Abstract
Recent evidence suggests that chronic exposure to lactobacilli, which are part of the normal intestinal flora, inhibits the development of allergic disorders. Allergy is mediated by Th2 cells, which produce high levels of IL4 and IL5, and suppressive effects of lactic acid bacteria on the development of allergy have been attributed to their Th1-inducing properties. On the other hand, lactic acid bacteria have also been shown to suppress autoimmune disorders which are mediated by Th1 cells producing high levels of IFNγ. To study this apparent discrepancy, the immunomodulatory potential of lactobacilli was evaluated using recombinants that express an immunodominant T-cell epitope of Der p 1 of house dust mites. Mucosal immunization of C57BL/6 J mice with such recombinants resulted in the induction of T cells which produced low amounts of IFNγ. Immunization with the house dust mite peptide followed by treatment with recombinant Lactobacillus plantarum resulted in the inhibition of both IFNγ and IL5 production. The effect on IFNγ production was shown to be a non-specific effect of L. plantarum. The effect on IL5 production, however, was only observed when the recombinant expressing the Der p 1 peptide, but not the control recombinant, was used for treatment. Neither of the recombinants had an effect on the antibody response. Taken together, these data suggest that recombinant L. plantarum may be a suitable candidate for the treatment of allergic disorders.
Keywords: lactobacilli, Th1/Th2, allergy, mucosal immunity
Introduction
Lactobacilli are Gram-positive non-pathogenic commensal organisms which constitute an important part of the intestinal microflora [1]. It is presumed that by colonizing the intestine, they can compete with pathogens. Apart from their protective effect against several infectious diseases [2], lactic acid bacteria have also been shown to exhibit strong anti-tumour activity [3]. These effects may be due to the capacity of lactobacilli to enhance cell-mediated immunity, and in vitro studies have indeed indicated that components of lactobacilli can induce IL12 production [4–6], a cytokine which is crucial in the development of Th1 responses. Since it has been shown that Th1 and Th2 cells can antagonize each other, the immunomodulatory effects of lactic acid bacteria may include interference in pathological Th2 responses such as those occurring in allergic disorders.
Allergy is characterized by an inappropriate immune response to environmental or food allergens, and involves activation of Th2 cells producing IL4 and IL5 [7]. This leads to the induction of IgE synthesis [8] and the activation and recruitment of eosinophils, two factors that mediate most of the clinical symptoms of allergy. Indirect evidence for protective effects of lactic acid bacteria against allergy came from a comparison of the intestinal flora of children in Sweden and Estonia. It appeared that children in Estonia, where prevalence of allergy is much lower than in Sweden, have higher amounts of lactic acid bacteria in their bowel flora [9]. Recently, this study was extended by the observation that both in Sweden and in Estonia, lower amounts of lactic acid bacteria are found in allergic children than in non-allergic controls [10]. In addition, lactic acid bacteria have been shown to inhibit IgE production [11,12]. Taken together, these data suggest that they may be highly suitable candidates for modulating allergic responses.
In contrast to the findings described above, lactic acid bacteria have also been shown to exert inhibitory effects on the development of Th1-mediated diseases like diabetes, arthritis [14] and colitis [15], suggesting that they may modulate immune responses by a mechanism other than polarizing immune responses towards Th1. For instance, it could be postulated that they interfere in processes that regulate tolerance induction. Such mechanisms, which are operating at mucosal surfaces, ensure the development of well-balanced, non-pathological T-cell responses to harmless environmental antigens. Indirect evidence for protective effects of lactic acid bacteria on the development of allergy can therefore also be explained by a mechanism involving stimulation of the normal immune regulatory mechanisms.
In order to gain more insight into the immunomodulatory properties of lactobacilli, we expressed an immunodominant T-cell epitope of Der p 1, one of the major allergens of house dust mites (HDM), in Lactobacillus plantarum, a species which is found in the intestinal microflora of both humans and rodents. Mucosal immunization with such recombinant lactobacilli leads to simultaneous exposure of the immune system to lactobacilli and allergen, and enables direct evaluation of their effects on the development Der p 1-specific T-cell responses. This particular peptide was chosen because it induces a dominant Th2 response, characterized by the production of high levels of IL5. However, small amounts of the Th1 cytokine IFNγ are also detectable and therefore, this model is suitable for studying the effects of lactic acid bacteria on the development of both the Th2 and the Th1 component of the T-cell response.
