Abstract
Local immune reactivity in the lungs of BALB/c mice was studied following (i) intranasal (i.n.) vaccination with Mycobacterium bovis BCG, (ii) intravenous (i.v.) challenge with a virulent M. bovis field isolate and (iii) i.n. vaccination with M. bovis BCG followed by i.v. challenge with an M. bovis field isolate. The results demonstrated that i.n. vaccination with BCG induced a high degree of protection against systemic M. bovis challenge, and that this protection correlated with a rapid production of IFN-γ after M. bovis challenge by lung T cells from vaccinated mice.
Keywords: intranasal vaccination, M. bovis infection, immunity in the lung
Introduction
Bovine tuberculosis has severe implications for animal welfare, and affected farms suffer painful economic losses. In recent years, the control strategy for cattle in Great Britain has been based on tuberculin testing and slaughter of infected animals. This test and slaughter strategy has reduced the numbers of affected herds dramatically since its implementation in the UK in the 1950s. However, it has failed to eradicate the disease completely, and since the mid-1980s a steady and dramatic rise in the incidence of tuberculosis in cattle has been reported in the UK. A recent independent review of this problem commissioned by the UK government has concluded that vaccination of cattle holds the best long-term prospect for tuberculosis control in British herds [1].
For the development of new effective vaccines, it is necessary to understand the mechanisms of the protective immune response. During the last decade, anti-mycobacterial immune responses in peripheral lymphoid organs have been characterized in some detail [2–5]. However, the predominant natural infection route with mycobacteria is via the respiratory tract and therefore, the characterization of immune responses occurring at the site of infection, the lung, is of prime importance. Lung immune responses exhibit unique features, and results obtained in peripheral lymphatic tissues like lymph node or spleen cannot necessarily be transferred to the situation in the lung [6,7]. Therefore, we [8,9] and others [10–13] have recently started to extensively characterize immune responses in the lungs of inbred mice challenged with virulent and vaccine strains of mycobacteria via different routes. In the present study, we describe local immunity in the lungs of BALB/c mice challenged with M. bovis BCG and/or virulent M. bovis field isolate.
Materials and methods
Animals
BALB/cJCit (BALB/c) mice were bred under conventional conditions at the Animal Facilities of the Central Institute for Tuberculosis (Moscow, Russia), according to the rules of Russian Academy of Medical Sciences, with water and food provided ad libitum. Male mice 2–4 months of age were used.
Vaccination and infection
The method for preparing clump-free, mid-log-phase mycobacterial suspensions was described earlier in detail [14], and was used for all mycobacterial preparations throughout the experiments. For vaccination, 105 colony-forming units (cfu) of mid-log-phase Mycobacterium bovis BCG (strain Pasteur, collection of the Central Institute for Tuberculosis, Moscow) were given under light anaesthesia either i.n. or s.c in 0·02 ml or 0·5 ml saline, respectively. To determine the efficacy of vaccination, mice were challenged i.v. with 2 × 103 cfu of mid-log-phase M. bovis (field isolate 2122/97 [15]), 5 weeks following vaccination. To assess mycobacterial load in spleens and lungs, 0·15 ml of serial 10-fold dilutions of whole spleen or lung lobe homogenates (see footnote to Fig. 1) were plated onto Dubos agar, and colonies were counted following 18–20 days of incubation at 37°C.
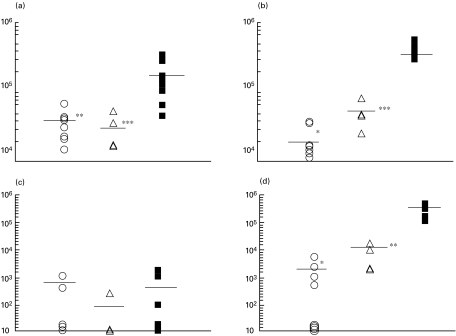
Fig. 1.
