Abstract
This study was performed to determine whether or not IL-18, formerly called IFN-γ-inducing factor, is involved in the pathogeneses of allergic disorders. Peripheral blood mononuclear cells (PBMC) were obtained from patients with allergic bronchial asthma (BA), patients with atopic dermatitis (AD) and controls who did not have any allergic disease, and then cultured with lipopolysaccharide (LPS) or phytohaemagglutinin (PHA). The concentrations of IL-18, IFN-γ and IL-13 in supernatant fluids were determined by enzymatic immunoassaying, and the expression of IFN-γ messenger (m) RNA in the cells was measured by colorimetric microplate assaying. IL-18 secretion in the BA patients (geometric mean (gm) = 189 pg/ml) and AD patients (gm = 172 pg/ml) was significantly higher than that in non-allergic controls (gm = 118 pg/ml). In contrast, IFN-γ secretion in the BA patients (gm = 7·3 IU/ml) and AD patients (gm = 6·8 IU/ml) was significantly lower than that in non-allergic controls (gm = 20·7 IU/ml). The amounts of IL-13 in supernatant fluids and IFN-γ mRNA in cells were not statistically different among the BA patients, AD patients and non-allergic controls. The possible involvement of IL-18 in allergic disorders is discussed.
Keywords: bronchial asthma, atopic dermatitis, IL-18, IFN-γ
Introduction
It is widely accepted that helper T (Th) cells consist of two functionally different subsets, Th1 and Th2. These subsets are characterized by the profile of cytokines involved: Th1 cells mainly produce IFN-γ, while Th2 cells selectively secrete IL-4, IL-5 and IL-13. There is overwhelming evidence that abnormal Th1 and Th2 functions contribute not only to the production of IgE but also to the pathogeneses of allergic disorders. Studies on peripheral blood mononuclear cells (PBMC) from patients with bronchial asthma (BA) and patients with atopic dermatitis (AD) have shown decreases in polyclonally-stimulated IFN-γ release [1–9]. IL-18, also designated as IFN-γ-inducing factor, is a novel cytokine that plays an important role in the Th1 cell response, primarily through its ability to induce IFN-γ production, especially in collaboration with IL-12 [10,11]. Recent animal studies, however, indicated a more complicated pleiotrophic role for IL-18 than simply induction of IFN-γ production, and IL-18 was reported to induce the production of IgE and Th2 cytokines [12–15]. Since the assessment of in vitro IL-18 production in allergic patients has not been reported, the present study was undertaken to examine the in vitro IL-18 production in different allergic disorders.
Patients and methods
Subjects
The study groups comprised 18 patients with allergic bronchial asthma without any skin disorders, 16 patients with AD without any respiratory problems, and 19 non-allergic controls without any haematological, immunological or nutritional problems. All the subjects, aged between 4 and 22 years, visited the Department of Child Development, Kumamoto University School of Medicine (Table 1). The asthmatic patients met the diagnostic criteria of the Guidelines for the Diagnosis and Management of Bronchial Asthma of the Japanese Society of Allergology [16]. All the asthmatic patients were sensitive to house-dust mites, as judged from the skin prick test reactivity and mite-specific IgE serum level, and had the moderate, persistent type of asthma according to the disease severity grading of the guidelines described above. They had been managed by means of inhaled cromoglycate, inhaled β-agonist, oral theophylline and/or inhaled corticosteroids. Subjects on more than 400 µg/day of prophylactic-inhaled corticosteroids were excluded to avoid any possible systemic effects of absorbed steroids on cytokine production. AD was diagnosed according to the criteria of Hanifin and Rajka [17], and all the AD patients were affected by an exacerbated form of chronic dermatitis, i.e. more than 18% of the skin of the face, limbs and trunk was involved, and thus were graded as having the moderate or severe type of AD according to the guidelines for the SCORAD index [18]. None of the allergic donors was undergoing systemic corticosteroid therapy. The test was performed during the remission phase not only in the patients with BA, but also in those with AD, and all the subjects were free from infection. Heparinized peripheral blood (5 ml) was obtained at the time of routine blood examination, and this study was approved by the Ethics Committee of the Kumamoto Society for Paediatrc Allergy. Informed consent for the investigation was obtained for all allergic and non-allergic subjects, the parents' consent being obtained for children.