Materials and methods
Strains and plasmids
Lactobacillus plantarum 256 was grown on MRS plates or in MRS medium (Difco, Detroit, MI, USA) and, when appropriate, erythromycin (Sigma, St. Louis, MO, USA) was added at 5 µg/ml. Transformation of lactobacilli was carried out by electroporation. Plasmid pLP503 [16] encodes the l-(+)-lactate dehydrogenese gene (ldh) promoter of L. casei, which allows constitutive expression of the β-glucuronidase gene (uidA) of Escherichia coli in the cytosol of lactobacilli. Plasmid pLP503-P1 contains a linker, encoding peptide 111–139 of Der p 1 of the house dust mite inserted in the BamHI and NcoI sites upstream of the uidA gene. Introduction of this plasmid in L. plantarum resulted in expression of a protein composed of the Der p 1 peptide fused to the E. coli β-glucuronidase protein. This recombinant was designated L. plantarum-p1 [17]. Peptide 111–139 contains a CD4, a CD8 and a B-cell epitope and is the major target for the T-cell response in H-2b mice [18]. Plasmid pLP503-OVA contains a linker encoding ovalbumin peptide 323–339 in front of the uidA gene. This construct was also introduced into L. plantarum and the resulting construct was designated L. plantarum-c.
Analysis of expression of heterologous antigens
For analysis of protein expression, sonicated extracts of recombinant L. plantarum were analysed by Western immuno-blotting. Blots were developed with a polyclonal antibody against β-glucuronidase, or against peptide 111–139 of Der p 1, and visualized by alkaline phosphatase detection. The polyclonal antibody against peptide 111–139 of Der p 1 was obtained from mice immunized with peptide 111–139 in IFA.
Preparation of antigens
For immunization purposes, recombinant L. plantarum expressing heterologous antigen peptide 111–139 of Der p 1 fused to β-glucuronidase (denoted L. plantarum-p1) or, as a control, expressing peptide 323–339 of ovalbumine fused to β-glucuronidase (denoted L. plantarum-c), were grown in Lactobacilli Carrier Medium (LCM) with 1% glucose and 5 µg/ml erythromycin to an optimal density of 0·6 (mid-log phase), which corresponds to approximately 5 × 108 bacteria/ml. The bacteria were harvested, washed and resuspended at the appropriate concentration in phosphate-buffered saline (PBS). The soluble fraction of sonicated extracts of L. plantarum 256 in PBS was used in ELISA. Peptide 111–139 of Der p 1 (FGISNYCQIYPPNANKIREALAQTHSALA) and peptide 323–339 of ovalbumin (ISQAVHAAHAEINEAGR) were made on an ABIMED 422 synthesizer (ABIMED, Langenfeld, Germany) using the simultaneous peptide synthesis method. The purity of the peptide was verified by reverse phase C18 HPLC (Lichrospher, Merck, Darmstadt, Germany) and was shown to be routinely over 75%.
Animals and immunization protocols
Female C57Bl/6 J (H-2b) mice (6–8-week-old) from Charles River/Broekman Institute (Someren, the Netherlands) were used in this study. Two groups of three to four mice, were immunized intranasally, twice with a 4‐week interval, with recombinant L. plantarum-p1 or L. plantarum-c (5 × 109) for three consecutive days. Serum, spleens and lymph nodes were harvested 4 weeks later. To investigate immunomodulatory properties of lactobacilli, three groups of four to five mice were immunized subcutaneously with 3 µg peptide 111–139 in Incomplete Freund's Adjuvant (IFA) in the flank. One week after priming, the mice were treated intranasally with either PBS, L. plantarum-c or L. plantarum-p1 (5 × 109) for 3 consecutive days. Serum and spleens were harvested 10 days after the last intranasal immunization.
Cell culture
Single cell suspensions of splenocytes or lymph node cells were cultured in 96-flat-well microtitre plates (Nunc, Denmark) at 2 × 106 cells/ml in 200 µl/well RPMI 1640 supplemented with 5% fetal calf serum, 2 mm l-glutamine, 20 IU/ml penicillin, 20 µg/ml streptomycin (Gibco, Grand Island, NY, USA) and 50 µm 2-mercaptoethanol (Sigma) at 37°C in humidified air containing 5% CO2. Cells were incubated in triplicate wells either alone, or with varying concentrations of antigens, for the assessment of proliferation or the level of cytokines in supernatant fluids at 72 h (previously determined as the optimum time-point). Proliferation was measured by pulsing for the last 16 h with 0·6 µCi/well 3H-thymidine (Amersham, UK), harvesting the contents of each well onto glass fibre mats and determining the incorporation of 3H-TdR using a β-plate counter (Canberra Packard, Meriden, CT, USA).