Protection against Mycobacterium bovis infection conferred by i.n. and s.c. administration of M. bovis BCG (cfu counts per organ). Mice were vaccinated with M. bovis BCG (105 cfu per mouse), either i.n. (n = 7–8 per time-point) or s.c. (n = 4 per time-point) or left unvaccinated (n = 6–9 per time-point). Five weeks later, mice were challenged i.v. with 2 × 103 M. bovis. Two and 5 weeks later, spleens and lungs were extracted and lungs were weighed. Suspensions were prepared individually from approximately one third of the whole lung and from the whole spleen in a total volume of 2 ml. Serial dilutions of suspensions were prepared and plated in a volume of 0·15 ml onto Dubos agar for cfu counting. The rest of the lung tissue was enzymatically digested and suspensions used in immunological assays. (a) Spleen, week 2; (b) spleen, week 5; (c) lungs, week 2; (d) lungs, week 5. (○) BCG i.n.; (▵) BCG s.c.; (▪) control. *P < 0·001; **P < 0·005; ***P < 0·05 compared with non-vaccinated controls.
Lung cell suspensions
Two, 5 and 8 weeks following challenge, mice were euthanized by injecting an overdose of thiopental (Biochemie GmbH, Vienna, Austria). Lung cells were isolated using the method described by Holt et al. [16] with our modifications [8,9]. Briefly, lung blood vessels were washed out using 0·02% EDTA-HBSS solution, and lung tissue was digested in the presence of collagenase (200 U/ml, Sigma, St. Louis, MO, USA) and DNase (50 U/ml, Sigma). Lung cell suspensions were further enriched for T lymphocytes by sequential depletion of plastic-adherent and nylon-wool-adherent cells, which resulted in approximately 75% CD3+ cell purity [8]. Viability of cells, as determined by trypan blue exclusion, was more than 93%. In some experiments, CD4+ and CD8+ lung cells were separated using MACS beads (Miltenyi, Germany) following the manufacturer's instructions.
Proliferative response
T-cell-enriched lung cells (105) mixed with 3 × 105 syngenic irradiated splenic APC were cultured in a 96-well flat-bottommed plate (Costar, Badhoevedorp, the Netherlands) at 37°C, 5% CO2, in supplemented RPMI-1640 medium [8] in the presence of either BCG culture filtrate, M. bovis sonicate, M. tuberculosis H37Rv sonicate (10 mg/ml for all antigens) or live M. bovis BCG (4 × 105 cfu/well). Mycobacterium bovis sonicate and H37Rv sonicate were kindly provided by Dr V. Avdienko (Central Institute for Tuberculosis, Moscow, Russia). All cultures were performed in triplicate and non-stimulated wells served as controls. [3H]-thymidine incorporation by proliferating cells was estimated 72 h later and the results expressed as the stimulation index (SI, calculated as cpm in the presence of antigen/cpm without antigen).
Staining of cell surface molecules
Lung cells (3–5 × 105) were double-stained with directly-conjugated anti-CD4 or anti-CD8 MoAbs combined with anti-CD44 MoAbs (Pharmingen, San Diego, CA, USA) according to the manufacturer's instructions. Stained cells were analysed using an EPICS ‘ELITE’ flow cytometer (Coulter Corporation, Miami, FL, USA) equipped with a CYONICS argon laser (Uniphase, San Jose, CA, USA) as described earlier [9]. Unstained controls were analysed at each time point.