Table 1.
Profiles of subjects
| Control | BA | AD | |
|---|---|---|---|
| Number | 19 | 18 | 16 |
| Age (years) | 14 ± 6 | 13 ± 5 | 13 ± 5 |
| (4–22) | (4–20) | (4–21) | |
| Sex (M/F) | 11/8 | 11/7 | 9/7 |
| IgE (U/ml) | 93 | 933 | 1349 |
| (50–174) | (537–1622) | (741–2455) |
Age (years) is shown as the mean ± 1 standard deviation and range. The levels of IgE are presented as the geometric means and 95% confidence intervals. BA, bronchial asthma; AD, atopic dermatitis.
Cell culture
PBMC were isolated by density gradient centrifugation, washed three times, and then adjusted to 1 × 106 cells/ml with RPMI 1640 medium containing 10% heat-inactivated fetal calf serum and 2 mm l-glutamine. The cells were then cultured in round-bottomed Falcon tubes (No. 2054; Beckton Dickinson, Lincoln Park, NJ, USA) with 50 ng/ml lipopolysaccharide (LPS) or 5 µg/ml phytohaemagglutinin (PHA), or without any stimulation, at 37°C under 5% CO2. In some experiments, PBMC were cultured with LPS for 1 h in the presence of cycloheximide (100 µg/ml; Sigma, St. Louis, MO, USA). The kinetics of IL-18, IFN-γ and IL-13 secretion were similar in allergic and non-allergic subjects, with maximal secretion of IL-18 at 6 h in LPS-stimulated cultures, and maximal secretion of IFN-γ and IL-13 at 3 days in PHA-stimulated cultures. Since there was moderate secretion of IL-18 within 1 h of stimulation with LPS, and moderate secretion of IFN-γ and IL-13 after 18 h of stimulation with PHA, the PBMC cultures were suspended at 1 h for the IL-18 assay, and at 18 h for the IFN-γ and IL-13 assays.
Measurement of IgE, IL-18, IFN-γ,IL-13 and IL-12
Plasma IgE was measured with an enzyme-linked immunosorbent assay (ELISA) kit, IgE-MP Mitsui, from Mitsui-Seiyaku, Tokyo, Japan. This kit includes a monoclonal antibody (MoAb) as the first antibody and a peroxidase-conjugated polyclonal anti-IgE antibody as the second antibody, and is capable of detecting 0·65–1000 U/ml of IgE. The ELISA kit for IL-18 was obtained from Medical & Biological Laboratories, Nagoya, Japan, and those for IFN-γ and IL-13 were purchased from Immunotech, Marseille, France. Each kit includes two non-competing MoAbs for each cytokine. Mouse MoAb #125–2H and rat MoAb #159–12B were used in the human IL-18 ELISA kit, and both MoAbs were confirmed to neutralize IFN-γ production by IL-18 [19]. The minimum sensitivities for the IL-18, IFN-γ and IL-13 assays were 12·5 pg/ml, 0·08 IU/ml and 1·5 pg/ml, respectively. The levels of IL-12 were determined with a kit involving the multiple sandwich principle from R & D Systems, Minneapolis, MN, USA. This kit includes a MoAb as the first antibody and alkaline phosphatase-conjugated polyclonal anti-IL-12 as the second antibody, and a colour-amplification system consisting of NADH, diaphorase and tetrazolium. The standard curve was linear from 0·5 to 40 pg/ml of IL-12 with this assay kit.
Western blot analysis for IL-18
Western blotting was performed, following the protocol recommended by Cell Signalling Technology, Inc. (Beverly, MA, USA). Briefly, samples of cell lysates containing 20 µg protein were heat-denatured in the presence of 62·5 mm Tris-HCl, pH 6·8, 2% SDS, 10% glycerol and 50 mm DTT, and then fractionated by 12·5% SDS-PAGE. The protein was then transferred to a Hybond-P membrane (Amersham Pharmacia Biotech, Inc., UK). The rabbit anti-human IL-18 antibody (H-173; Santa Cruz Biotechnology, CA, USA), which detects both 24 kD precursor and 18 kD mature form IL-18, was diluted to a concentration of 1:1000 before use. The bound antibody was detected with horseradish peroxidase-conjugated anti-rabbit IgG (Cell Signalling Technology) and visualized with an ECL kit (Amersham Pharmacia Biotech) according to the manufacturer's instructions.
