Abstract
Circulating polymorphonuclear neutrophils (PMNs) are known to increase in number and are functionally activated in the acute phase of Kawasaki disease (KD). In the present study, we investigated whether the apoptosis of PMNs is deregulated in KD. When the isolated PMNs were cultured in vitro, the proportions of spontaneous apoptotic PMNs (annexin V+ cells and cells with fragmented DNA) were found to be significantly lower (P < 0·01) in the patients with KD (n = 25) than in the patients with a bacterial infection (n = 20) or a viral infection (n = 20), or in healthy children (n = 20). The proportion of circulating Fas-positive PMNs was also significantly lower (P < 0·01) in the acute KD patients than in the other groups. In the acute phase of KD, the proportion of spontaneous apoptotic PMNs showed a significant positive correlation (P < 0·01) with the proportions of circulating Fas-positive PMNs. Furthermore, the agonistic anti-Fas MoAb (CH-11) induced a significant increase in the proportion of apoptotic PMNs in the patients with a viral infection and healthy children, but not in either the patients with KD or the patients with a bacterial infection. In the intracellular expression of anti- and pro-apoptotic proteins, the A1/Bax ratio was significantly higher in acute KD than in the other groups. These findings indicate that PMN apoptosis is inhibited during the acute phase of KD and also suggest that both the resistance against the Fas-mediated death signal and the down-regulation of the mitochondrial apoptotic signalling pathway due to an altered balance of Bcl-2 protein expression are responsible for the delayed PMN apoptosis.
Keywords: A1/Bax, apoptosis, Fas, Kawasaki disease, neutrophils
Introduction
Human polymorphonuclear neutrophils (PMNs) are constitutively programmed to undergo apoptosis, and their life-span is short: peripheral PMNs have a circulatory half-life of 6–10 h in vivo, and about half of isolated PMNs die within the first 24 h ex vivo [1–3]. Fas antigen is constitutively expressed on the surfaces of PMNs [4,5], while FasL is not, based on the findings of recent studies [6,7]. An anti-Fas monoclonal antibody (MoAb) is reported to accelerate the apoptotic PMN death [4], and the Fas system is considered to play an important role in the regulation of spontaneous PMN apoptosis [5]. As an alternative pathway, the process of apoptosis is regulated by different members of Bcl-2 family protein [8]. The balance of expression between pro-apoptotic protein (Bax) and antiapoptotic proteins (A1 and Bcl-XL) determines the fate of the PMNs [9–11]. Although PMNs have been shown to play an important role in acute inflammation as the host defence, the abnormal activation of PMNs can lead to host tissue injury by secreting a large number of toxic mediators and enzymes [12,13]. Since the prolonged survival of activated PMNs may accelerate tissue damage, PMN apoptosis appears to represent a suitable mechanism for limiting the autotoxic potential of PMNs and thereby resolving inflammation [14]. In clinical studies, spontaneous PMN apoptosis is reported to be inhibited in systemic inflammatory response syndrome (SIRS) [15,16], severe sepsis [17] and acute respiratory distress syndrome [18], thus suggesting that delayed PMN apoptosis may be involved in the pathogenesis of these diseases.
Kawasaki disease (KD) is an acute febrile illness characterized by multi-systemic vasculitis and is seen mainly in infants and young children [19]. This disease has a worldwide distribution and is generally self-limiting. Although treatment with a combination of aspirin and intravenous immunoglobulin is generally effective, from 5 to 15% of the patients develop coronary artery lesions that may lead to myocardial infarction [20]. The immune system shows marked activation during the acute phase of KD, thus suggesting that the increased production of cytokines leads to the activation and damage of endothelial cells [21]. Although KD is believed widely to be caused by an infectious agent, its aetiology remains unclear. Toxic neutrophils, morphologically characterized by cytoplasmic vacuolization and toxic granulation with leukocytosis and a shift to the left, are present in the acute phase of KD [22,23]. PMNs also secrete a large amount of autotoxic mediators such as reactive oxygen species (ROS) [24] and elastase [25] during the acute phase of KD, thus suggesting that such activated PMNs contribute to the pathogenesis of KD vasculitis. The aim of the present study is to investigate whether the apoptotic potential of PMNs changes in the acute phase of KD and, if so, to determine the mechanism of the deregulated signal transduction in the Fas-and Bcl-2 protein family dependent pathways.
Materials and methods
Patients
The patients and healthy controls enrolled in this study were classified into four groups consisting of patients with KD, patients with bacterial infection (BI), patients with viral infection (VI) and healthy controls (HC). The patient profiles are shown in Table 1. The patients were hospitalized at the National Defense Medical College Hospital between January 1999 and August 2000. Twenty-five KD patients were enrolled within 7 days of the onset of illness, with day 1 defined as the first day of fever, and all patients met the diagnostic criteria for KD established by the Japanese Kawasaki Disease Research Committee [26]. No bacterial species were identified in blood cultures from the KD patients. All patients were scheduled to receive both aspirin (30 mg/kg/day) and intravenous immunoglobulin (IVIG, 1 g/kg or 2 g/kg). No patients with KD had a coronary aneurysm. Serial blood samples were obtained from all KD patients in the acute phase (before IVIG therapy, days 3–7) and in the convalescent phase (days 21–36), when the C-reactive protein (CRP) of each patient was < 0·3 mg/dL. The BI group included 20 children: 14 microbiologically documented and six clinically documented. Six had a urinary tract infection with Escherichia coli, three had pneumonia with Streptococcus pneumoniae, three had pneumonia with Haemophilus influenzae and two had enterocolitis with Salmonella (O9). These organisms were isolated from urine, pharyngeal mucus and stool cultures. Six patients had pneumonia with high CRP levels of more than 8·0 mg/dL, although the causative microorganisms were not identified. The VI group included 10 patients consisting of five with an upper respiratory infection, 20 with bronchitis and five with enterocolitis. Although no viral culture was carried out, the CRP levels were less than 0·3 mg/dL in all patients in this group. The HC group consisted of 20 healthy children serving as controls. They had neither any underlying diseases nor were receiving any medications. Informed consent was obtained from the parents of all children, and the protocol was approved by the institutional review board.
