Abstract
To elucidate the in vivo mechanisms involved in the impairment in pulmonary defence as the result of treatment with glucocorticoids, we established fatal pneumonia with bacteraemia in dexamethasone (DEX)-treated mice by means of an intratracheal challenge of Pseudomonas aeruginosa. An increased neutrophil influx was observed in bronchoalveolar lavage (BAL) fluids from both untreated and DEX-treated mice. The complete suppression of an inducible isoform of nitric oxide synthase (iNOS) mRNA expression and tumour necrosis factor alpha (TNF-α) production during the early phase of pneumonia, but not CXC chemokine production, were found in the case of the DEX-treated mice. An immunohistochemical study with a specific antibody also revealed negative staining for nitrotyrosine in the lung tissue of DEX-treated mice, while the formation of nitrotyrosine, which indirectly indicates the generation of peroxynitrite with a potent bactericidal activity, was detected clearly in the bronchial epithelium as well as alveolar phagocytic cells of lung tissue from untreated mice. Furthermore, an intraperitoneal administration of S-methyl-isothiourea (SMT), a potent inhibitor of NOS, significantly decreased the survival and increased bacterial density in the case of untreated mice. In contrast, no significant effects on the survival and bacterial density in the lung and blood were found as the result of treatment with SMT in DEX-treated mice.
Collectively, a complete repression of iNOS gene expression and a lack of the generation of peroxynitrite as well as an inhibition of TNF-α production in the lung appeared to be responsible for the progression of the fatal pneumonia due to P. aeruginosa in DEX-treated mice.
Keywords: glucocorticoids, iNOS gene, peroxynitrite, pulmonary defence, TNF-α
Introduction
Nosocomial pneumonia caused by Pseudomonas aeruginosa is frequently associated with immunocompromised hosts [1]. It is generally thought that glucocorticoids represent a class of compounds that are able to induce these conditions [2]. These agents, however, are frequently used for the long-term treatment of chronic pulmonary diseases, such as asthma and idiopathic pulmonary fibrosis [3,4]. Previous in vitro studies have reported a suppression of bactericidal activity in monocytes and neutrophils as the result of the presence of glucocorticoids [5]. Decreased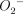 production and chemotaxis have been reported in neutrophils obtained from the bronchoalveolar spaces of glucocorticoid-treated rabbits infected with P. aeruginosa [6].
production and chemotaxis have been reported in neutrophils obtained from the bronchoalveolar spaces of glucocorticoid-treated rabbits infected with P. aeruginosa [6].
The role of tumour necrosis factor alpha (TNF-α) and nitric oxide (NO) synthesis in pulmonary defence or innate immunity against bacterial infections has been highlighted in mouse models [7,8]. In addition, a recent study has documented the existence of TNF-α or an inducible isoform of nitric oxide synthase (iNOS) gene as one of the critical host genes for respiratory infection due to P. aeruginosa using gene-targeted mice [9]. The production of antibacterial molecules in the lung as a result of NO production, however, has not been determined to date. It has also been suggested that murine CXC chemokines, such as macrophage inflammatory protein-2 (MIP-2) and KC, play a protective role in murine models of Klebsiella pneumonia [10,11].
In an earlier study, Mukaida et al. reported that a glucocorticoid, dexamethasone (DEX), interfered with the binding of the most essential transcription factor, nuclear factor-κB (NF-κB), to its cognate cis-element in vitro [12]. Because NF-κB has been shown to regulate the gene transcription of TNF-α, iNOS and IL-8, a human CXC chemokine [13–17], an alteration in the binding of NF-κB to the DNA binding site by glucocorticoids would be expected to directly impair pulmonary defence. However, in vivo mechanisms on alterations in pulmonary defence caused by glucocorticoid remain unclear at this time.