Cytokine assays
IFNγ was measured by ELISA using the rat anti-mouse coating antibody R4–6A2 and biotinylated detector antibody XMG1·2 pair (Pharmingen, San Diego, CA, USA). IL4 and IL5 were also measured by ELISA using coating MoAbs 11B11 or TRFK-5 and biotinylated detector MoAbs BvD6–24G2 or TRFK-4 (Pharmingen). The binding of biotinylated antibody was detected with alkaline phosphatase-conjugated streptavidin (Amersham, UK), followed by p-nitrophenylphosphate (Sigma, Poole, UK) at 1 mg/ml in Tris/HCl buffer, pH 9·7;6, as substrate. Optical density at 405 nm (O.D. 405 nm) of the product was measured using a Biorad ELISA reader. Recombinant murine IFNγ, IL4 and IL5 (Pharmingen) were used to generate a standard curve.
ELISA for specific antibodies
Der p 1 peptide p111–139 (5 µg/ml) was coated onto Maxisorp microtitre plates (Nunc) in bicarbonate coating buffer (Sigma), overnight at 4°C. After blocking (1 h, PBS-1% BSA) and washing, serum dilutions were incubated for 2 h at 37°C. After washing, the amounts of specific IgG isotypes bound were detected using alkaline phosphatase-conjugated rat antimouse IgG1 and IgG2a (Pharmingen) for 1 h at 37°C. The enzyme substrate p-nitrophenylphosphate was added and the soluble product measured as for the cytokine ELISAs. To measure IgE levels, rat anti-mouse IgE antibody (R35–72, Pharmingen) was coated onto Maxisorp microtitre plates in bicarbonate coating buffer overnight at 4°C. After blocking (1 h, PBS-5% skim milk) and washing, serum dilutions were incubated for 2 h at 37°C. The amount of IgE or peptide-specific IgE was detected using biotinylated rat anti-mouse IgE (R35–92, Pharmingen) or biotinylated peptide 111–139. The binding of biotinylated antibody/peptide was detected with alkaline phosphatase-conjugated streptavidin and by p-nitrophenylphosphate, as described for the cytokine ELISAs.
Statistics
Statistical analysis was performed using the Mann–Whitney test and a P-value < 0·05 was considered significant.
Results
Expression of fusion proteins in L. plantarum
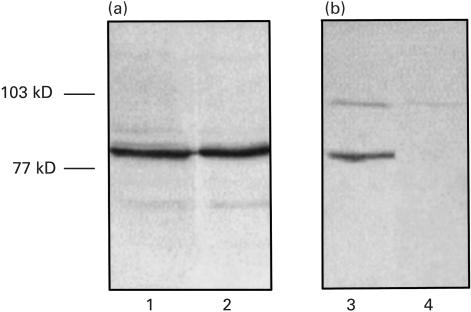
To investigate the immunomodulatory properties of lactobacilli, a house dust mite peptide and an ovalbumin peptide were expressed in L. plantarum, a strain that was previously shown to colonize the murine intestine for approximately 12 days (C. Havenith, unpublished observation). Both fusion proteins, which are expressed in the cytoplasm of the lactobacilli, are visible as a band of 80 kDa, the calculated molecular weight, when an antibody specific for β-glucuronidase is used for staining (Fig. 1a). When an antibody specific for peptide 111–139 is used for staining, as expected, only the fusion protein expressed by L. plantarum-p1 is visible. The fusion protein constitutes between 0·2 and 0·5% of a soluble extract of lactobacilli, as determined on CBB-stained gels using BSA as a standard for comparison (data not shown).
Fig. 1.
Expression of β-glucoronidase fusions by recombinant Lactobacillus plantarum. Western blot analysis of sonicated extracts of L. plantarum-p1 (lanes 1 and 3) and L. plantarum-c (lanes 2 and 4). Blots were developed with a polyclonal antibody directed to β-glucoronidase (a) or a polyclonal antibody to Der p 1 : 111–139 (b).