Determination of IFN-γ mRNA expression
For RNA extraction, 1 × 106 PBMC were cultured with PHA for 3 h, and then mRNA was isolated using a poly(A)+ RNA isolation kit with oligo dT-latex beads (mini-Message Marker; Novagen Inc., Madison, WI, USA). Samples were then hybridized with hIFN-γ-specific biotin-labelled capture oligonucleotide probes and digoxigenin-labelled detection probes on streptavidin-coated microplates (Colorimetric mRNA Quantification Kit; R & D Systems) according to the manufacturer's instructions. The standard curve was linear from 4·7 to 300 amol/ml IFN-γ mRNA.
Statistical analysis
All samples were coded and read blind on assaying. The plasma IgE level and in vitro cytokine amount data were logarithmically transformed before statistical analysis, and were expressed as gm and 95% confidence intervals (ci). Group means were compared by means of the unpaired Student's t-test. Correlation was determined by multiple and single regression analyses. Frequencies were compared as to their statistical significance with the Fisher's exact test. All statistical analyses were performed with StatView Statistical Package 4·5 (SAS Institute, Cary, NC, USA). Probability (P) values of less than 0·05 were considered significant.
Results
The levels of IgE in plasma from the BA patients and AD patients were extremely elevated compared with those in age, sex-matched controls who did not have any allergic disease (Table 1). However, no statistical difference in the plasma IgE levels was observed between the BA patients and AD patients.
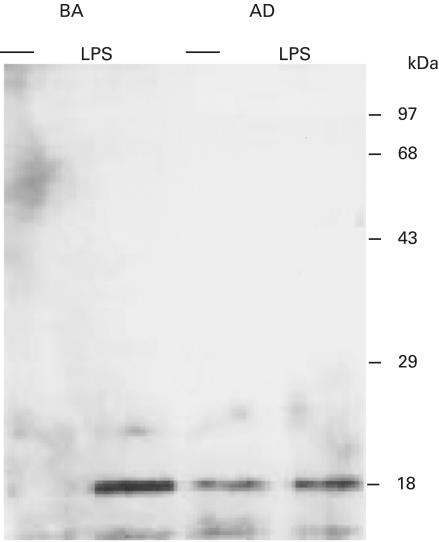
Spontaneous secretion of IL-18, IFN-γ and IL-13 by unstimulated PBMC was very low or unmeasurable in all cultures. The means and standard error of the mean (s.e.m.) of the IL-18 concentration in the supernatant fluids of LPS-stimulated PBMC cultured for 1 h were 114 ± 17 pg/ml without cycloheximide and 122 ± 9 with cycloheximide in three non-allergic controls, and 173 ± 15 pg/ml without cycloheximide and 157 ± 10 pg/ml with cycloheximide in three allergic patients. Western blot analysis was performed using extracts of PBMC cultured with or without LPS for 1 h from three BA patients, three AD patients and three non-allergic controls. The results showed that only mature form IL-18 was present in cultured PBMC with LPS in BA patients and non-allergic controls, although mature form IL-18 was detected in both unstimulated PBMC and LPS-stimulated PBMC from AD patients. Representative results are shown in Fig. 1. There was moderate enhancement of the level of mature form IL-18 within 1 h of stimulation with LPS in an AD patient.
Fig. 1.
Detection of IL-18 in peripheral blood mononuclear cells (PBMC) cultured without stimulation (–) or with lipopolysaccharide (LPS) for 1 h. Lysates of PBMC were subjected to SDS/PAGE and Western blotting with an anti-human IL-18 antibody (H-173), which detects both 24 kDa precursor form IL-18 and 18 kDa mature form IL-18. Representative results for a BA patient (BA) and an AD patient (AD) are shown in this figure.
