6%) was almost the same as that for LGC (sarcoidosis: 18·5 ± 11·1%, OGD: 19·7 ± 5·9%). The FI for total MGC in these diseases (sarcoidosis: 38·1 ± 12·8%, OGD: 41·1 ± 11·7%) was slightly but not significantly higher than that in healthy controls (30·6 ± 16·1%). These results indicate that there was no difference in the capacity of PBMC to produce MGC-inducing factors by Con A-stimulation between these three groups.
Fig. 1.
MGC formation by normal monocytes cultured in conditioned medium of sarcoidosis patients, OGD patients or healthy controls. Peripheral mononuclear cells from sarcoidosis patients, OGD patients or healthy controls were stimulated with 16 µg/ml Con A. Culture supernatants (conditioned medium) were obtained 72 h after stimulation. There was no significant difference in the ability of conditioned medium to form MGC between these three groups. Results are expressed as the mean ± s.d. of 10 patients or controls. ▪, LGC;  , FGC; □, total.
, FGC; □, total.
MGC formation from monocytes of sarcoidosis patients
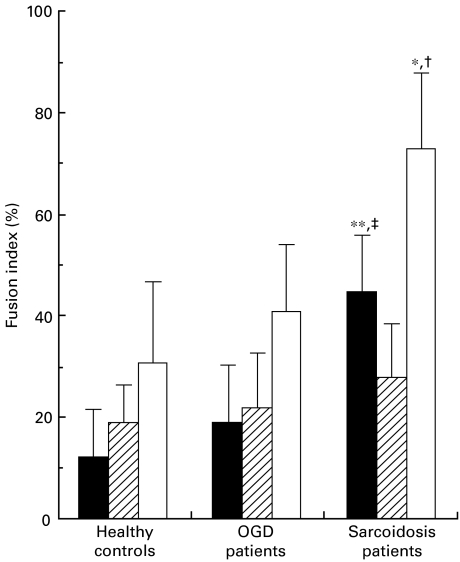
MGC formation from monocytes of sarcoidosis patients was compared with that of OGD patients and healthy controls. Monocytes of sarcoidosis patients showed a significantly higher FI for total MGC than those of other groups (P < 0·005; Fig. 2). The much higher FI for LGC in sarcoidosis patients was attributed to the difference in FI for total MGC between the patients and other two groups (27·8 ± 10·3% in FGC and 44·7 ± 11·1% in LGC in sarcoidosis patients, 22·0 ± 10·8% in FGC and 18·7 ± 11·6% in LGC in OGD patients and 18·7 ± 7·8% in FGC and 12·0 ± 9·6% in LGC in healthy controls). There was no significant difference between OGD patients and healthy controls.
Fig. 2.
MGC formation by monocytes of sarcoidosis patients, OGD patients or healthy controls cultured in conditioned medium of healthy controls. LGC were induced significantly higher from monocytes of sarcoidosis patients than those of OGD patients and healthy controls. *P < 0·005 and **P < 0·0005 compared with healthy controls. †P < 0·005 and ‡P < 0·001 compared with OGD patients. Results are expressed as the mean ± s.d. of 10 patients or controls. ▪, LGC;  , FGC; □, total.
, FGC; □, total.
Generation of MGC from macrophages
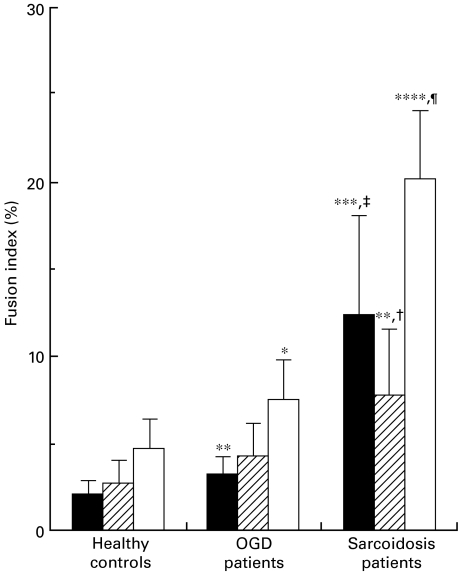
Next, the ability of macrophages to fuse was examined in patients and controls. Macrophages induced by treatment of monocytes with 1000 U/ml M-CSF for 4 days were cultured in conditioned medium. Macrophages showed a much lower ability to form MGC than monocytes in all groups (Fig. 3). Only a small number of MGC were produced from macrophages in healthy controls (2·0 ± 0·9% in LGC and 2·7 ± 1·4% in FGC) and OGD patients (3·2 ± 1·1% in LGC and 4·3 ± 1·9% in FGC). Macrophages from sarcoidosis patients showed a significantly higher FI for MGC than those from healthy controls and OGD patients (12·4 ± 5·7% in LGC (P < 0·001 vs. healthy controls and OGD patients), 7·8 ± 3·8% in FGC (P < 0·01 vs. healthy controls and P < 0·05 vs. OGD patients) and 20·2 ± 3·9% in total MGC (P < 0·0001 vs. healthy controls and OGD patients)). Macrophages from OGD patients also showed a significantly higher FI for LGC and total MGC than those from healthy controls (P < 0·01 in LGC and P < 0·05 in total MGC).