Induction of Der p1 specific T-cell responses with L. plantarum-p1
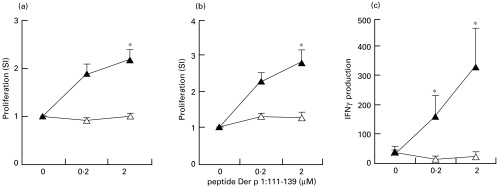
In order to investigate qualitative and quantitative aspects of the T-cell response raised with L. plantarum, C57BL/6 mice were immunized intranasally with recombinant lactobacilli, as described in Materials and methods. Intranasal immunization with L. plantarum-p1 leads to priming of peptide 111–139-specific T-cell proliferation both in draining lymph node and in spleen cells (Fig. 2a,b). This response was shown to be specific since spleen and lymph node cells of L. plantarum-c-immunized mice did not respond to this peptide. Cell supernatant fluids were assayed for the presence of IFNγ, IL5 and IL10. No cytokine production was detectable in lymph node cell cultures. In spleen cell cultures, low levels of IFNγ could be detected in response to peptide 111–139 (Fig. 2c). These results indicate that intranasal immunization with L. plantarum-p1 results in priming of T cells which can produce small amounts of IFNγ, indicating that these cells have some Th1 characteristics but are not strongly polarized.
Fig. 2.
T-cell responses to recombinant Lactobacillus plantarum. Mice (n = 8) were immunized intranasally with recombinant L. plantarum-c (open triangles) or L. plantarum-p1 (closed triangles), twice with a 4-week interval. Four weeks after the second immunization, proliferative responses of lymph node cells (a) and spleen cells (b) to peptide Der p 1 : 111–139 were determined. IFNγ in response to peptide was only detectable in spleen cell cultures (c). *P < 0·05 as compared with the L. plantarum-c group.
Modulation of T-cell responses with recombinant L. plantarum
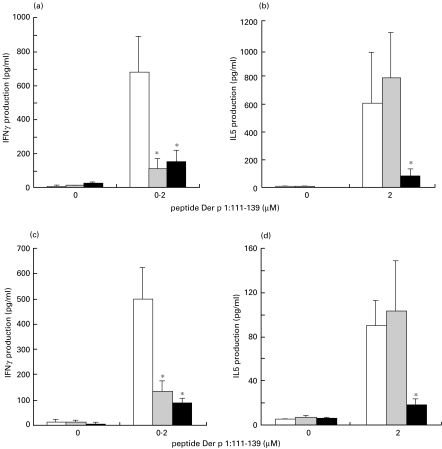
To study immunomodulatory properties of lactobacilli, we used a system in which C57BL/6 mice were immunized subcutaneously with Der p 1 peptide 111–139 in Incomplete Freund's Ajuvant. This immunization protocol leads to the induction of a T-cell response that is characterized by the production of both IL5 and IFNγ (see PBS group in Fig. 3). To determine whether lactobacillli can modulate the Th1 or the Th2 component of this response, mice were treated intranasally seven days after priming for three consecutive days with PBS, L. plantarum-c or L. plantarum-p1. This treatment regimen was chosen to mimic some aspects of classical immunotherapy protocols used in humans. A representative experiment is shown in Fig. 3a,b. Surprisingly, treatment with both L. plantarum-c and L. plantarum-p1 resulted in down-regulation of IFNγ production, indicating that this regimen does not lead to enhanced cell-mediated immune responses but, in contrast, dramatically inhibits the Th1 component of the response. Since both recombinants have the same effect, this inhibition is a result of the lactobacilli themselves and is not dependent on the presence of the Der p 1 peptide. IL5 production was also decreased, but this decrease was dependent on the presence of the Der p 1 peptide in the recombinants since control lactobacilli did not alter IL5 production. In contrast to the reported Th1-inducing properties of lactobacilli, the treatment regimen used in this study led to non-specific down-regulation of the Th1 component of the response and antigen-specific down-regulation of the Th2 component. IL4 production was not detectable and IL10 production was similar in all three groups (data not shown). Proliferative responses to the peptide were similar in all three groups, indicating that the recombinants do not induce tolerance.
Fig. 3.