Although previous investigators have not demonstrated conclusively that treatment with glucocorticoid is an important cause of fatal pneumonia due to P. aeruginosa in guinea pigs [18], we report here on a fatal pneumonia model due to P. aeruginosa in mice that are receiving high-doses of DEX. The goal of the present study was to investigate the issue of whether iNOS and nitrogen-derived oxidants are responsible for impaired pulmonary defence against P. aeruginosa in DEX-treated mice. The roles of TNF-α and CXC chemokines were also evaluated in this model.
Materials and methods
Pneumonia model
After an overnight growth of Fisher immunotype 1 (It-1) P. aeruginosa on brain heart infusion agar (Difco, Detroit, MI, USA) at 37°C the cultures were harvested in normal saline, resuspended in brain heart infusion broth (Difco) containing 2% skim milk, and stored at − 80°C prior to use [19]. Specific pathogen-free, 5-week-old female CBA/J mice (18–20 g) were obtained from Charles River Japan, Kanagawa, Japan. Mice were given sterile food and water ad libitum in an environmentally controlled room. Pneumonia was produced by intratracheal challenging with P. aeruginosa according to a published method [19], and the survival of the mice was monitored for 7 days after bacterial challenge. Mice were pretreated with an intraperitoneal administration of dexamethason (DEX; Banyu Pharmaceutical Co., Tokyo, Japan) at a dose of 10 mg/kg twice a day for 5 days prior to the intratracheal challenge of P. aeruginosa. An intratracheal challenge of P. aeruginosa at a dose of 2·4 × 105 colony-forming units (CFU) induced 100% mortality in DEX-treated mice within 48 h, whereas all the untreated mice survived after a bacterial challenge of the same dose. The 50% lethal dose (LD50) in untreated and DEX-treated mice for P. aeruginosa It-1 strain was determined to be 5 × 105 CFU and 9 × 103 CFU/mouse, respectively. In some experiments, S-methyl-isothiourea (SMT), a selective inhibitor of NOS, at a dose of 1·0 mg per mouse, dissolved in 0·2 ml of sterile saline, was intraperitoneally administrated at 0 h, 3 h and 6 h after intratracheal challenge [20]. This reagent has been reported to function as a competitive inhibitor of NOS at the l-arginine binding site [21]. Quantitative bacterial cultures of venous blood and lung tissue from mice which had been euthanized with pentobarbital were performed at the indicated times. The lungs were removed aseptically and homogenized in 9 ml of sterile saline per gram of lung tissue prior to culturing. Bronchoalveolar lavage (BAL) was performed for the euthanized mice after intraracheal challenge of P. aeruginosa at the indicated times as described previously [19]. Cell morphology was determined on cell monolayers prepared by Cytospin 2 (Shandon Southern Products, Astomore, UK) and stained with a modified Wright stain (Diff-Quik, Kokusaishiyaku, Japan). The recovered supernatant from the BAL fluid was sterilized by filtration and stored at − 80°C until used. The experimental protocol was approved by the Ethics Review Committee for Animal Experimentation of Nagasaki University School of Medicine.
Elisa
The concentrations of murine MIP-2 or TNF-α in supernatants of BAL fluids were determined by a sandwich ELISA, as described previously [22,23]. We also determined the levels of murine KC in supernatants of BAL fluids using a commercially available kit (R&D system, Minneapolis, MN, USA).
RNA preparation and RT-PCR
Cellular RNA was extracted from whole lungs and RT-PCR was performed as described previously [24]. The iNOS primer pairs amplified the 500 bp PCR product and was composed of the following sequences; 5′-GTCGACCTTCCGAAGTTTCTTGTGGCAGCAGCG-3′ and 5′-GTCGAC GACGAGCCTCGTGGCTTTGGGCTCCTC-3′ [25]. The control G3PDH primer pairs amplified a 805 bp of PCR product and were composed of the following sequences: 5′-AAACCCATCAC CATCTTCCA-3′ and 5′-CAGGGGTTTCTTACTCCTTG-3′. The PCR products were analysed on ethidium bromide-stained 1% agarose gels.