Table 1.
Patient characteristics and laboratory findings
| Group | Acute KD | Conv. KD | BI | VI | HC |
|---|---|---|---|---|---|
| Median age | 26 months | 25 months | 27 months | 25 months | |
| (range) | (8 months−2 years) | (3 months−4 years) | (6 months−5 years) | (3 months−6 years) | |
| Number | 25 | 20 | 20 | 20 | |
| (male/female) | (18/7) | (13/7) | (10/10) | (17/3) | |
| CRP (mg/dl) | 11·2 ± 1·6* | <0·3 | 12·3 ± 1·8* | <0·3 | <0·3 |
| Leucocytes (/μl) | 14464 ± 854* | 8887 ± 1996 | 16050 ± 2117* | 8115 ± 806 | 8350 ± 657 |
| Neutrophils (PMNs) (/μl) | 10491 ± 708* | 3910 ± 1638 | 11478 ± 1684* | 5213 ± 727 | 4138 ± 383 |
| Immature PMNs (/μl) | 2811 ± 482* | 164 ± 258 | 3225 ± 539* | 317 ± 156 | 111 ± 91 |
| Monocytes (/μl) | 704 ± 134 | 478 ± 190 | 1053 ± 267 | 324 ± 124 | 407 ± 38 |
KD, Kawasaki disease; BI, bacterial infection; VI, viral infection; HC, healthy controls; CRP, C-reactive protein; conv., convalescent.
P <0.01 vs. conv. KD, VI and HC.
Reagents
The following reagents were prepared: propidium iodide (PI) from Molecular Probe, Eugene, OR, USA; Triton X-100, trypan blue, ethidium bromide and bovine serum albumin (BSA) from the Sigma Chemical Co., St Louis, MO, USA; annexin V-FITC kit, PE-conjugated anti-Fas MoAb (clone UB2), blocking anti-Fas MoAb (clone ZB4) from Immunotech Co., Marseille, France; agonistic anti-Fas MoAb (clone CH-11), anti-Bax MoAb (clone 4F11) from MBL Medical and Biological Laboratories Co., Nagoya, Japan; anti-A1 polyclonal antibody (goat IgG) from Santa Cruz Biotechnology, Santa Cruz, CA, USA.
Sample preparations and culture of PMNs
Peripheral blood PMNs were immediately isolated by density gradient centrifugation using a one-step Polymorph (Accurate Chemical and Scientific Corp, Westbury, NY, USA) and washed with PBS containing 1% BSA. Contaminated erythrocytes were removed by hypotonic lysis. The PMNs were then washed twice with PBS. The purity of the PMNs was more than 95%, as assessed by a flow cytometer using forward and side scatter. The viability of the PMNs were more than 98%, as evaluated by trypan blue dye exclusion. The cells were then resuspended in RPMI-1640 containing 10% fetal bovine serum (FBS) at a concentration of 1 × 106/ml and were cultured at 37°C in a 5% CO2 atmosphere for 24 and 48 h.
To investigate the effect of anti-Fas MoAb on PMN apoptosis, an agonistic anti-Fas MoAb (CH-11, 500 ng/ml) or a blocking anti-Fas MoAb (ZB4, 500 ng/ml) was added to the culture medium, followed by incubation for 24–48 h. When both antibodies were added to the same culture medium, the ZB4 was added 1 h before the addition of the CH-11.
Analysis of apoptotic cells by a flow cytometer
After culturing PMNs for 24 and 48 h, the cells were suspended in the binding buffer (10 mm HEPES, 140 mm NaCl, 2 mm CaCl2, PH 7·4), followed by the staining of both Annexin V-FITC and PI. After a 10-min incubation at 4°C, the cells were analysed using the FACSCalibur flow cytometer (Becton Dickinson, San Jose, CA, USA). After setting the gate around the PMN population, the data were analysed by CellQuest software (Becton Dickinson). The results were presented as two-colour fluorescent diagrams of PMNs stained with antiannexin V-FITC and PI.
We also measured the proportion of PMNs with fragmented DNA. After the PMNs were treated with 0·1% Triton-X 100/PBS and RNase, the cells were then stained with PI for 1 h at 4°C. Apoptotic PMNs were was quantified by the flow cytometric determination of the proportion of cells with hypodiploid DNA (sub-G1 cells), as described previously by others [27].
Morphological assessment of apoptotic PMNs
Small aliquots of each sample (100 µl) were removed and spun in a cytospin, followed by staining with May–Giemsa. The smears were examined by oil immersion light microscopy at a final magnification of 400 × 0. The apoptotic PMNs were detected as cells showing features associated with chromatin condensation and fragmented nuclei.
Analysis of the Fas expression on the surface of PMNs by flow cytometer
The freshly isolated PMNs were stained with PE-conjugated anti-Fas MoAb (clone UB2) and isotype control MoAb for 30 min at 4°C. The cells were then analysed using a flow cytometer. The positive cells were defined as cells which had more than a 95% fluorescence intensity in the isotype control MoAb.
Intracellular expression of A1 and Bax in PMNs
The freshly isolated PMNs were treated with 2% paraformaldehyde/PBS, followed by treatment with 0·05% Triton-X 100/PBS. After blocking the cells with 5% BSA/PBS, the cells were incubated with anti-A1 polyconal antibody or anti-Bax MoAb for 30 min at 4°C. The cells were then stained with FITC-conjugated rabbit antigoat antibody or FITC-conjugated goat antimouse antibody for 30 min at 4°C. Finally, the cells were analysed with a flow cytometer.