Modulation of Der p 1 : 111–139-specific T-cell responses. Mice were immunized subcutaneously (n = 9) (a, b) or intraperitoneally (n = 6) (c, d) with Der p 1 : 111–139 in IFA. On days 8, 9 and 10, mice were treated intranasally with PBS, Lactobacillus plantatrum-c or L. plantarum-p1, and spleen cell responses to Der p 1 : 111–139 were measured 10 days later. Both IFNγ production (a and c) and IL5 production (b and d) were determined. The optimal cytokine response (to 0·2 and 2 µm peptide for IFNγ and IL5, respectively) is plotted. *P < 0·05 as compared with the PBS group. (□), PBS; ( ), L. plantarum-c; (
), L. plantarum-c; ( ), L. plantarum-p1.
), L. plantarum-p1.
Modulation of antibody responses with L. plantarum
Sub-cutaneous immunization with peptide 111–139 in IFA resulted in high IgG1 production and low IgG2a production (see PBS group in Table 1), which is a reflection of the Th2 dominant response. Treatment with L. plantarum-p1 or L. plantarum-c did not alter the antibody response, although a trend in inhibition of IgG1 was observed (Table 1). This inhibition never reached statistical significance (P-value around 0·1).
Table 1.
Antibody responses of mice treated with recombinant lactobacilli
| Antibodies specific for Der p1 : 111–139 | |||||
|---|---|---|---|---|---|
| Group | Route | IgG1 (titre) | IgG2a (titre) | IgE (arbitrary units) | Total IgE (ng/ml) |
| Naive | – | – | – | – | 50 ± 21 |
| PBS | s.c. | 3·39 ± 0·31 | 1·89 ± 0·26 | ND* | 234 ± 18 |
| L. plantarum-c | s.c. | 2·61 ± 0·27 | 1·39 ± 0·23 | ND | 263 ± 33 |
| L. plantarum-p1 | s.c. | 2·78 ± 0·19 | 1·28 ± 0·17 | ND | 134 ± 21 |
| PBS | i.p. | 4·08 ± 0·08 | 1·67 ± 0·31 | 515 ± 120 | 1449 ± 179 |
| L. plantarum-c | i.p. | 4·00 ± 0·13 | 1·75 ± 0·21 | 333 ± 60 | 1283 ± 116 |
| L. plantarum-p1 | i.p. | 3·92 ± 0·20 | 1·58 ± 0·24 | 339 ± 73 | 1030 ± 178 |
ND, below the detection limit.
Effect of lactobacilli on IgE production
A parameter which truly reflects the presence of Th2 cells is the production of IgE [8,19]. Subcutaneous immunization with Der p 1 peptide 111–139 in IFA did result in an increase in total IgE production (Table 1), but production of peptide-specific IgE was not detectable. In order to study the effects of lactobacilli on Der p 1 : 111–139-specific IgE production, a different immunization protocol was used in which mice were immunized intraperitoneally with peptide 111–139 in IFA. This resulted in the production of very high levels of IgG1 and led to a greater increase in total serum IgE than subcutaneous immunization (Table 1). Also, peptide 111–139-specific IgE could be detected using this immunization protocol (Table 1). The effects of treatment with recombinant lactobacilli on the T-cell response were similar to those described above (Fig. 3c,d). However, total IgE levels were not altered (Table 1) by treatment with recombinant lactobacilli strains, indicating that the inhibition of the T-cell response is not a general immune suppression. Peptide-specific IgE production was somewhat reduced in the L. plantarum-treated groups. However, this reduction was not statistically significant and was probably due to the large variation found in peptide-specific IgE.
Discussion
Lactobacilli have been shown to exhibit strong (immuno)modulatory properties including prevention of infections, anti-tumour activity, modulation of autoimmune diseases and allergic disorders [2,3,14,21]. Although it has been reported that lactobacilli can induce IL12 production in vitro, the mechanisms by which lactobacilli can modulate immune responses in vivo are largely unknown, and the present study was designed to gain a better insight into this process.
First, the adjuvant properties of L. plantarum were studied. It appeared that L.plantarum-p1, when delivered intranasally, induced Der p1 : 111–139-specific T-cell proliferative responses. Probably, lactobacilli or fragments of lactobacilli, reach the nasal-associated lymphoid tissue where antigen presentation takes place and T cells are primed. These data confirm previous observations that recombinant lactobacilli expressing fragment C of tetanus toxin intracellularly induce systemic immune responses [22].