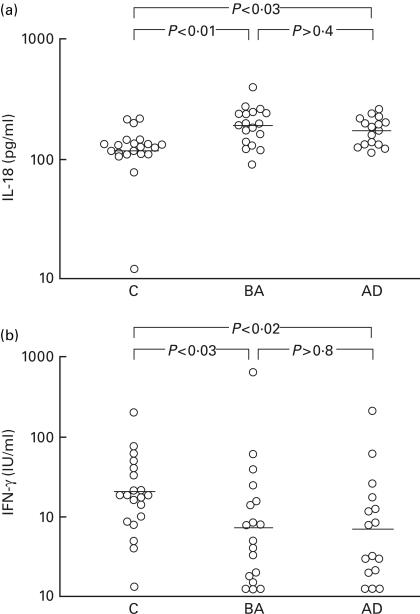
As shown in Fig. 2, increased IL-18 secretion by LPS-stimulated PBMC was observed in the BA patients (gm 189 pg/ml, 95% ci 161–223 pg/ml) and AD patients (gm 172 pg/ml, 95% ci 151–196 pg/ml) compared with controls who did not have any allergic disease (gm 118 pg/ml, 95% ci 91–153 pg/ml). In contrast, secretion of IFN-γ by PHA-stimulated PBMC from the BA patients (gm 7·3 IU/ml, 95% ci 3·5–15·5 IU/ml) and AD patients (gm 6·8 IU/ml, 95% ci 3·3–13·9 IU/ml) was significantly decreased compared with controls who did not have any allergic disease (gm 20·7 IU/ml, 95% ci 13·6–53·3 IU/ml). The amounts of IL-18 and IFN-γ in the culture supernatant fluids did not differ markedly between the BA patients and AD patients. The amounts of IL-13 secreted by PHA-stimulated PBMC were comparable among the BA patients (gm 27·9 pg/ml, 95% ci 11·1–70·0 pg/ml), AD patients (gm 31·3 pg/ml, 95% ci 11·3–86·9 pg/ml) and non-allergic controls (gm 54·6 pg/ml, 95% ci 34·8–85·5 pg/ml). No significant differences in IFN-γ mRNA expression in PHA-stimulated PBMC were observed among the BA patients (mean 18·3 amol/106 cells, 95% ci 15·1–21·4 amol/106 cells), AD patients (mean 20·6 amol/106 cells, 95% ci 14·6–26·5 amol/106 cells) and non-allergic controls (mean 15·3 amol/106 cells, 95% ci 11·1–19·5 amol/106 cells).
Fig. 2.
Secretion of (a) IL-18 by LPS-stimulated PBMC cultures at 1 h and (b) IFN-γ by phytohaemagglutinin (PHA)-stimulated PBMC cultures at 18 h was measured by enzymatic immunoassaying. Horizontal lines represent geometric means. C, non-allergic controls; BA, bronchial asthma; AD, atopic dermatitis.
All plasma samples gave results above the detection limit of the IL-18 assay in this study, and the mean ± s.e.m. plasma IL-18 levels were 192 ± 17 pg/ml in BA patients, 216 ± 29 pg/ml in AD patients and 150 ± 18 pg in non-allergic controls. Although the plasma IL-18 levels in allergic patients were more increased than those in non-allergic controls, the differences were not significant (BA patients versus controls, P = 0·107; AD patients versus controls, P = 0·074). Six plasma samples (three from 18 BA patients, two from 16 AD patients and one from 19 non-allergic controls) gave results above the detection limit of the IL-12 assay, and the frequencies of subjects showing results above the sensitivity of the IL-12 assay were not statistically different among the three groups.
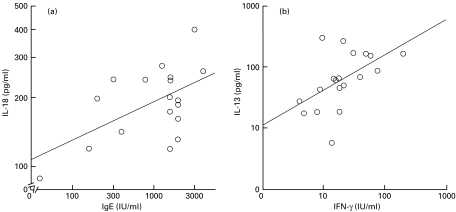
The cytokine levels in stimulated PBMC cultures were used as independent variables, and regressed against the plasma IgE concentration as the dependent variable for multiple regression analysis. IL-18 secretion was significantly correlated with the plasma IgE concentration in BA patients (P = 0·026), although IL-18 secretion was not correlated with the plasma IgE concentration in AD patients (P = 0·121) or non-allergic controls (P = 0·748). IFN-γ secretion, IL-13 secretion and IFN-γ mRNA expression were not statistically correlated with the plasma IgE concentration in the three groups, respectively. IFN-γ secretion was chosen as the dependent variable and analysed with IL-18 secretion, IL-13 secretion and IFN-γ mRNA expression as independent variables for multiple regression analysis. IL-13 secretion was significantly correlated with IFN-γ secretion in non-allergic controls (P = 0·031). However, IL-13 secretion was not statistically correlated with IFN-γ secretion in BA patients (P = 0·146) or AD patients (P = 0·204). Figure 3 depicts the positive correlation between the plasma IgE level and IL-18 secretion in BA patients (r = 0·556, P = 0·017), and the positive correlation between IFN-γ secretion and IL-13 secretion in non-allergic controls (r = 0·533, P = 0·019), observed on single regression analysis, respectively. IL-18 secretion was chosen as the dependent variable and analysed with IFN-γ secretion, IL-13 secretion and IFN-γ mRNA expression as the independent variables for multiple regression analysis. IL-18 secretion was correlated with neither IFN-γ secretion, IL-13 secretion nor IFN-γ mRNA expression in the three groups.