Statistical analysis
All data are expressed as mean ± s.e., and differences were analysed using the Mann–Whitney test. Correlations between the proportions of spontaneous apoptotic PMNs and the peripheral PMN count or the proportions of circulating Fas-positive PMN were assessed using Spearman's rank correlation coefficient. A P-value < 0·05 was considered to be significant.
Results
Laboratory findings
The laboratory findings of four groups (KD, BI, VI and HC) are demonstrated in Table 1. The mean levels of CRP and the mean counts of leucocytes, PMNs and immature cells (band cells plus myelocytes) were significantly higher in the patients with acute KD and BI than in the patients with convalescent KD, acute VI and HC. There were no significant differences in the laboratory data between the acute KD and BI.
Spontaneous apoptosis of PMNs
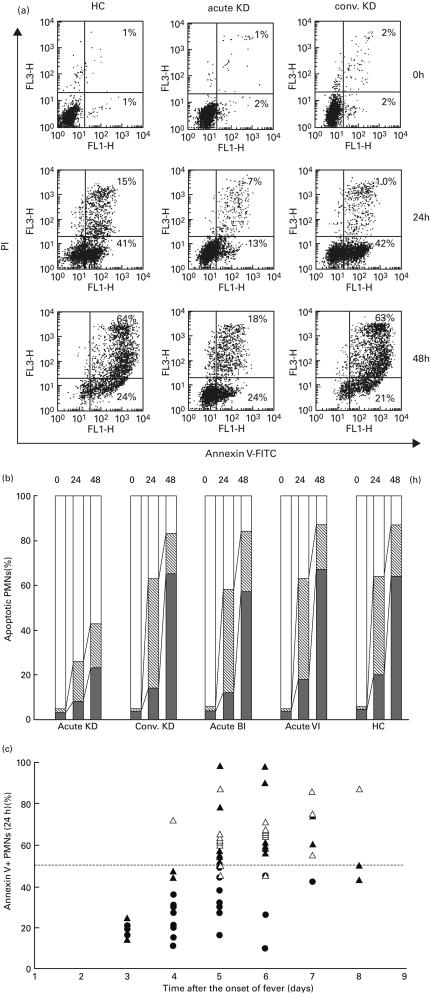
After the isolated PMNs were cultured in vitro for 24 and 48 h, the spontaneous apoptotic PMNs were analysed using a flow cytometer. Figure 1a demonstrates the dot-plot diagrams for representative cases. In the 24-h culture, the percentages (%) of annexin V-positive PI-negative (annexin V+PI−, early apoptotic) cells increased in the HC and a patient with convalescent KD (> 40%), in comparison to the patients with acute KD (13%). In the 48-h culture, the percentage of double-positive (annexin V+PI+, late apoptotic) cells increased in the HC and a patient with convalescent KD (> 60%), in comparison to the patients with acute KD (18%).
Fig. 1.
Serial changes of apoptotic PMNs measured by annexin V and propidium iodide (PI). (a) After isolated PMNs were cultured in vitro with FBS for 24 and 48 h, the spontaneous apoptotic PMNs (annexin V+PI+ and annexin V+PI− cells) were analysed by a dot-plot using a flow cytometer. The numbers in the quadrants of each plot indicate the percentage of positive cells. (b) The mean percentage of annexin V+PI+, annexin V+PI− and annexin V− PI− cells in each group was demonstrated in bar graphs. □, Annexin V− PI− cells; ▒, annexin V+ PI− cells; ▪, annexin V+ PI+ cells. (c) The percentage of annexin V+ cells (y-axis) in 24-h culture was plotted according to the days (x-axis) after the onset of fever, in patients with acute KD (•), acute BI (▴) and acute VI(▵).
The mean percentage of annexin V+PI+, annexin V+PI− and annexin V−PI− cells in each group is plotted in Fig. 1b. The mean percentage of annexin V+PI− cells in the 24-h culture and the mean percentage of annexin V+PI+ cells in the 48-h culture were significantly lower (P < 0·01) in acute KD than in the other groups. The mean percentage of annexin V+ (annexin V+PI+ plus annexin V+PI−) cells in both the 24- and 48-h cultures was significantly lower (P < 0·01) in acute KD than in the other groups.
The percentage of annexin V+ PMNs in 24-h culture was plotted on the days after the onset of fever in each patient with acute KD, BI and VI (Fig. 1c). From days 3–4, the percentage of annexin V+ PMNs was lower than 50% in all acute KD (n = 12) and BI (n = 4) patients, except for one patient with VI. However, from days 5–6, the percentage of annexin V+ PMNs was still lower than 50% in all KD patients (n = 10), while it was higher than 50% in all acute BI patients (n = 12) and in 12 of 15 acute VI patients. Therefore, the delayed apoptosis of PMNs might be a common phenomenon during the early stage (< day 4) of acute KD and BI. After day 5, however, the rate of PMN apoptosis in KD may continue to be delayed, while that in BI may recover to the normal range.