When the T-cell response was further characterized, it was shown to have some Th1 properties, but was not strongly polarized. This indicates that mucosally-delivered L. plantarum does not have strong Th1 promoting properties. These data are consistent with those described by Maassen et al. [23] showing that oral administration of L. plantarum does not induce Th1 cytokine expression in the intestinal mucosa. In contrast, Shida et al. [11] have shown that L. casei-Shirota can induce IFNγ. However, since the latter study described in vitro effects of lactobacilli, the results cannot be directly compared with our study.
In order to investigate immunomodulatory properties of lactobacilli, a simple model was used in which parameters of both Th1 and Th2 responses could be evaluated. Mucosally-delivered lactobacilli inhibited IFNγ production to peptide 111–139 of Der p 1 in an antigen non-specific manner. IL5 production was also reduced, although this effect was dependent on the presence of the peptide in the bacteria. Since IL4 production could not be detected in this model, the presence of IgE was used as an indirect way of assessing the production of this cytokine. No difference in total IgE and antigen-specific IgE production could be detected after treatment with L. plantarum, suggesting that IL4 production was unaffected. This is in contrast to the inhibitory effects of lactobacilli on IgE production in vitro [12] and in vivo when given intraperitoneally in a heat-killed form [11]. The possibility cannot be excluded that the antibody response induced using peptide in IFA was too strong to be susceptible to modulation using our treatment protocol. An alternative explanation for the discrepancy is that the time-frame used in our study may not have been optimal for the induction and modulation of IgE responses.
Previous studies by Hoyne et al. have shown that intranasal immunization with soluble Der p 1 peptide alone induces tolerance in mice previously primed with peptide in CFA [24,25]. This tolerance was characterized by inhibition of a proliferative response to the peptide, and at least one dose of 100 µg of peptide was required to obtain this effect. The calculated amount of peptide present in each dose of recombinant lactobacilli is 25–50 ng. In addition, this peptide is given as a particulate antigen as it is expressed in the cytoplasm of the lactobacilli. Since treatment with recombinant lactobacilli does not inhibit the proliferative response to the peptide, our data are not reminiscent of the intranasal tolerance described by Hoyne et al.
Taken together, these data indicate that intranasal administration of L. plantarum inhibits non-specifically the Th1 component of the response, and this may be an explanation for the beneficial effects of lactobacilli on the development of Th1-mediated autoimmune disorders such as arthritis, colitis and diabetes [13–15]. Specific inhibitory effects on IL5 production are consistent with the observed effects of the presence of lactobacilli in the intestinal flora on development of allergy [9,10]. In our model, however, the immunomodulatory properties of lactobacilli do not seem to be attributable to their Th1-promoting properties. In fact, mucosal delivery of recombinants did not result in the induction of a strongly polarized Th1 response. Rather, our data support the hypothesis that lactobacilli stimulate immunoregulatory mechanisms which are operative at mucosal surfaces, and ensure the development of a well balanced, non-pathological immune response to harmless antigens encountered via the mucosa.
The data described here indicate that recombinant lactobacilli expressing allergens may be candidates for the modulation of allergic disorders. However, their effectiveness in relieving clinical symptoms of allergic disease in model systems where such parameters can be monitored [26] still needs to be established. In addition, the long-term effects of treatment with lactobacilli need to be studied. The fact that no effects on IgE levels were detected may not limit their use for the treatment of allergy, since classical immunotherapy, which is an effective way of relieving clinical symptoms of allergic disease, also does not decrease IgE levels [27–29]. In addition, the non-specific effect of lactobacilli on the Th1 component of the immune response may explain their beneficial effects in animal models of autoimmunity [13–15].
Acknowledgments
The authors would like to acknowledge Drs M. Luca and A. Nagelkerken for helpful discussion.