Cytokine assays
Sandwich ELISA assays were performed to detect IL-4, IL-5, IL-10, IL-12, TNF-α and IFN-γ in 48 h culture supernatant fluids. Capture and detecting (biotinylated) MoAbs specific for mouse cytokines were purchased from Pharmingen: for IFN-γ, clones R4–6A2 and XMG1·2 (sensitivity, 160 pg/ml); for IL-4, clones 11B11 and BVD6–24G2 (sensitivity 62 pg/ml); for IL-5, clones TRFK5 and TRFK4 (sensitivity 62 pg/ml); for IL-10, clones JES5–2A5 and JES5–16E3 (sensitivity 312 pg/ml); for IL-12, clones C 17·8 and C 15·6 (sensitivity 125 pg/ml); for TNF-α, clone MP6-XT22 and polyclonal antibodies (sensitivity 312 pg/ml). Assays were performed following the manufacturer's instructions. A standard curve for each assay was generated with known concentrations of mouse rIL-4, rIL-5, rIL-12 and rTNF-α (all Pharmingen), rIL-10 (Sigma) and rIFN-γ (Genzyme, Boston, MA, USA).
DTH response
DTH to tuberculin was assessed as a 24 h footpad swelling reaction described earlier [17,18].
Statistical analysis
Significance of differences was estimated by Student's t-test and Mann–Whitney test. P < 0·05 was considered statistically significant.
Results
Local and systemic responses following i.n. vaccination with BCG
In the first set of experiments, BALB/c mice were vaccinated i.n. with 105 cfu of M. bovis BCG. One, 2, 3 and 5 weeks following vaccination, lung T-cell composition and lung T-cell proliferation and cytokine production were determined.
To evaluate the accumulation of T lymphocytes, lung cells were stained with anti-CD4 and anti-CD8 monoclonal antibodies and analysed by flow cytometry. Total lung cell counts as well as the number of CD4+ and CD8+ cells were compared with those in non-vaccinated mice. At weeks 1 and 2, no significant increase in numbers of total lung cells, CD4+ and CD8+ lymphocytes was observed (data not shown). In contrast, 3 and 5 weeks after BCG vaccination, significantly more total lung cells and CD4+ T cells were recovered from the lungs of vaccinated compared with naïve mice (total cell counts at week 5: 26·0 ± 1·0 × 106 per vaccinated and 16·9 ± 1·1 × 106 per naïve mouse, respectively, P < 0·005; CD4+ cell counts: 7·3 ± 0·3 × 106 and 4·3 ± 0·4 × 106 per vaccinated and naïve mouse, respectively, P < 0·05). Although the number of CD8+ lymphocytes also increased (3·6 ± 0·08 and 2·5 ± 0·03 × 106 CD8 cells per infected and naïve mouse, respectively), the differences between vaccinated and naïve mice were not statistically significant (P > 0·05).
To assess proliferative responses in the lung of vaccinated mice, lung T cells were co-cultured with APC and the mycobacterial antigen preparations listed in the Materials and Methods section. Similar results were obtained with all these antigen preparations so therefore, only results obtained with M. bovis sonicate are shown. While no antigen-specific proliferation was observed at weeks 1 and 2, a specific lung T-cell proliferative response was readily seen at week 3 and later time points (see Table 1 for results obtained at weeks 0, 1, 2 and 5). Following magnetic separation of CD4 and CD8 T cells, we could demonstrate that the proliferative responses of lung-derived T cells was restricted to the CD4+ subset, whereas CD8+ cells did not proliferate (SI at week 3: 5·9, 7·6, 1·0 for pan T cells, CD4+ and CD8+ lung cells, respectively). This is in agreement with the observed accumulation of CD4 T cells following BCG vaccination described above.
Table 1.