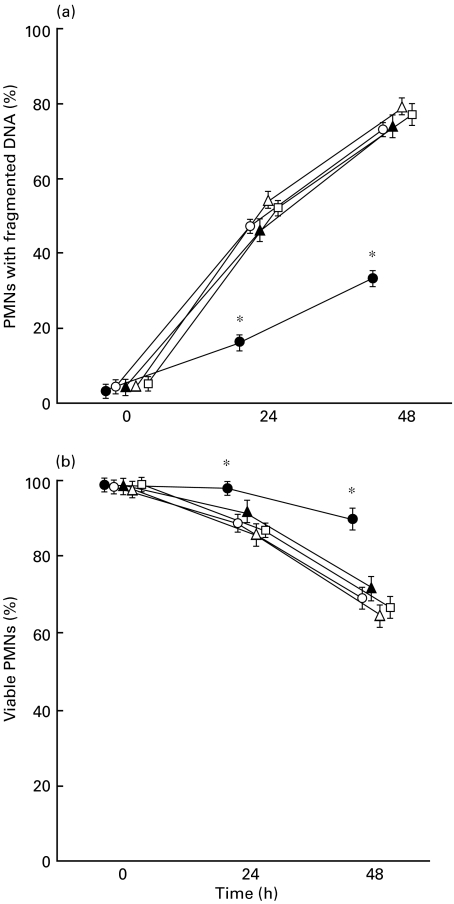
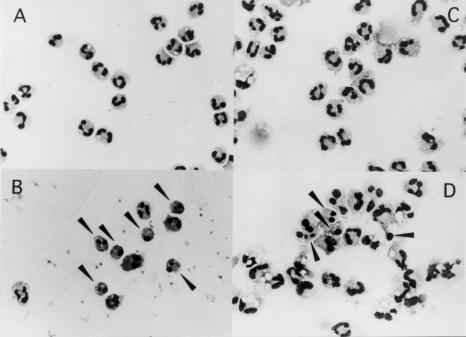
Furthermore, we measured the proportion of PMNs with fragmented DNA using a flow cytometric analysis (Fig. 2a). The mean percentage of PMNs with fragmented DNA in both the 24- and 48-h cultures were significantly lower in the acute KD than the convalescent KD, the acute BI, the acute VI and HC. When the viability of PMNs was measured microscopically (Fig. 2b), the percentage of viable PMNs was significantly higher in acute KD than in the other groups. Based on the morphological findings, the proportion of apoptotic PMNs was lower in acute KD than in HC, when the cells were cultured for 24 h (Fig. 3).
Fig. 2.
Serial changes in the PMNs with fragmented DNA (a) and viable PMNs (b). (a) After the isolated PMNs were cultured in vitro for 24 and 48 h, the proportion of PMNs with fragmented DNA was measured using a flow cytometric analysis. (b) The proportion of viable PMNs were measured using trypan blue in a total of 200 cells.*P < 0·01 vs. convalescent KD, acute BI, acute VI and HC. •, Acute KD; ○, convalescent KD; ▴, acute BI; ▵, acute VI; □, HC.
Fig. 3.
Microscopic findings of apoptotic PMNs. After the isolated PMNs were cultured in vitro for 24 h, the morphological changes of PMNs were observed under a microscope: 0-h (A) and 24-h (B) cultures of PMNs from a healthy child (HC), and 0-h (C) and 24-h (D) cultures of PMNs from an acute KD patient. The arrowheads indicate the apoptotic PMNs with chromatin condensation and fragmented nuclei.
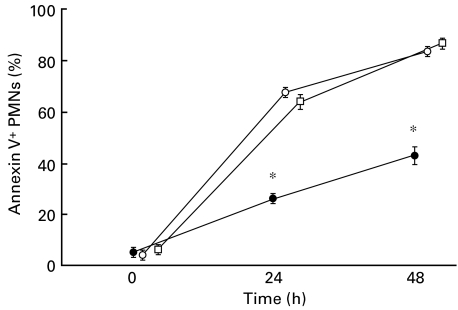
To investigate whether serum factors in acute KD may affect the PMN apoptosis, PMNs isolated from HC were incubated with acute KD serum instead of FBS (Fig. 4). The results showed that acute KD serum did not change the apoptotic rate of normal PMNs.
Fig. 4.
Serial changes of annexin V+ cells in normal PMNs cultured with acute KD serum. After isolated PMNs from healthy children and KD patients were cultured in vitro with acute KD serum or FBS for 24 and 48 h, the spontaneous apoptotic PMNs (annexin V+PI+ cells) were analysed using a flow cytometer. The data were expressed as the mean of samples ± s.e. from five experiments. •, Acute KD PMNs + FBS; ○, HC PMNs + acute KD serum; □, HC PMNs + FBS.
Fas expressions on PMNs
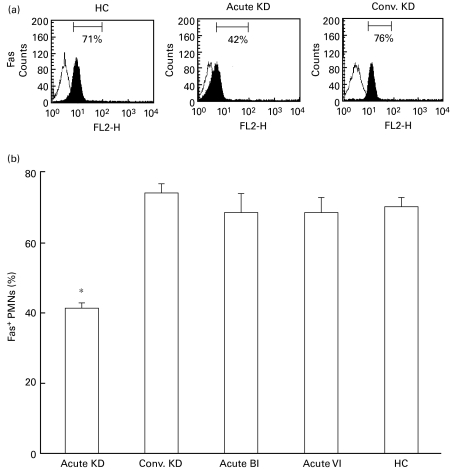
The cell surface expression of Fas on PMNs was analysed by a flow cytometer, and the results for representative cases were shown in Fig. 5a. The expression level of Fas increased in a patient with acute KD in comparison with HC and a patient with convalescent KD. The mean percentage of Fas-positive (Fas+) PMNs was significantly lower (P < 0·01) in acute KD than in convalescent KD, the acute BI and the acute VI and HC (Fig. 5b).
Fig. 5.
Fas expression on the surfaces of PMNs. (a) After PMNs were isolated, the cell surface expression of Fas on PMNs was immediately measured by a flow cytometer using the anti-Fas MoAb (shaded histograms) or isotype-matched MoAb (solid lines) in a healthy child (HC) and the acute and convalescent (conv.) phase of a KD patient. (b) The mean percentage of Fas-positive (Fas+) PMNs in each group was plotted in bar graphs. *P < 0·01 vs. convalescent KD, acute BI, acute VI and HC.