Fig. 3.
Correlation between plasma IgE concentration and in vitro IL-18 secretion by LPS-stimulated PBMC in BA patients (a), and that between IFN-γ secretion and IL-13 secretion in PHA-stimulated PBMC cultures in non-allergic controls (b). The differences in the slopes between groups were assessed by single regression analyses. A significant positive correlation between IL-18 secretion and plasma IgE was found (r = 0·556, P = 0·017). Secretion of IFN-γ was significantly correlated with secretion of IL-13 (r = 0·533, P = 0·019).
Discussion
The original characteristics of PBMC may be considerably altered during in vitro incubation with stimulants. In this study, cytokine secretion by PBMC was measured in short-term cultures to minimize alterations in the nature of PBMC, the results probably reflecting the state of in vivo cytokine production.
Spontaneous secretion of IL-18 by unstimulated PBMC was very low or unmeasurable, and mature form IL-18 was not present in lysates of unstimulated PBMC from BA patients and non-allergic controls. Puren et al. [20] reported that mature form IL-18 was detected in freshly obtained PBMC from healthy donors. Mature form IL-18 was found in unstimulated PBMC from AD patients in this study, suggesting that de-novo IL-18 synthesis occurs in these cells without any stimulation. Since the amounts of IL-18 in the supernatant fluids of 1 h-stimulated PBMC with LPS were not statistically different between cycloheximide-treated and untreated cultures, it is probable that IL-18 in the supernatant fluids might have been preformed IL-18 released by the cells.
The present study clearly showed that IL-18 secretion by stimulated PBMC was increased in BA patients and AD patients. The amount of IL-18 did not differ markedly between the BA patients and AD patients. This implies that the increased IL-18 secretion is related to the allergic state per se rather than to the clinical features of allergic diseases. The effects of IL-18 in allergic disorders have been previously assessed in murine asthma models with different outcomes [14,15,21]. In the study by Hofsta et al. [21], the combined in vivo administration of IL-18 and IL-12 inhibited allergen-induced airway hyperresponsiveness, lung production of eosinophilia and serum IgE, although the administration of IL-18 alone failed to modulate any of these allergic responses. However, Kumano et al. [14] and Wild et al. [15] showed that in vivo IL-18 administration alone enhanced allergen-induced eosinophilic recruitment to the lungs, Th2 cytokine production and IgE production in sensitized mice. The latter findings in murine models are in accord with the results of the present study for BA patients.
In the recent study by Hoshino et al. [22], the administration of IL-18 alone in vivo significantly increased the serum IgE level in non-sensitized mice, and the administration of IL-18 plus IL-2 induced a 70-fold higher serum level of IgE than that in control mice. Alternatively, human IL-18 may be involved in the increased IgE production in bronchial asthma, since the IL-18 secretion by LPS-stimulated PBMC was associated with the plasma IgE level in BA patients in the present study.
An important question is whether the cytokine imbalance demonstrated in the in vitro study was the cause or the result of the disease process. All the patients were examined during the remission phase in the present study, suggesting that an imbalance of IL-18 and IFN-γ secretion is not simply related to the disease process. The IFN-γ deficiency in PBMC precedes the allergic state, rather than resulting from it, as suggested by the finding of decreased IFN-γ production in cord blood cells of neonates who subsequently developed allergic diseases [23]. Therefore, defective IFN-γ secretion by PBMC in vitro is likely to underlie allergic disorders.