Immune responses in the lungs and DTH reaction following i.n. vaccination of BALB/c mice with Mycobacterium bovis BCG
| Weeks post-vaccination | |||||
|---|---|---|---|---|---|
| Response | Antigen | 0 | 1 | 2 | 5 |
| IL-12 | + | 1090 ± 78 | 2210 ± 370* | 1370 ± 490 | 1790 ± 310 |
| (pg/ml) | – | 970 ± 300 | 1380 ± 355 | 1160 ± 400 | 1695 ± 200 |
| IFN-γ | + | 458 ± 140 | 300 ± 130 | 253 ± 150 | 1930 ± 364* |
| (pg/ml) | – | 370 ± 150 | 260 ± 85 | 200 ± 80 | 1170 ± 250 |
| IL-5 | + | 50 ± 50 | 407 ± 72* | 178 ± 61 | 86 ± 30 |
| (pg/ml) | – | 21 ± 21 | 335 ± 69* | 190 ± 0·05* | 64 ± 25 |
| Proliferation | + | ND | 1·5 | 1·4 | 4·0* |
| (SI) | |||||
| DTH | + | 0·03 ± 0·02 | ND | ND | 0·54 ± 0·06** |
| (mm) | |||||
To measure cytokine production by ELISA in cultural supernatant fluids, lung cells from 3–4 mice per time-point were cultured individually in the wells of 24-well plates (2 × 106 cells/well) in the presence or absence of M. bovis sonicate (10 mg/ml). For proliferation assays, lung cells from 3–4 mice were pooled, enriched for T lymphocytes and cultured in 96-well plates at 1 × 105/well in the presence of irradiated splenic antigen-presenting cells (3 × 105/well) and M. bovis sonicate (10 mg/ml). [3H]-thymidine incorporation is expressed as stimulation indices SI. The results of one of two similar experiments are presented. DTH reaction was measured at week 5 following vaccination in 7 mice. Cytokine levels and DTH reaction are expressed as mean ± s.e.m.
P < 0·05;
P < 0·001 as compared with week 0.
To assess cytokine production, total lung cells were cultured in the presence of M. bovis sonicate. As shown in Table 1, we observed a significant early increase in the antigen-induced production of IL-5 and IL-12 (week 1 post-vaccination) which was followed later (week 5) by a significant increase in antigen-driven IFN-γ production not evident during the first 2 weeks following vaccination. Interestingly, the levels of IL-5 produced in the absence of antigen were also significantly elevated 1 and 3 weeks following vaccination. Thus, intranasal administration of M. bovis BCG stimulated an early local production of IL-12 and IL-5, followed by accumulation of antigen-specific CD4+ cells in the lung associated with an increase in IFN-γ production.
To determine whether i.n. vaccination, apart from lung responses, also induced systemic immune responses, we estimated the DTH response to tuberculin, which is the in vivo test widely used to detect exposure to mycobacteria in humans and cattle. The results presented in Table 1 demonstrate that i.n. vaccination resulted in strong tuberculin skin responses and thus, showed that this vaccination route resulted not only in local but also systemic specific immune responses.
Intranasal vaccination with BCG induces a high degree of protection against subsequent challenge with virulent M. bovis
To determine whether intranasal BCG vaccination protected mice against subsequent challenge with virulent M. bovis, mice were challenged i.v. with an M. bovis field isolate. Two and 5 weeks following challenge, mycobacterial burdens in lungs and spleens were determined and compared with those in non-vaccinated infected controls. Since mice vaccinated subcutaneously with the same BCG dose are known to be well protected against M. tuberculosis infection [17], a group of subcutaneouly vaccinated mice was included as a positive control of the efficacy of vaccination.
Within the first 2 weeks of infection, mycobacteria rapidly replicated in the spleens (Fig. 1a), reaching cfu counts that exceeded the size of inoculum up to 100-fold in non-vaccinated mice. Both i.n. and s.c. vaccination resulted in a significant decrease in splenic cfu counts (P < 0·005 and P < 0·05, respectively, compared with the non-vaccinated control). In the lungs, low numbers of mycobacteria could be detected in some but not all animals at this time point (Fig. 1c), indicating that the pulmonary infection only started to develop. Evaluation of vaccination efficacy at week 5 following challenge indicated that i.n. vaccination was highly protective and significantly reduced the M. bovis burden in spleens and lungs (Fig. 1b,d,P < 0·001 compared with non-vaccinated controls). Encouragingly, the degree of protection induced by intranasal vaccination 5 weeks post-challenge was slightly better than that induced by s.c. vaccination (i.n. vaccination compared with s.c. vaccination at week 5: P = 0·055 and P = 0·044 for lungs and spleens, respectively).