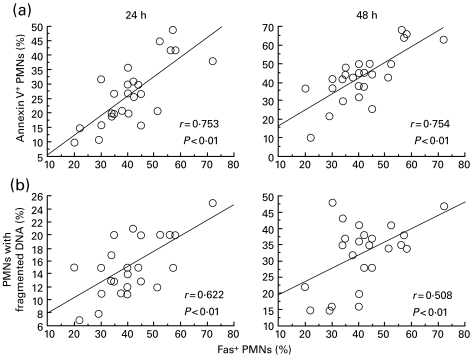
In addition, significant positive correlations (P < 0·01) were observed between the percentage of Fas+ PMNs and the percentage of annexin V+ PMNs or PMNs with fragmented DNA at the incubation times of both 24 and 48 h in the acute phase of KD (Fig. 6), but not in the acute BI, the acute VI and HC (data not shown). Therefore, a reduced expression of Fas on the peripheral PMNs may be associated with a decreased susceptibility of PMNs toward spontaneous apoptosis during acute KD.
Fig. 6.
Correlation between the proportion of Fas+ PMN and (a) the proportion of annexin V+ PMNs and (b) the proportion of PMNs with fragmented DNA in 24- and 48-h cultures in the acute phase of KD. Solid lines indicate the linear regression lines.
Anti-Fas MoAb-induced apoptosis of PMNs
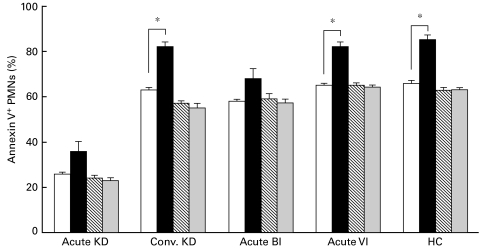
After the PMNs were cultured with an agonistic (clone CH-11) and a blocking (clone ZB4) anti-Fas MoAb for 24 h, the mean percentage of apoptotic PMNs (annexin V+ cells) was measured (Fig. 7). CH-11 induced a significant increase in the percentage of annexin V+ PMNs in convalescent KD, acute VI and HC, but not in acute KD or BI. Furthermore, ZB4 did not induce an increase in the percentage of annexin V+ PMNs but did block the CH-11-induced apoptosis of PMNs. These findings indicate that the anti-Fas MoAb-mediated apoptosis of PMNs is inhibited in the acute phases of both KD and BI.
Fig. 7.
Effect of anti-Fas MoAb (CH-11 and ZB4) on PMN apoptosis. After the isolated PMNs were cultured with an agonistic (clone CH-11) and/or a blocking (clone ZB4) anti-Fas MoAbs for 24 h, the percentage of annexin V+ cells was measured using a flow cytometer. The control indicates the condition without either of the anti-Fas MoAbs (medium only). *P < 0·05 vs. control. □, Control; ▪, CH-11; ▒, ZB4; ░, ZB4 + CH-11.
A1 and Bax expressions and the A1/Bax ratio in PMNs
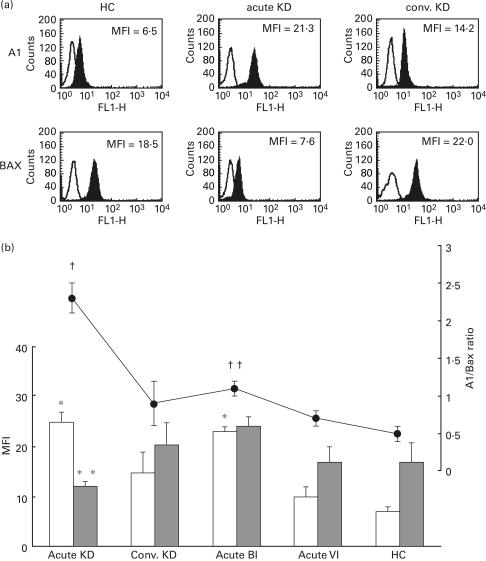
Figure 8a shows the immunofluorescence histograms demonstrating the A1 and Bax expressions in the intracellular PMNs for representative cases. The expression level of A1 increased, while the expression level of Bax decreased, in a patient with acute KD in comparison to a HC and a patient with convalescent KD. The mean fluorescence intensity (MFI) for A1 and Bax expression and the mean A1/Bax ratio in each group are plotted in Fig. 8b. The MFI of A1 was significantly higher (P < 0·01) in acute KD and acute BI than in acute VI and HC. The MFI of Bax was significantly lower (P < 0·01) in acute KD than either convalescent KD or acute BI. The A1/Bax ratio was also significantly higher in acute KD than in the other groups. Therefore, these findings demonstrate that the balance of A1/Bax expression changed markedly during the acute phase of KD.
Fig. 8.
Intracellular expressions of A1 and Bax and the A1/Bax ratio in PMNs. (a) After PMNs were isolated, the intracellular expressions of A1/Bax were immediately measured by a flow cytometer using an anti-A1 polyconal antibody and anti-Bax MoAb in a healthy child (HC) and the acute and convalescent (conv.) phase of a KD patient. (b) The mean fluorescence intensity (MFI) of A1 and Bax in each group was plotted in bar graphs, and the ratio of A1/Bax was plotted in a polygonal line graph. *P < 0·01 vs. convalescent KD, acute VI and HC. **P < 0·01 vs. convalescent KD and BI. †P < 0·01 vs. convalescent KD, acute BI, acute VI and HC. ††P < 0·01 vs. acute VI and HC. □, A1; ▪, Bax; •, A1/Bax ratio.
Discussion
Laboratory findings during the acute phase of KD show a marked increase in the peripheral PMN count, but a decrease in the peripheral lymphocyte count [16]. The apoptosis of peripheral lymphocytes is reported to be up-regulated in the acute phase of KD, thus suggesting a decrease in the peripheral lymphocyte levels [28]. PMN apoptosis is accelerated in the patients with systemic lupus erythematosus (SLE) and neutropenia [29]. In contrast, the present study revealed that the apoptosis of peripheral PMNs was down-regulated persistently during the acute phase of KD (days 3–6 after the onset of fever). It is likely that an increase in number of immature PMNs in acute KD may be associated with the delayed PMN apoptosis. However, the apoptotic rate of PMNs in acute KD was significantly inhibited in comparison with that in acute BI, especially after day 5, in spite of similar number of immature PMNs in both acute KD and BI groups. Therefore, different factor(s) may also contribute to the delayed apoptosis of PMNs in acute KD.