References
- 1.Molin G, Jeppsson B, Johansson ML, et al. Numerical taxonomy of Lactobacillus spp. associated with healthy and diseased mucosa of the human intestines. J Appl Bacteriol. 1993;74:314–23. doi: 10.1111/j.1365-2672.1993.tb03031.x. [DOI] [PubMed] [Google Scholar]
- 2.Wagner RD, Pierson C, Warner T, et al. Biotherapeutic effects of probiotic bacteria on candidiasis in immunodeficient mice. Infect Immun. 1997;65:4165–72. doi: 10.1128/iai.65.10.4165-4172.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kato I, Endo K, Yokokura T. Effects of oral administration of Lactobacillus casei on antitumor responses induced by tumor resection in mice. Int J Immunopharmacol. 1994;16:29–36. doi: 10.1016/0192-0561(94)90116-3. [DOI] [PubMed] [Google Scholar]
- 4.Kato I, Tanaka K, Yokokura T. Lactic acid bacterium potently induces the production of interleukin-12 and interferon-gamma by mouse splenocytes. Int J Immunopharmacol. 1999;21:121–31. doi: 10.1016/s0192-0561(98)00072-1. 10.1016/S0192-0561(98)00072-1. [DOI] [PubMed] [Google Scholar]
- 5.Miettinen M, Matikainen S, Vuopio-Varkila J, et al. Lactobacilli and streptococci induce interleukin-12 (IL-12), IL-18, and gamma interferon production in human peripheral blood mononuclear cells. Infect Immun. 1998;66:6058–62. doi: 10.1128/iai.66.12.6058-6062.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hessle C, Hanson LA, Wold AE. Lactobacilli from human gastrointestinal mucosa are strong stimulators of IL-12 production. Clin Exp Immunol. 1999;116:276–82. doi: 10.1046/j.1365-2249.1999.00885.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mosmann TR, Coffman RL. TH1 and TH2 cells: different patterns of lymphokine secretion lead to different functional properties. Annu Rev Immunol. 1989;7:145–73. doi: 10.1146/annurev.iy.07.040189.001045. [DOI] [PubMed] [Google Scholar]
- 8.Gascan H, Gauchat JF, Roncarolo MG, Yssel H, Spits H, de Vries JE. Human B cell clones can be induced to proliferate and to switch to IgE and IgG4 synthesis by interleukin 4 and a signal provided by activated CD4+ T cell clones. J Exp Med. 1991;173:747–50. doi: 10.1084/jem.173.3.747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sepp E, Julge K, Vasar M, Naaber P, Bjorksten B, Mikelsaar M. Intestinal microflora of Estonian and Swedish infants. Acta Paediatr. 1997;86:956–61. doi: 10.1111/j.1651-2227.1997.tb15178.x. [DOI] [PubMed] [Google Scholar]
- 10.Bjorksten B, Naaber P, Sepp E, Mikelsaar M. The intestinal microflora in allergic Estonian and Swedish 2-year-old children. Clin Exp Allergy. 1999;29:342–6. doi: 10.1046/j.1365-2222.1999.00560.x. 10.1046/j.1365-2222.1999.00560.x. [DOI] [PubMed] [Google Scholar]
- 11.Shida K, Makino K, Morishita A, et al. Lactobacillus casei inhibits antigen-induced IgE secretion through regulation of cytokine production in murine splenocyte cultures. Int Arch Allergy Immunol. 1998;115:278–87. doi: 10.1159/000069458. [DOI] [PubMed] [Google Scholar]
- 12.Murosaki S, Yamamoto Y, Ito K, et al. Heat-killed Lactobacillus plantarum L-137 suppresses naturally fed antigen-specific IgE production by stimulation of IL-12 production in mice. J Allergy Clin Immunol. 1998;102:57–64. doi: 10.1016/s0091-6749(98)70055-7. [DOI] [PubMed] [Google Scholar]
- 13.Matsuzaki T, Nagata Y, Kado S, et al. Prevention of onset in an insulin-dependent diabetes mellitus model, NOD mice, by oral feeding of Lactobacillus casei. APMIS. 1997;105:643–9. doi: 10.1111/j.1699-0463.1997.tb05066.x. [DOI] [PubMed] [Google Scholar]
- 14.Kato I, Endo-Tanaka K, Yokokura T. Suppressive effects of the oral administration of Lactobacillus casei on type II collagen-induced arthritis in DBA/1 mice. Life Sci. 1998;63:635–44. doi: 10.1016/s0024-3205(98)00315-4. 10.1016/S0024-3205(98)00315-4. [DOI] [PubMed] [Google Scholar]
- 15.Madsen KL, Doyle JS, Jewell LD, Tavernini MM, Fedorak RN. Lactobacillus species prevents colitis in interleukin 10 gene-deficient mice [see comments] Gastroenterology. 1999;116:1107–14. doi: 10.1016/s0016-5085(99)70013-2. [DOI] [PubMed] [Google Scholar]