Immune responses in the course of M. bovis infection in vaccinated versus non-vaccinated mice
In order to elucidate possible immunological correlates of protection, we compared immune responses in the lungs of vaccinated and non-vaccinated mice following M. bovis challenge. Lung cells were obtained 2 and 5 weeks post-infection and T-cell activation, proliferation and cytokine production were determined as described above.
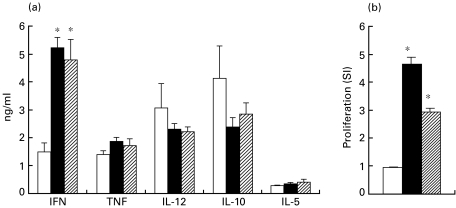
T-cell activation, estimated as the increased percentage of CD44hi cells within CD4+ and CD8+ subsets, occurred as early as 2 weeks following challenge and was higher within CD4+ (77–80% of CD44hi cells in challenged compared with 26% in control mice) than within the CD8+ subset (28–47% of CD44hi cells in challenged compared with 15% in control mice). Although T lymphocytes recovered from the lungs of both non-vaccinated and vaccinated mice 2 weeks after challenge were similarly activated, the former did not proliferate in response to mycobacterial antigens at this early time-point post-challenge (Fig. 2). In contrast, T lymphocytes from vaccinated mice proliferated vigorously 2 weeks after M. bovis infection (Fig. 2). Moreover, lung cells from vaccinated mice produced significantly more IFN-γ (Fig. 2, P < 0·001) compared with non-vaccinated mice early (2 weeks) after infection. No major differences in IL-12, TNF-α, IL-10 and IL-5 production was found at any stage of infection, and IL-4 was undetectable in all animals (data not shown). When these parameters of the local immune response were compared between mice vaccinated by the i.n. and s.c. routes, no major differences were observed (Fig. 2). After 5 weeks post-challenge, proliferative and IFN-γ responses of lung T cells in non-vaccinated mice increased and reached levels comparable with those in vaccinated animals (SI: 11·1 and 14·7 in non-vaccinated and vaccinated mice, respectively; IFN-γ: 8000 ± 1780 pg/ml and 4850 ± 1700 pg/ml in non-vaccinated and vaccinated mice, respectively, P > 0·05).
Fig. 2.
Cytokine production (a) and antigen-specific proliferative response (b) of lung cells 2 weeks after Mycobacterium bovis infection. Non-vaccinated (□), i.n. vaccinated (▪) or s.c. vaccinated ( ) mice were challenged with virulent M. bovis (see legend to Fig. 1). Two weeks later, lung cells were isolated and cytokine production and proliferation were estimated (see legend to table 1). Asterisks indicate significant differences between vaccinated and non-vaccinated mice (P < 0·005).
) mice were challenged with virulent M. bovis (see legend to Fig. 1). Two weeks later, lung cells were isolated and cytokine production and proliferation were estimated (see legend to table 1). Asterisks indicate significant differences between vaccinated and non-vaccinated mice (P < 0·005).
In conclusion, the main differences between BCG vaccinated (protected) mice and non-vaccinated (non-protected) mice were found to be in the ability of lung T cells from vaccinated animals to produce IFN-γ and to proliferate in response to mycobacterial antigens early after M. bovis challenge.
Discussion
In this work, we have followed the development of immune responses in the lungs of mice vaccinated i.n. with M. bovis BCG. It was found that this vaccination route elicits immune responses associated with considerable protection against systemic infection with virulent M. bovis. The latter was demonstrated by the reduction of bacterial loads in lungs (200-fold) and spleens (50-fold) of vaccinated mice (Fig. 1). These data, as well as a strong DTH response to tuberculin (Table 1), suggest that i.n. administration of the vaccine induces not only a local but also a systemic immune defence. Falero-Diaz et al. [19] have recently reported a similar degree of protection against M. tuberculosis H37Rv infection in mice conferred by i.n. delivery of BCG vaccine. We have also compared the degree of protection conferred by the i.n. and s.c. vaccination routes, and demonstrated that i.n. vaccination conferred as good, if not better, protection against M. bovis challenge as BCG vaccination by the s.c. route.