Many factors can affect PMN apoptosis [2,3]. Lipopolysaccharide (LPS), bacteria-derived endotoxin, can induce either a reduction of PMN apoptosis or an acceleration of lymphocyte apoptosis [30,31], while viruses such as influenza [32], RS [33] and EB [34] can induce an acceleration of PMN apoptosis. Cytokines such as G-CSF, GM-CSF, IL-1β, IL-6, IFN-γ and TNF-α can inhibit PMN apoptosis [3,35]. These cytokines are also reported to increase in acute KD [21,36–38], thus suggesting that cytokines are involved in the delayed PMN apoptosis in this disease. PMN apoptosis is also delayed in the patients with SIRS who have high levels of serum cytokines [12,13]. However, when the PMNs from healthy children were incubated with acute KD serum, the spontaneous apoptosis of PMNs was not delayed (Fig. 4). Furthermore, supernatants from culture medium of acute KD PMNs did not change the apoptotic rates of normal PMNs (data not shown). Therefore, delayed PMN apoptosis cannot be explained by the serum factors (e.g. cytokines) alone. Kim et al. suggested that putative aetiological factors may activate T cells during the early phase of KD, followed by the death of T cells through an apoptotic process [28]. We therefore cannot rule out the possibility that an aetiological agent may mediate the negative signal of PMN apoptosis during the early phase after the onset of KD.
The peripheral PMNs are terminally differentiated cells which are destined to undergo rapid cell death via apoptosis [9–11]. After the transendothelial migration of PMNs into an inflammatory focus the spontaneous apoptosis of PMNs is delayed, thus indicating the appropriate adaptive response of the host against injury and infection [39]. However, once PMNs ingest bacteria, the cells die immediately due to apoptosis and are subsequently recognized and ingested by the surrounding mononuclear phagocytes [1,40]. On the other hand, the excessively activated PMNs, which have a prolonged life-span in such uncontrolled inflammatory syndromes as ARDS and SIRS, may induce tissue injury by releasing toxic mediators into the surrounding microenvironment [3,7,13–18]. PMN apoptosis may thus play an important role in the normal resolution of inflammation by limiting the autotoxic potential of these cells [3,41]. It has been noted recently that the neutrophil-mediated injury of endothelial cells was mediated mainly by protease, especially elastase [42]. A large amount of elastase and ROS secreted by the activated PMNs is suggested to be involved in the endothelial cell injury and vascular damage in KD [24,25]. As a result, the prolonged life-span of activated PMNs is suggested to contribute to the pathogenesis of KD vasculitis by secreting an excessive amount of these toxic mediators during the acute phase of KD. Furthermore, the use of PMN apoptosis-inducing drugs may also be a new therapeutic strategy for KD in the future.
The Fas system is believed to play a fundamental role in the regulation of spontaneous apoptosis [4–6]. SLE patients with an accelerated apoptosis of PMNs are reported to have an increased expression of Fas on their surfaces [29]. In contrast, the present study revealed that the reduced apoptosis of PMNs observed in KD patients was associated with a decreased expression of Fas. The decreased expression of Fas also showed a positive correlation with the percentage of spontaneous apoptotic PMNs. A low expression of Fas on the surface of PMNs is therefore linked with the delayed apoptosis in KD. Furthermore, the anti-Fas MoAb(CH-11)-induced apoptosis of PMNs was inhibited, thus suggesting that the resistance against Fas signal transduction in PMN apoptosis is present during the acute phase of KD. However, since anti-Fas MoAb-induced apoptosis was also inhibited in acute BI, the involvement of an additional pathway is suggested in the delayed PMN apoptosis in KD.
Many Bcl-2 family proteins are predominantly located in the outer mitochondrial membrane, and the relatively abundant expression of pro-and anti‐apoptotic proteins determines the susceptibility to cell death [43]. A1, an anti-apoptotic protein, is known to play an important role in the maintenance of the life span of PMNs [9,11]. On the other hand, the expression of Bax, a pro-apoptotic protein, in PMNs is down-regulated in the GM-CSF-induced prevention of apoptosis [10]. In the present study, we therefore measured the expression levels of A1 and Bax and the A1/Bax ratio in each group. These results indicate that the A1/Bax ratio in acute KD increased significantly in comparison to the other groups, thus suggesting that an altered balance between the antiand pro-apoptotic proteins in the Bcl-2 family may be involved in the delayed apoptosis of PMNs in patients with this disease.
In summary, the apoptosis of circulating PMNs is inhibited in the acute phase of KD, and the delayed apoptosis of PMNs may be associated with the increased number of peripheral PMNs and the decreased expression of Fas on their surfaces. Furthermore, we demonstrated that the PMNs in the acute KD are resistant to death signal transduction via Fas receptor and the increase in the A1/Bax expression in Bcl-2 family proteins may help to prevent mitochondrial-dependent apoptosis. These findings may provide us with new insight into the pathogenesis of KD.