Fig. 3.
MGC formation by macrophages of sarcoidosis patients, OGD patients or healthy controls. Macrophages were induced by culturing monocytes with 1000 U/ml rhM-CSF for 4 days. Macrophages of sarcoidosis patients had a higher ability to generate MGC than those of OGD patients and healthy controls. *P < 0·05, **P < 0·01, ***P < 0·001 and ****P < 0·0001 compared with healthy controls. † < 0·05, ‡P < 0·001 and ¶P < 0·0001 compared with OGD patients. Results are expressed as the mean ± s.d. of 10 patients or controls. ▪, LGC;  , FGC; □, total.
, FGC; □, total.
The sensitivity of monocytes of sarcoidosis patients to ATP
To confirm the previous report [18] showing that the P2×7 receptor is involved in MGC formation in the human system, we examined whether oATP inhibits MGC formation from monocytes of healthy controls, OGD patients and sarcoidosis patients. Monocytes were cultured in 25% conditioned medium with or without 300 µm oATP for 3 days. oATP inhibited both types of MGC formation from monocytes of all groups (data not shown). Because the sustained activation of the P2×7 receptor leads to cell death, we next tested for the release of the cytoplasmic enzyme LDH in response to BzATP, which is a potent P2×7 receptor agonist [19] (Fig. 4). Freshly isolated monocytes from both patients and controls did not release a significant amount of LDH by stimulation of BzATP. When monocytes were cultured in 25% conditioned medium for 24 h and treated with BzATP for 1 h, LDH release in sarcoidosis patients was significantly higher than that in healthy controls (P < 0·0001) and the OGD patients (P < 0·01). LDH release in OGD patients was also significantly higher than that in healthy controls (P < 0·05). The averages of LDH release were 60·3 ± 13·6% in sarcoidosis patients, 35·1 ± 16·8% in OGD patients and 18·2 ± 11·2% in healthy controls.
Fig. 4.
The susceptibility to BzATP of monocytes cultured in conditioned medium. Monocytes were cultured in 25% conditioned medium for 24 h and stimulated with 300 µm BzATP for 1 h. LDH release in the supernatants was measured and expressed as U/l. Percentage of specific lysis was calculated as (BzATP – spontaneous)/(Tween 20 – spontaneous) × 100 and was presented as average for triplicate samples. Sarcoidosis patients showed a higher susceptibility to BzATP than OGD patients and healthy controls. *P < 0·05 and **P < 0·0001 compared with healthy controls. †P < 0·01 compared with OGD patients. Results are expressed as the mean ± s.d. of 10 patients or controls.
Discussion
We examined whether or not there is any difference in the ability of monocytes to form MGC between sarcoidosis patients and OGD patients or healthy controls. Monocytes of sarcoidosis patients had a more heightened ability to form MGC than those of OGD patients and control subjects. The higher FI in sarcoidosis patients was due mainly to the enhanced formation of LGC, which are the predominant form of MGC in sarcoidal lesions. MGC formation from macrophages, induced by M-CSF treated-monocytes, was also significantly greater in sarcoidosis patients than in other groups. These findings suggest that peripheral blood monocytes in sarcoidosis patients may have the potential to form MGC easily in response to inflammatory stimuli.
On the other hand, there was no significant difference in the ability of the conditioned medium to produce MGC between sarcoidosis patients and OGD patients or healthy subjects. The conditioned medium used here to produce MGC was the supernatant of Con A-stimulated mononuclear cells which contains a number of cytokines and inflammatory mediators. In our previous study [12], when anti-IFN-γ, anti-IL-3 or anti-GM-CSF monoclonal antibody (MoAb) was added to the culture system, anti-IFN-γ MoAb completely abrogated MGC generation and both anti-GM-CSF and IL-3 MoAbs inhibited LGC generation. These findings indicated that IFN-γ is a key cytokine to induce MGC and that IL-3 and GM-CSF are important factors for induction of LGC. We measured the protein levels of IFN-γ, IL-3 and GM-CSF in the conditioned medium, but there was no difference in these protein levels between sarcoidosis patients and OGD patients or normal controls (data not shown).