To establish immunological correlate(s) of protection, we compared cytokine production in the lungs of vaccinated and non-vaccinated mice challenged with M. bovis. The major difference between vaccinated and non-vaccinated mice was the capacity of the lung cells of the former group to produce IFN-γ early after infection. This underlines the significance of early IFN-γ production for anti-mycobacterial defense, and is in agreement with the data obtained by Caruso et al. [20]. This is also in line with numerous results pointing to the role of type 1 cytokines, IFN-γ and IL-12 in the first instance, in the development of adaptive protective immunity against mycobacterial infections [21–26]. Wakeham et al. [13] have recently shown that BALB/c mice are characterized by relatively low levels of type 1 cytokines in bronchial alveolar lavages following intratracheal challenge with M. bovis BCG. Thus, the capacity of lung cells to mount early IFN-γ production, induced by vaccination, may also have a significant impact on the control of tuberculosis.
The data presented here show accumulation of CD4+ T lymphocytes in the lung by week 5 following i.n. vaccination, whereas the increase in CD8+ cell numbers was not statistically significant. This is in full agreement with the data of Fulton et al. [12], who reported that intratracheal administration of 105 cfu of M. bovis BCG induced an influx of CD4+ cells into the lungs between weeks 3 and 5, while the CD8+ T-cell numbers increased only slightly. However, for M. tuberculosis experimental infections, accumulation of both CD4+ and CD8+ lymphocytes in the lungs has been reported [9–11,27].
Antigen-experienced T lymphocytes, able to proliferate in response to mycobacterial antigens, could be detected in the lungs no earlier than 3 weeks following challenge with either M. bovis BCG (i.n.) or with virulent M. bovis (i.v.). Thus, the development of a primary immune response in the lungs may be considered as relatively slow, since some proliferation of auxilliary lymph node cells could already be seen at week 2 following M. bovis challenge (data not shown). These observations are in line with the results of Cooper et al. [28], who observed a delay in the establishment of anti-mycobacterial immunity in the lungs, even in memory-immune mice. Feng et al. [10] have also reported a delay between the peak of infection and T-cell response in the lung following aerosol infection of mice with M. tuberculosis. In our experiments, i.n. vaccination accelerated the dynamics of the response in the lungs of infected mice. This early recall immune response may be extremely important for the display of a successful anti-tuberculous immune response.
In conclusion, the work described here demonstrated that i.n. BCG vaccination induced protective immunity against systemic M. bovis challenge in mice. The protective immune response was characterized by the ability of lung T cells from vaccinated mice to produce IFN-γ rapidly after M. bovis challenge. The success of BCG delivery by the intranasal route is highly encouraging and could prove to be of particular use for the delivery of TB vaccines designed to protect wildlife.
Acknowledgments
This work was funded by the UK Ministry of Agriculture, Fisheries and Food, and by the Wellcome Trust Collaborative Research Initiative grant (to ASA).