References
- 1.Savill J, Dransfield I, Hogg N, Haslett C. Vitronectin receptor-mediated phagocytosis of cells undergoing apoptosis. Nature. 1990;343:170–3. doi: 10.1038/343170a0. [DOI] [PubMed] [Google Scholar]
- 2.Payne CM, Glasser L, Tischer ME, et al. Programmed cell death of the normal human neutrophil: an in vitro model of senescence. Microsc Res Tech. 1994;28:327–44. doi: 10.1002/jemt.1070280408. [DOI] [PubMed] [Google Scholar]
- 3.Homburg CHE, Roos D. Apoptosis of neutrophils. Curr Opin Hematol. 1996;3:94–9. doi: 10.1097/00062752-199603010-00014. [DOI] [PubMed] [Google Scholar]
- 4.Iwai K, Miyawaki T, Takizawa T, et al. Differential expression of bcl-2 and susceptibility to anti-Fas-mediated cell death in peripheral blood lymphocytes, monocytes, and neutrophils. Blood. 1994;84:1201–8. [PubMed] [Google Scholar]
- 5.Liles WC, Klebanoff SJ. Regulation of apoptosis in neutrophils-Fas track to death? J Immunol. 1995;155:3289–91. [PubMed] [Google Scholar]
- 6.Brown SB, Savill J. Phagocytosis triggers macrophage release of Fas ligand and induces apoptosis of bystander leukocytes. J Immunol. 1999;162:480–5. [PubMed] [Google Scholar]
- 7.Savill J, Haslett C. Granulocytes. In: Winker JD, editor. Apoptosis and inflammation. Progress in inflammation research. Basel, Switzerland: Birkhäuser; 1999. [Google Scholar]
- 8.Nagata S. Apoptosis by death factor. Cell. 1997;88:355–65. doi: 10.1016/s0092-8674(00)81874-7. [DOI] [PubMed] [Google Scholar]
- 9.Chuang PI, Yee E, Karsan A, Winn RK, Harlan JM. A1 is a constitutive and inducible Bcl-2 homologue in mature human neutrophils. Biochem Biophys Res Commun. 1998;249:361–5. doi: 10.1006/bbrc.1998.9155. [DOI] [PubMed] [Google Scholar]
- 10.Weinmann P, Gaehtgens P, Walzog B. Bcl-Xl- and Bax-alpha-mediated regulation of apoptosis of human neutrophils via caspase-3. Blood. 1999;93:3106–15. [PubMed] [Google Scholar]
- 11.Santos-Beneit AM, Mollinedo F. Expression of genes involved in initiation, regulation, and execution of apoptosis in human neutrophils and during neutrophil differentiation of HL-60 cells. J Leukoc Biol. 2000;67:712–24. doi: 10.1002/jlb.67.5.712. [DOI] [PubMed] [Google Scholar]
- 12.Henson PM, Johnson RB., Jr Tissue injury in inflammation. Oxidants, protease, and cationic protein. J Clin Invest. 1987;79:669–74. doi: 10.1172/JCI112869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Malech HL, Gallin JI. Neutrophils in human diseases. N Engl J Med. 1987;317:687–93. doi: 10.1056/NEJM198709103171107. [DOI] [PubMed] [Google Scholar]
- 14.Savill J. Apoptosis in resolution of inflammation. J Leukoc Biol. 1997;61:375–80. doi: 10.1002/jlb.61.4.375. [DOI] [PubMed] [Google Scholar]
- 15.Jimenez MF, Watson WG, Parodo J, et al. Dysregulated expression of neutrophil apoptosis in the systemic inflammatory response syndrome. Arch Surg. 1997;132:1263–70. doi: 10.1001/archsurg.1997.01430360009002. [DOI] [PubMed] [Google Scholar]
- 16.Fanning NF, Kell MR, Shorten GD, et al. Circulating granulocyte macrophage colony-stimulating factor in plasma of patients with the systemic inflammatory response syndrome delays neutrophil apoptosis through inhibition of spontaneous reactive oxygen species generation. Shock. 1999;11:167–74. doi: 10.1097/00024382-199903000-00003. [DOI] [PubMed] [Google Scholar]
- 17.Keel M, Steckholzer U, Niederer E, Hartung T, Trentz O, Ertel W. Interleukin-10 counterregulates proinflammatory cytokine-induced inhibition of neutrophil apoptosis during severe sepsis. Blood. 1997;90:3356–63. [PubMed] [Google Scholar]
- 18.Matute-Bello G, Liles WC, Radella IIF, et al. Neutrophil apoptosis in the acute respiratory distress syndrome. Am J Respir Crit Care Med. 1997;156:1969–77. doi: 10.1164/ajrccm.156.6.96-12081. [DOI] [PubMed] [Google Scholar]
- 19.Kawasaki T. Acute febrile mucocutaneous syndrome with lymphoid involvement with specific desquamation of the fingers and toes in children. Clinical observation of 50 cases. Jpn J Allergy. 1967;16:178–222. [PubMed] [Google Scholar]
- 20.Furusho K, Kamiya T, Nakano H, et al. High-dose intravenous gammaglobulin for Kawasaki disease. Lancet. 1984;2:1055–8. doi: 10.1016/s0140-6736(84)91504-6. [DOI] [PubMed] [Google Scholar]
- 21.Leung DYM, Cotran RS, Kurt-Jones E, Burns JC, Newburger JW, Pober JS. Endothelial cell activation and high interleukin-1 secretion in the pathogenesis of acute Kawasaki disease. Lancet. 1989;2:1298–302. doi: 10.1016/s0140-6736(89)91910-7. [DOI] [PubMed] [Google Scholar]
- 22.Takeshita S, Sekine I, Fujisawa T, Yoshioka S. Studies of peripheral blood toxic neutrophils as a predictor of coronary risk in Kawasaki disease – the pathogenetic role of hematopoietic colony-stimulating factors (GM-CSF, G-GSF) Acta Paediatr Jpn. 1990;32:508–14. doi: 10.1111/j.1442-200x.1990.tb00871.x. [DOI] [PubMed] [Google Scholar]
- 23.Rowe PC, Quinlan A, Luke BKH. Value of degenerative change in neutrophils as a diagnostic test for Kawasaki disease. J Pediatr. 1991;119:370–4. doi: 10.1016/s0022-3476(05)82047-5. [DOI] [PubMed] [Google Scholar]