- 16.Pouwels PH, Leer RJ, Boersma WJ. The potential of Lactobacillus as a carrier for oral immunization: development and preliminary characterization of vector systems for targeted delivery of antigens. J Biotechnol. 1996;44:183–92. doi: 10.1016/0168-1656(95)00140-9. [DOI] [PubMed] [Google Scholar]
- 17.Hoyne GF, Callow MG, Kuo MC, Thomas WR. Characterization of T-cell responses to the house dust mite allergen Der p II in mice. Evidence for major and cryptic epitopes. Immunology. 1993;78:65–73. [PMC free article] [PubMed] [Google Scholar]
- 18.Hoyne GF, Callow MG, Kuo MC, Thomas WR. Comparison of antigen presentation by lymph node cells from protein and peptide-primed mice. Immunology. 1993;78:58–64. [PMC free article] [PubMed] [Google Scholar]
- 19.Vercelli D, Jabara HH, Arai K, Geha RS. Induction of human IgE synthesis requires interleukin 4 and T/B cell interactions involving the T cell receptor/CD3 complex and MHC class II antigens. J Exp Med. 1989;169:1295–307. doi: 10.1084/jem.169.4.1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yip HC, Karulin AY, Tary-Lehmann M, et al. Adjuvant-guided type-1 and type-2 immunity: infectious/noninfectious dichotomy defines the class of response. J Immunol. 1999;162:3942–9. [PubMed] [Google Scholar]
- 21.Wheeler JG, Shema SJ, Bogle ML, et al. Immune and clinical impact of Lactobacillus acidophilus on asthma. Ann Allergy Asthma Immunol. 1997;79:229–33. doi: 10.1016/S1081-1206(10)63007-4. [DOI] [PubMed] [Google Scholar]
- 22.Shaw DM, Gaerthe B, Leer RJ, et al. Engineering the microflora to vaccinate the mucosa: serum immunoglobulin G responses and activated draining cervical lymph nodes following mucosal application of tetanus toxin fragment C-expressing lactobacilli. Immunology. 2000;100:510–8. doi: 10.1046/j.1365-2567.2000.00069.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Maassen CB, van Holten JC, Balk F, et al. Orally administered Lactobacillus strains differentially affect the direction and efficacy of the immune response. Vet Q. 1998;20(Suppl.):S81–3. :S81. S83. [PubMed] [Google Scholar]
- 24.Hoyne GF, O'Hehir RE, Wraith DC, Thomas WR, Lamb JR. Inhibition of T cell and antibody responses to house dust mite allergen by inhalation of the dominant T cell epitope in naive and sensitized mice. J Exp Med. 1993;178:1783–8. doi: 10.1084/jem.178.5.1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hoyne GF, Askonas BA, Hetzel C, Thomas WR, Lamb JR. Regulation of house dust mite responses by intranasally administered peptide: transient activation of CD4+ T cells precedes the development of tolerance in vivo. Int Immunol. 1996;8:335–42. doi: 10.1093/intimm/8.3.335. [DOI] [PubMed] [Google Scholar]
- 26.Hamada K, Goldsmith CA, Goldman A, Kobzik L. Resistance of very young mice to inhaled allergen sensitization is overcome by coexposure to an air-pollutant aerosol. Am J Respir Crit Care Med. 2000;161:1285–93. doi: 10.1164/ajrccm.161.4.9906137. [DOI] [PubMed] [Google Scholar]
- 27.Zenner HP, Baumgarten C, Rasp G, et al. Short-term immunotherapy: a prospective, randomized, double-blind, placebo-controlled multicenter study of molecular standardized grass and rye allergens in patients with grass pollen-induced allergic rhinitis. J Allergy Clin Immunol. 1997;100:23–9. doi: 10.1016/s0091-6749(97)70190-8. [DOI] [PubMed] [Google Scholar]
- 28.Bousquet J, Maasch H, Martinot B, Hejjaoui A, Wahl R, Michel FB. Double-blind, placebo-controlled immunotherapy with mixed grass-pollen allergoids. II. Comparison between parameters assessing the efficacy of immunotherapy. J Allergy Clin Immunol. 1988;82:439–46. doi: 10.1016/0091-6749(88)90017-6. [DOI] [PubMed] [Google Scholar]
- 29.Ebner C, Siemann U, Bohle B, et al. Immunological changes during specific immunotherapy of grass pollen allergy: reduced lymphoproliferative responses to allergen and shift from TH2 to TH1 in T-cell clones specific for Phl p 1, a major grass pollen allergen. Clin Exp Allergy. 1997;27:1007–15. doi: 10.1111/j.1365-2222.1997.tb01252.x. [DOI] [PubMed] [Google Scholar]