There have been reports of abnormal findings in monocyte-macrophage lineage cells of sarcoidosis patients. Romer et al. [20] demonstrated that patients exhibited an increased proportion of monocytes and a significantly increased antibody-dependent cell-mediated cytotoxicity of monocytes. They also showed that peripheral blood monocytes had a measurable, but low, ACE activity which was modestly higher in active sarcoidosis than in controls. Furthermore, the development of bronchoalveolar lavage contributes to understanding the important role of alveolar macrophages in pulmonary sarcoidosis. In contrast to the lesional monocyte-macrophage lineage cells such as alveolar macrophages, peripheral blood monocytes in sarcoidosis patients have been shown to have normal functions compared to those of healthy controls. For example, Terao et al. [21] showed that the amounts of TNF-α and IL-1β in culture supernatants of unstimulated alveolar macrophages from sarcoidosis patients were significantly higher than those from normal subjects, whereas there was no difference in the amounts of TNF-α and IL-1β in culture supernatants of peripheral blood monocytes between sarcoidosis patients and normal subjects. This suggests that some immunological and inflammatory properties of monocyte-macrophage lineage cells may be different between peripheral and lesional cells. Our present data demonstrated clearly that monocytes of sarcoidosis patients had a higher capability to form MGC in vitro, when cultured in conditioned medium, than those of OGD patients and healthy controls. Therefore, if an appropriate stimulus is administered, the difference in functional properties of peripheral blood monocytes may be discriminated between sarcoidosis patients and other groups.
P2X receptors are ligand-gated ion channels that are activated by extracellular ATP. In cells with high levels of expression of the P2×7 receptor, long exposures to extracellular ATP cause irreversible cell damage, death and release of LDH. Falzoni et al. [13] reported that formation of concanavalin A-induced MGC was inhibited by oATP and we confirmed it in conditioned medium-induced MGC formation. These findings suggest that expression of P2×7 receptor may play a role in the process of cell fusion that leads to MGC formation during granulomatous inflammations. The current study also showed that BzATP, a P2×7 receptor agonist, induced more cell lysis of monocytes cultured in conditioned medium for 24 h from sarcoidosis patients than cells from OGD patients and healthy controls. BzATP is a potent P2×7 receptor agonist but not selective for P2×7. Other purinergic receptors have been reported to be activated by BzATP [22–24]. Also oATP is not wholly selective for P2×7 receptors. However, the findings of cell lysis by BzATP together with the data of oATP blockade suggest that monocyte-macrophage lineage cells in sarcoidosis patients expressed higher levels of P2×7 receptor than those in OGD patients and healthy controls.
In conclusion, the ability of monocytes to form MGC was enhanced in sarcoidosis patients, suggesting that monocytes may be concerned with the pathogenesis of sarcoidosis and that P2×7 receptors may play a role in MGC formation in sarcoidosis.
Acknowledgments
This work was supported by grants-in-aid from the Ministry of Education, Science, Sports and Culture of Japan (11670855).
References
- 1.Newman LS, Rose CS, Maier LA. Sarcoidosis. N Engl J Med. 1997;336:1224–34. doi: 10.1056/NEJM199704243361706. [DOI] [PubMed] [Google Scholar]
- 2.Moller DR. Cells and cytokines involved in the pathogenesis of sarcoidosis. Sarcoidosis Vasc Diffuse Lung Dis. 1999;16:24–31. [PubMed] [Google Scholar]
- 3.Devergne O, Marfaing-Koka A, Schall TJ, et al. Production of the RANTES chemokine in delayed-type hypersensitivity reactions: involvement of macrophages and endothelial cells. J Exp Med. 1994;179:1689–94. doi: 10.1084/jem.179.5.1689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Center DM, Kornfeld H, Cruikshank WW. Interleukin 16 and its function as a CD4 ligand. Immunol Today. 1996;17:476–81. doi: 10.1016/0167-5699(96)10052-i. 10.1016/0167-5699(96)10052-I. [DOI] [PubMed] [Google Scholar]
- 5.Agostini C, Cassatella M, Zambello R, et al. Involvement of the IP-10 chemokine in sarcoid granulomatous reactions. J Immunol. 1998;161:6413–20. [PubMed] [Google Scholar]