References
- 1.Krebs JR, Anderson RM, Clutton‐Brock T, et al. London, UK: MAFF Publications; Bovine tuberculosisin cattle and badgers. Report to the Rt. Hon. Dr Jack Cunningham M.P. [Google Scholar]
- 2.Orme IM, Andersen P, Boom WH. T cell response to Mycobacterium tuberculosis. J Infect Dis. 1993;167:1481–97. doi: 10.1093/infdis/167.6.1481. [DOI] [PubMed] [Google Scholar]
- 3.Boom WH. The role of T-cell subsets in Mycobacterium tuberculosis infection. Infect Agents Dis. 1996;5:73–81. [PubMed] [Google Scholar]
- 4.Flynn JL, Ernst JD. Immune responses in tuberculosis. Curr Opin Immunol. 2000;12:432–6. doi: 10.1016/s0952-7915(00)00116-3. [DOI] [PubMed] [Google Scholar]
- 5.Daniel TM, Ellner JJ, Boom WH. Immunology of tuberculosis. In: Reichman LR, Herchfield ES, editors. TuberculosisA comprehensive international approach. New York: Marcel Dekker; 2000. pp. 187–214. [Google Scholar]
- 6.North RJ. Mycobacterium tuberculosis is strikingly more virulent for mice when given via the respiratory than via the intravenous route. J Infect Dis. 1995;72:1550–3. doi: 10.1093/infdis/172.6.1550. [DOI] [PubMed] [Google Scholar]
- 7.Feng CG, Britton WJ. CD4+ and CD8+ T cells mediate adoptive immunity to aerosol infection of Mycobacterium bovis bacillus Calmette-Guerin. J Infect Dis. 2000;181:1846–9. doi: 10.1086/315466. [DOI] [PubMed] [Google Scholar]
- 8.Lyadova IV, Yeremeev VV, Majorov KB, et al. An ex vivo study of T lymphocytes recovered from the lungs of I/St mice infected with and susceptible to Mycobacterium tuberculosis. Infect Immun. 1998;66:4981–8. doi: 10.1128/iai.66.10.4981-4988.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lyadova IV, Eruslanov EB, Yeremeev VV, et al. Comparative analysis of T lymphocytes recovered from the lungs of mice genetically susceptible, resistant and hyperresistant to Mycobacterium tuberculosis-triggered disease. J Immunol. 2000;165:5921–31. doi: 10.4049/jimmunol.165.10.5921. [DOI] [PubMed] [Google Scholar]
- 10.Feng CG, Bean AG, Hooi H, Briscoe H, Britton WJ. Increase in gamma interferon-secreting CD8+, as well as CD4+, T cells in lungs following aerosol infection with Mycobacterium tuberculosis. Infect Immun. 1999;67:3242–7. doi: 10.1128/iai.67.7.3242-3247.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Serbina NV, Flynn JL. Early emergence of CD8+ T cells primed for production of type 1 cytokines in the lungs of Mycobacterium tuberculosis-infected mice. Infect Immun. 1999;67:3980–8. doi: 10.1128/iai.67.8.3980-3988.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fulton SA, Martin TD, Redline RW, Boom WH. Pulmonary immune responses during primary Mycobacterium bovis-Calmette-Guerin Bacillus infection in C57Bl/6 mice. Am J Respir Cell Mol Biol. 2000;22:333–43. doi: 10.1165/ajrcmb.22.3.3776. [DOI] [PubMed] [Google Scholar]
- 13.Wakeham J, Wang J, Xing Z. Genetically determined disparate innate and adaptive cell-mediated immune responses to pulmonary Mycobacterium bovis BCG infection in C57BL/6 and BALB/c mice. Infect Immun. 2000;68:6946–53. doi: 10.1128/iai.68.12.6946-6953.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nikonenko BV, Averbakh MM, Jr, Lavebratt C, Schurr E, Apt AS. Comparative analysis of mycobacterial infections in susceptible I/St and resistant A/Sn inbred mice. Tuber Lung Dis. 2000;80:15–25. doi: 10.1054/tuld.1999.0225. 10.1054/tuld.1999.0225. [DOI] [PubMed] [Google Scholar]