IL-18 has been described as a potent inducer of IFN-γ [10,11], and reduced IFN-γ secretion with increased secretion of IL-18 may imply a defect in IL-18 responsiveness in allergic patients. Supporting this possibility, Jung et al. [24] reported that a deficiency of IFN-γ production in severe AD patients could not be reversed by IL-18 in vitro.
There is an absolute requirement for IL-12 for development of the Th1 cell response, and it is well established that in the presence of low picomolar concentrations of IL-12, IL-18 becomes essential for the production of IFN-γ [25]. Another interpretation is that the regulatory cytokines involved in IFN-γ production may differ between allergic patients and non-allergic controls. However, the frequencies of cases showing results above the detection limit of the IL-12 assay (0·5 pg/ml) were not statistically different among BA patients, AD patients and non-allergic controls.
In contrast to the reduced in vitro IFN-γ secretion in the BA patients and AD patients, however, equivalent expression of IFN-γ mRNA in stimulated PBMC was observed for the allergic patients compared with the controls. Comparable studies have demonstrated that expression of IFN-γ mRNA is not necessarily correlated to secretion, as children with AD exhibit comparable or increased expression of IFN-γ mRNA in stimulated PBMC compared with controls, despite exhibiting reduced IFN-γ secretion [5,9]. The discrepancy between IFN-γ mRNA expression and secretion demonstrated here for allergic patients suggests that reduced secretion of IFN-γ is not related to abnormal regulation of transcription, but rather to a post-transcriptional defect of IFN-γ production.
This study demonstrated a positive correlation between IFN-γ secretion and Th2 cytokine IL-13 secretion in cultures of PHA-stimulated PBMC in non-allergic controls. IL-18 has been reported to have the ability to stimulate the production of IFN-γ by T cells, NK cells and B cells [11,25], and appeared recently to induce the production of IL-13 by T cells and NK cells in vitro [12]. The present study did not reveal any correlation between IL-18 secretion by LPS-stimulated PBMC cultures and IFN-γ secretion or IL-13 secretion by PHA-stimulated PBMC cultures in non-allergic controls. IFN-γ was found not to be generally co-expressed with IL-13 in human T cells in an intracellular cytokine staining assay, and IFN-γ does not affect IL-13 production in stimulated human T-cell cultures [26]. However, both IFN-γ and IL-13 are cytokines with long-lasting kinetics on secretion from T cells [26]. It is tempting to speculate that IFN-γand IL-13 play roles in ongoing immune responses in humans without any disorders.
IL-18 is considered to possess biological properties other than induction of IFN-γ, IgE or Th2 cytokines, such as up-regulation of the expression of intercellular adhesion molecule-1 (ICAM-1) and Fas-ligand in human myelomonocytic KG-1 cells [27,28]. Circulating ICAM-1 levels have been reported to increase gradually with the severity of AD [29], and successful phototherapy for AD patients has been found to result from ultraviolet A radiation-induced apoptosis of skin-infiltrating activated T cells through the Fas/Fas-ligand system [30]. The plasma levels of IL-18 were not different between the remission-phase allergic patients and the non-allergic controls. Therefore, further study is in progress in our laboratory to confirm the biological significance of IL-18 during the allergic inflammatory process.
Acknowledgments
This work was supported by a Grant-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology, Japan (project no. 13670818).