- 6.Steffen M, Petersen J, Oldigs M, et al. Increased secretion of tumor necrosis factor-alpha, interleukin-1-beta, and interleukin-6 by alveolar macrophages from patients with sarcoidosis. J Allergy Clin Immunol. 1993;91:939–49. doi: 10.1016/0091-6749(93)90352-g. [DOI] [PubMed] [Google Scholar]
- 7.Bergeron A, Bonay M, Kambouchner M, et al. Cytokine patterns in tuberculous and sarcoid granulomas: correlations with histopathologic features of the granulomatous response. J Immunol. 1997;159:3034–43. [PubMed] [Google Scholar]
- 8.Melis M, Gjomarkaj M, Pace E, et al. Increased expression of leukocyte function associated antigen-1 (LFA-1) and intercellular adhesion molecule-1 (ICAM-1) by alveolar macrophages of patients with pulmonary sarcoidosis. Chest. 1991;100:910–6. doi: 10.1378/chest.100.4.910. [DOI] [PubMed] [Google Scholar]
- 9.Langhans T. Ueber Riesenzellen mit wandstandigen Kernen in Tuberkeln und die fibrose Forn des Tuberkels. Virchows Arch Pathol Anat. 1868;42:382–404. [Google Scholar]
- 10.Postlethwaite AE, Jackson BK, Beachey EH, et al. Formation of multi-nucleated giant cells from human monocyte precursors. Mediation by a soluble protein from antigen- and mitogen-stimulated lymphocytes. J Exp Med. 1982;155:168–78. doi: 10.1084/jem.155.1.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Möst J, Spötl L, Mayr D, et al. Formation of multi-nucleated giant cells in vitro is dependent on the stage of monocyte to macrophage maturation. Blood. 1997;89:662–71. [PubMed] [Google Scholar]
- 12.Mizuno K, Okamoto H, Horio T. Muramyl dipeptide and mononuclear cell supernatant induce Langhans‐type cells from monocytes. J Leukoc Biol. [PubMed]
- 13.Falzoni S, Munerati M, Ferrari D, et al. The purinergic P2z receptor of human macrophage cells. Characterization and possible physiological role. J Clin Invest. 1995;95:1207–16. doi: 10.1172/JCI117770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Di Virgilio F, Falzoni S, Chiozzi P, et al. ATP receptors and giant cell formation. J Leukoc Biol. 1999;66:723–6. doi: 10.1002/jlb.66.5.723. [DOI] [PubMed] [Google Scholar]
- 15.Di Virgilio F, Pizzo P, Zanovello P, et al. Extracellular ATP as a possible mediator of cell-mediated cytotoxicity. Immunol Today. 1990;11:274–7. doi: 10.1016/0167-5699(90)90111-l. [DOI] [PubMed] [Google Scholar]
- 16.Becker S, Warren MK, Haskill S. Colony-stimulating factor-induced monocyte survival and differentiation into macrophages in serum-free cultures. J Immunol. 1987;139:3703–9. [PubMed] [Google Scholar]
- 17.Wroblewski F, John SL. Lactic dehydrogenase activity in blood. Proc Soc Exp Biol Med. 1955;90:210–3. doi: 10.3181/00379727-90-21985. [DOI] [PubMed] [Google Scholar]
- 18.Buell G, Chessell IP, Michel AD, et al. Blockade of human P2X7 receptor function with a monoclonal antibody. Blood. 1998;92:3521–8. [PubMed] [Google Scholar]
- 19.Humphreys BD, Dubyak GR. Modulation of P2X7 nucleotide receptor expression by pro- and anti-inflammatory stimuli in THP-1 monocytes. J Leukoc Biol. 1998;64:265–73. doi: 10.1002/jlb.64.2.265. [DOI] [PubMed] [Google Scholar]
- 20.Romer FK, Christiansen SE, Kragballe K, et al. Studies of peripheral blood monocytes in pulmonary sarcoidosis. Clin Exp Immunol. 1984;58:357–63. [PMC free article] [PubMed] [Google Scholar]
- 21.Terao I, Hashimoto S, Horie T. Effect of GM-CSF on TNF-alpha and IL-1-beta production by alveolar macrophages and peripheral blood monocytes from patients with sarcoidosis. Int Arch Allergy Immunol. 1993;102:242–8. doi: 10.1159/000236532. [DOI] [PubMed] [Google Scholar]
- 22.Bianchi BR, Lynch KJ, Touma E, et al. Pharmacological characterization of recombinant human and rat P2X receptor subtypes. Eur J Pharmacol. 1999;376:127–38. doi: 10.1016/s0014-2999(99)00350-7. [DOI] [PubMed] [Google Scholar]
- 23.Erb L, Lustig KD, Sullivan DM, et al. Functional expression and photoaffinity labeling of a cloned P2U purinergic receptor. Proc Natl Acad Sci USA. 1993;90:10449–53. doi: 10.1073/pnas.90.22.10449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Communi D, Robaye B, Boeynaems JM. Pharmacological characterization of the human P2Y11 receptor. Br J Pharmacol. 1999;128:1199–206. doi: 10.1038/sj.bjp.0702909. [DOI] [PMC free article] [PubMed] [Google Scholar]