- 15.Rhodes SG, Gavier-Widen D, Buddle BM, et al. Antigen specificity in experimental bovine tuberculosis. Infect Immun. 2000;68:2573–8. doi: 10.1128/iai.68.5.2573-2578.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Holt PG, Degebrodt A, O'Leary C, Krska K, Plozza T. T cell activation by antigen-presenting cells from lung tissue digests: suppression by endogenous macrophages. Clin Exp Immunol. 1985;62:586–93. [PMC free article] [PubMed] [Google Scholar]
- 17.Apt AS, Avdienko VG, Nikonenko BV, Kramnik IB, Moroz AM, Skamene E. Distinct H-2 complex control of mortality, and immune responses to tuberculosis infection in virgin and BCG-vaccinated mice. Clin Exp Immunol. 1993;94:322–9. doi: 10.1111/j.1365-2249.1993.tb03451.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Abou-Zeid C, Gares M-P, Inwald J, et al. Induction of a type 1 immune response to a recombinant antigen from Mycobacterium tuberculosis expressed in Mycobacterium vaccae. Infect Immun. 1997;65:1856–62. doi: 10.1128/iai.65.5.1856-1862.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Falero-Diaz G, Challacombe A, Banerjee D, Douce G, Boyd A, Ivanyi J. Intranasal vaccination of mice against infection with Mycobacterium tuberculosis. Vaccine. 2000;18:3223–9. doi: 10.1016/s0264-410x(00)00134-1. 10.1016/s0264-410x(00)00134-1. [DOI] [PubMed] [Google Scholar]
- 20.Caruso M, Serbina N, Klein E, Triebold K, Bloom BR, Flynn AL. Mice deficient in CD4 T cells have only transiently diminished levels of IFN-γ, yet succumb to tuberculosis. J Immunol. 1999;165:5407–16. [PubMed] [Google Scholar]
- 21.Flynn JL, Chan J, Triebold KJ, Dalton DK, Stewart TA, Bloom BR. An essential role for IFN-γ in resistance to M. tuberculosis infection. J Exp Med. 1993;178:2249–54. doi: 10.1084/jem.178.6.2249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cooper AM, Dalton DK, Stewart TA, Griffin JP, Russel DG, Orme IM. Disseminated tuberculosis in interferon-γ gene-disrupted mice. J Exp Med. 1993;178:2243–7. doi: 10.1084/jem.178.6.2243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cooper AM, Roberts AD, Rhoades ER, Callahan JE, Getzy DM, Orme IM. The role of intereleukin-12 in acquired immunity to Mycobacterium tuberculosis infection. Immunology. 1995;84:423–32. [PMC free article] [PubMed] [Google Scholar]
- 24.Flynn JL, Goldstein MM, Triebold KJ, Sypek J, Wolf S, Bloom BR. IL-12 increases resistance of BALB/c mice to Mycobacterium tuberculosis infection. J Immunol. 1995;155:2515–24. [PubMed] [Google Scholar]
- 25.Dalton DK, Pitts-Meek S, Keshav S, Figari IS, Bradley A, Stewart TA. Multiple defects of immune cell function in mice with disrupted interferon-γ genes. Science. 1993;259:1739–42. doi: 10.1126/science.8456300. [DOI] [PubMed] [Google Scholar]
- 26.Wakeham J, Wang J, Magram J, et al. Lack of both types 1 and 2 cytokines, tissue inflammatory responses, and immune protection during pulmonary infection by Mycobacterium bovis Bacille Calmette-Guérin in IL-12-deficient mice. J Immunol. 1998;160:6101–11. [PubMed] [Google Scholar]
- 27.Howard AD, Trask OJ, Weisbrode SE, Zwilling BS. Phenotypic changes in T cell populations during the reactivation of tuberculosis in mice. Clin Exp Immunol. 1998;111:309–15. doi: 10.1046/j.1365-2249.1998.00489.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cooper AM, Callahan JE, Keen M, Belisle JT, Orme IM. Expression of memory immunity in the lung following reexposure to Mycobacterium tuberculosis. Tuber Lung Dis. 1997;78:67–73. doi: 10.1016/s0962-8479(97)90017-4. [DOI] [PubMed] [Google Scholar]