References
- 1.Tang ML, Coleman J, Kemp AS. Interleukin-4 and interferon- gamma production in atopic and non-atopic children with asthma. Clin Exp Allergy. 1995;25:515–21. doi: 10.1111/j.1365-2222.1995.tb01088.x. [DOI] [PubMed] [Google Scholar]
- 2.Hoekstra MO, Hoekstra Y, DeReus D, Rutgers B, Gerritsen J, Kauffman HF. Interleukin-4, interferon-gamma and interleukin-5 in peripheral blood of children with moderate atopic asthma. Clin Exp Allergy. 1997;27:1254–60. [PubMed] [Google Scholar]
- 3.Nurse B, Haus M, Puterman AS, Weinberg EG, Potter PC. Reduced interferon-gamma but normal IL-4 and IL-5 release by peripheral blood mononuclear cells from Xhosa children with atopic asthma. J Allergy Clin Immunol. 1997;100:662–8. doi: 10.1016/s0091-6749(97)70171-4. [DOI] [PubMed] [Google Scholar]
- 4.Tang M, Kemp A. Production and secretion of interferon-gamma (IFN-gamma) in children with atopic dermatitis. Clin Exp Immunol. 1994;95:66–72. doi: 10.1111/j.1365-2249.1994.tb06016.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Koning H, Neijens HJ, Baert MRM, Oranje AP, Savelkoul HFJ. T cell subsets and cytokines in allergic and non-allergic children. I. Analysis of IL-4, IFN-γ and IL-13 mRNA expression and protein production. Cytokine. 1997;6:416–26. doi: 10.1006/cyto.1996.0184. [DOI] [PubMed] [Google Scholar]
- 6.Kimura M, Tsuruta S, Yoshida T. Differences in cytokine production by peripheral blood mononuclear cells (PBMC) between patients with atopic dermatitis and bronchial asthma. Clin Exp Immunol. 1999;118:192–6. doi: 10.1046/j.1365-2249.1999.01055.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lonati A, Licenziati S, Canaris AD, et al. Reduced production of both the Th1 and Tc1 lymphocyte subsets in atopic dermatitis (AD) Clin Exp Immunol. 1999;115:1–5. doi: 10.1046/j.1365-2249.1999.00773.x. 10.1046/j.1365-2249.1999.00773.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Campbell DE, Gryga AS, Bol S, Kemp AS. Intracellular interferon- gamma (IFN-γ) production in normal children and children with atopic dermatitis. Clin Exp Immunol. 1999;115:377–82. doi: 10.1046/j.1365-2249.1999.00814.x. 10.1046/j.1365-2249.1999.00814.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tang MLK, Varigos G, Kemp AS. Reduced interferon-gamma (IFN-γ) secretion with increased IFN-γ mRNA expression in atopic dermatitis: evidence for a post-transcriptional defect. Clin Exp Immunol. 1994;97:483–90. doi: 10.1111/j.1365-2249.1994.tb06114.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Okamura H, Tsutsui H, Komatsu T, et al. Cloning of a new cytokine that induces interferon-γ. Nature. 1995;378:88–91. doi: 10.1038/378088a0. [DOI] [PubMed] [Google Scholar]
- 11.Yoshimoto T, Okamura H, Tagawa Y, et al. Interleukin 18 together with interleukin 12 inhibits IgE production by induction of interferon-γ production from activated B cells. Proc Natl Acad Sci USA. 1997;94:3948–53. doi: 10.1073/pnas.94.8.3948. 10.1073/pnas.94.8.3948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hoshino T, Wiltrout RH, Young HA. IL-18 is a potent coinducer of IL-13 in NK and T cells: a new potential role for IL-18 in modulating the immune response. J Immunol. 1999;162:5070–7. [PubMed] [Google Scholar]
- 13.Yoshimoto T, Tsutsui H, Tominaga K, et al. IL-18, although antiallergic when administered with IL-12, stimulates IL-4 and histamine release by basophils. Proc Natl Acad Sci USA. 1999;96:13962–6. doi: 10.1073/pnas.96.24.13962. 10.1073/pnas.96.24.13962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kumano K, Nakano A, Kanajima H, et al. Interleukin-18 enhances antigen-induced eosinophil recruitment into the mouse airways. Am J Respir Crit Care Med. 1999;160:873–8. doi: 10.1164/ajrccm.160.3.9805026. [DOI] [PubMed] [Google Scholar]
- 15.Wild JS, Sigounas A, Sur N, et al. IFN-γ-inducing factor (IL-18) increases allergic sensitization, serum IgE, Th2 cytokines, and airway eosinophilia in a mouse model of allergic asthma. J Immunol. 2000;164:2701–10. doi: 10.4049/jimmunol.164.5.2701. [DOI] [PubMed] [Google Scholar]