- 24.Niwa Y, Sohmiya K. Enhanced neutrophilic functions in mucocutaneous lymph node syndrome, with special reference to the possible role of increased oxygen intermediate generation in the pathogenesis of coronary thromboarteritis. J Pediatr. 1984;104:56–60. doi: 10.1016/s0022-3476(84)80589-2. [DOI] [PubMed] [Google Scholar]
- 25.Takeshita S, Nakatani K, Kawase H, et al. The role of bacterial lipopolysaccharide-bound neutrophils in the pathogenesis of Kawasaki disease. J Infect Dis. 1999;179:508–12. doi: 10.1086/314600. 10.1086/314600. [DOI] [PubMed] [Google Scholar]
- 26.Japan Kawasaki Disease Research Committee. Diagnostic guidelines of Kawasaki disease. 4. Tokyo Japan: Kawasaki Disease Research Committee; 1984. [Google Scholar]
- 27.Nicoletti I, Migliorati G, Pagliacci MC, Grignani F, Riccardi C. A rapid and simple method for measuring thymocyte apoptosis by propidium iodide staining and flow cytometry. J Immunol Methods. 1991;139:271–9. doi: 10.1016/0022-1759(91)90198-o. [DOI] [PubMed] [Google Scholar]
- 28.Kim HY, Lee HG, Kim DS. Apoptosis of peripheral blood mononuclear cells in Kawasaki disease. J Rheumatol. 2000;27:801–6. [PubMed] [Google Scholar]
- 29.Courtney PA, Crockard AD, Williamson K, Irvine AE, Kennedy RJ, Bell AL. Increased apoptotic peripheral blood neutrophils in systemic lupus erythematosus: relations with disease activity, antibodies to double stranded DNA, and neutropenia. Ann Rheum Dis. 1999;58:309–14. doi: 10.1136/ard.58.5.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hachiya O, Takeda Y, Miyata H, Watanabe H, Yamashita T, Sendo F. Inhibition by bacterial lipopolysaccharide of spontaneous and TNF-alpha-induced human neutrophil apoptosis in vitro. Microbiol Immunol. 1995;39:715–23. doi: 10.1111/j.1348-0421.1995.tb03247.x. [DOI] [PubMed] [Google Scholar]
- 31.Castro A, Bemer V, Nobrega A, Coutinho A, Truffa-Bachi P. Administration to mouse of endotoxin from gram-negative bacteria leads to activation and apoptosis of T lymphocytes. Eur J Immunol. 1998;28:488–95. doi: 10.1002/(SICI)1521-4141(199802)28:02<488::AID-IMMU488>3.0.CO;2-R. 10.1002/(sici)1521-4141(199802)28:02<488::aid-immu488>3.3.co;2-i. [DOI] [PubMed] [Google Scholar]
- 32.Colamussi ML, White MR, Crouch E, Hartshorn KL. Influenza A virus accelerates neutrophil apoptosis and markedly potentiates apoptotic effects of bacteria. Blood. 1999;93:2395–403. [PubMed] [Google Scholar]
- 33.Wang SZ, Smith PK, Lovejoy M, Bowden JJ, Alpers JH, Forsyth KD. The apoptosis of neutrophils is accelerated in respiratory syncytial virus (RSV) -induced bronchiolitis. Clin Exp Immunol. 1998;114:49–54. doi: 10.1046/j.1365-2249.1998.00681.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Larochelle B, Flamand L, Gourde P, Beauchamp D, Gosselin J. Epstein–Barr virus infects and induces apoptosis in human neutrophils. Blood. 1998;92:291–9. [PubMed] [Google Scholar]
- 35.Squier MK, Sehnert AJ, Cohen JJ. Apoptosis in leukocytes. J Leukoc Biol. 1995;57:2–10. doi: 10.1002/jlb.57.1.2. [DOI] [PubMed] [Google Scholar]
- 36.Furukawa S, Matsubara T, Yone K, Hirano Y, Okumura K, Yabuta K. Kawasaki disease differs from anaphylactoid purpura and measles with regard to tumour necrosis factor-alpha and interleukin 6 in serum. Eur J Pediatr. 1992;151:44–7. doi: 10.1007/BF02073890. [DOI] [PubMed] [Google Scholar]
- 37.Lin CY, Lin CC, Hwang B, Chiang B. Serial changes of serum interleukin-6, interleukin-8, and tumor necrosis factor alpha among patients with Kawasaki disease. J Pediatr. 1992;121:924–6. doi: 10.1016/s0022-3476(05)80343-9. [DOI] [PubMed] [Google Scholar]
- 38.Igarashi H, Hatake K, Tomizuka H, Yamada M, Gunji Y, Momoi MY. High serum levels of M-CSF and G-CSF in Kawasaki disease. Br J Haematol. 1999;105:613–5. doi: 10.1046/j.1365-2141.1999.01381.x. [DOI] [PubMed] [Google Scholar]
- 39.Watson RW, Rotstein OD, Nathens AB, Parodo J, Marshall JC. Neutrophil apoptosis is modulated by endothelial transmigration and adhesion molecule engagement. J Immunol. 1997;158:945–53. [PubMed] [Google Scholar]
- 40.Watson RW, Redmond HP, Wang JH, Condron C, Bouchier-Hayes D. Neutrophils undergo apoptosis following ingestion of Escherichia coli. J Immunol. 1996;156:3986–92. [PubMed] [Google Scholar]
- 41.Smedly LA, Tonnesen MG, Sandhaus RA, et al. Neutrophil-mediated injury to endothelial cells. Enhancement by endotoxin and essential role of neutrophil elastase. J Clin Invest. 1986;77:1233–43. doi: 10.1172/JCI112426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Savill J, Haslett C. Granulocyte clearance by apoptosis in the resolution of inflammation. Semin Cell Biol. 1995;6:385–93. doi: 10.1016/s1043-4682(05)80009-1. [DOI] [PubMed] [Google Scholar]
- 43.Kroemer G. The proto-oncogene Bcl-2 and its role in regulating apoptosis. Nat Med. 1997;3:614–20. doi: 10.1038/nm0697-614. [DOI] [PubMed] [Google Scholar]