- 16.Makino S, Furusho K, Miyamoto T, Ohta K. Asthma prevention and management guidelines. Int Arch Allergy Immunol. 2000;121(Suppl.):1–78. doi: 10.1159/000053608. [DOI] [PubMed] [Google Scholar]
- 17.Hanifin JM, Rajka G. Diagnostic features of atopic dermatitis. Acta Derm Venereol (Stock) 1980;92(Suppl.):44–7. [Google Scholar]
- 18.European Task Force on Atopic Dermatitis. Severity scoring of atopic dermatitis. The SCORAD index. Dermatol. 1993;186:23–31. doi: 10.1159/000247298. [DOI] [PubMed] [Google Scholar]
- 19.Taniguchi M, Nagaoka K, Kunikata T, et al. Characterization of anti-human interleukin-18 (IL-18)/interferon-γ-inducing factor (IGIF) monoclonal antibodies and their application in the measurement of human IL-18 by ELISA. J Immunol Method. 1997;206:107–13. doi: 10.1016/s0022-1759(97)00094-x. [DOI] [PubMed] [Google Scholar]
- 20.Puren AJ, Fantizzi G, Dinarello CA. Gene expression, synthesis, and secretion of interleukin 18 and interleukin 1β are differentially regulated in human blood mononuclear cells and mouse spleen cells. Proc Natl Acad Sci USA. 1999;96:2256–61. doi: 10.1073/pnas.96.5.2256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hofstra CL, Ark IV, Hofman G, et al. Prevention of Th2-like cell responses by coadministration of IL-12 and IL-18 is associated with inhibition of antigen-induced airway hyperresponsiveness, eosinophilia, and serum IgE levels. J Immunol. 1998;161:5054–60. [PubMed] [Google Scholar]
- 22.Hoshino T, Yagita H, Ortaldo JR, et al. In vivo administration of IL-18 can induce IgE production through Th2 cytokine induction and up-regulation of CD40 ligand (CD154) expression on CD4+ T cells. Eur J Immunol. 2000;30:1998–2000. doi: 10.1002/1521-4141(200007)30:7<1998::AID-IMMU1998>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 23.Kondo N, Kobayashi Y, Shinoda S, et al. Reduced interferon-gamma production by antigen-stimulated cord blood mononuclear cells is a risk factor of allergic disorders – 6-year follow-up study. Clin Exp Allergy. 1998;28:1313–6. doi: 10.1046/j.1365-2222.1998.00418.x. [DOI] [PubMed] [Google Scholar]
- 24.Jung T, Witzak K, Dieckhoff K, et al. IFN-γ is only partially restored by co-stimulation with IL-12, IL-2, IL-15, IL-18 or engagement of CD28. Clin Exp Allergy. 1999;29:207–16. doi: 10.1046/j.1365-2222.1999.00482.x. [DOI] [PubMed] [Google Scholar]
- 25.Fantuzzi G, Puren AJ, Harding MW, Livingston DJ, Dinarello CA. IL-18 regulation of IFN-γ production and cell proliferation as revealed in interleukin-1β converting enzyme-deficient mice. Blood. 1998;91:2118–25. [PubMed] [Google Scholar]
- 26.Jung T, Wijdenes J, Neumann C, et al. Interleukin-13 is produced by activated human CD45RA+ and CD45RO+ T cells: modulation by interleukin-4 and interleukin-12. Eur J Immunol. 1996;26:571–7. doi: 10.1002/eji.1830260311. [DOI] [PubMed] [Google Scholar]
- 27.Kohda H, Yoshino T, Iwagaki H, et al. Interleukin-18/interferon-γ-inducing factor, a novel cytokine, upregulates ICAM-1 (CD45) expression in KG-1 cells. J Leukoc Biol. 1998;64:519–27. doi: 10.1002/jlb.64.4.519. [DOI] [PubMed] [Google Scholar]
- 28.Ohtsuka T, Micallef MJ, Kohno K, Tanimoto T, Ikeda M, Kurimoto M. Interleukin-18 enhances Fas ligand expression and induces apoptosis in Fas-expressing human myelomonocytic KG-1 cells. Anticancer Res. 1997;17:3253–8. [PubMed] [Google Scholar]
- 29.Kojima T, Ono A, Aoki T, Kameda-Hayashi N, Kobayashi Y. Circulating ICAM-1 levels in children with atopic dermatitis. Ann Allergy. 1994;73:351–5. [PubMed] [Google Scholar]
- 30.Morita A, Werfel T, Stege H, et al. Evidence that singlet oxygen- induced human T helper cell apoptosis is the basic mechanism of ultraviolet-A radiation phototherapy. J Exp Med. 1997;186:1763–8. doi: 10.1084/jem.186.10.1763. [DOI] [PMC free article] [PubMed] [Google Scholar]