Immunohistochemical staining for nitrotyrosine
Paraffin-embedded specimens of whole lungs were cut into 3-μm sections, placed on silane-coated slides, dewaxed with xylene and dehydrated through graded concentrations of ethanol. The tissue was then incubated with 3% hydrogen peroxide in absolute methanol for 20 minutes to reduce endogenous peroxidase activity. Specific immunohistochemical studies were performed using a mouse monoclonal antinitrotyrosine IgG antibody (20 μg/ml; Cayman Chemical, Ann Arbor, MI, USA) or a mouse control IgG monoclonal antibody as primary antibodies for 2 h at ambient temperature. The tissue sections were then labelled using a Universal DAKO LSBA 2 kit containing an appropriate, biotinylated secondary antibody and streptoavidin-conjugated peroxidase (DAKO, Capinteria, CA, USA). For immunohistochemical visualization, 3,3′-diaminobenzidine (Sigma Chemical Co, St Louis, MO, USA) was used as a substrate, and the cell preparations and sections were counterstained with Harris's haematoxylin.
Statistical analysis
The significances in the survival of untreated or DEX-treated mice after treatment with SMT or saline were analysed by Kaplan–Mayer methods. The comparison of bacterial densities in blood and lung tissue, the cell number and the levels of MIP-2, KC and TNF-α in BAL fluids between untreated and DEX-treated mice were analysed by unpaired Student's t-test. Data were considered statistically significant if P-values were less than 0·05.
Results
Bacterial densities in pneumonia model
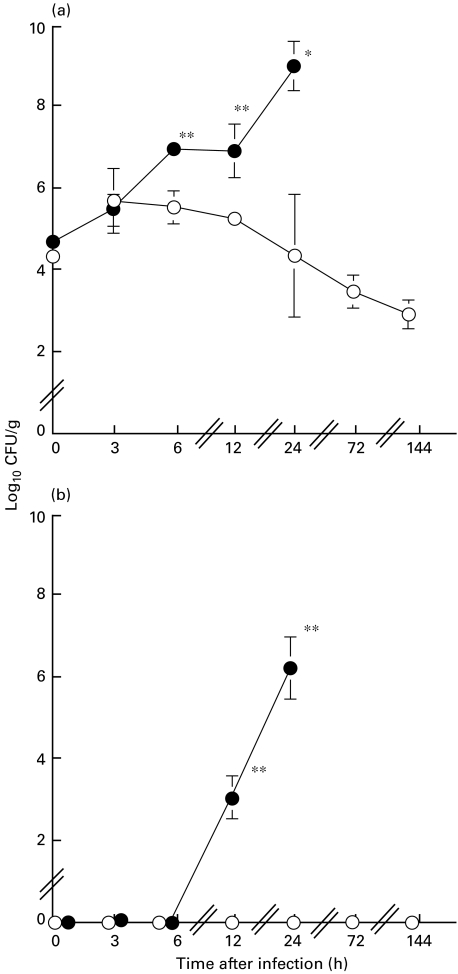
The mean bacterial number in lung tissue from untreated mice was found to be 5 × 105 CFU/g at 6 h postchallenge (Fig. 1a). The bacterial densities in the lung tissues from untreated mice decreased slowly, reaching a level of 4 × 103 CFU/g at 72 h postchallenge. In contrast, the bacterial densities in lung tissue of DEX-treated mice reached approximately 2 × 107 CFU/g of lung tissue by 6 h and 1 × 109 CFU/g by a 24-h postchallenge. No bacteraemia was detected in the untreated mice (Fig. 1b), while bacteraemia developed with a bacterial number of 1 × 103 and 2 × 106 CFU/ml in plasma of DEX-treated mice at 12 h and 24 h postchallenge, respectively.
Fig. 1.
Comparisons of bacterial numbers in lung tissues (a) and blood (b) for untreated mice (open circles) and dexamethasone-treated mice (closed circles) after an intratracheal challenge of P. aeruginosa It-1 strain at a dose of 2·4 × 105 CFU/mouse. Data represent the mean ± s.d. of five animals. *P < 0·05, **P < 0·01 (versus untreated mice).
Kinetics of BAL cells in pneumonia model
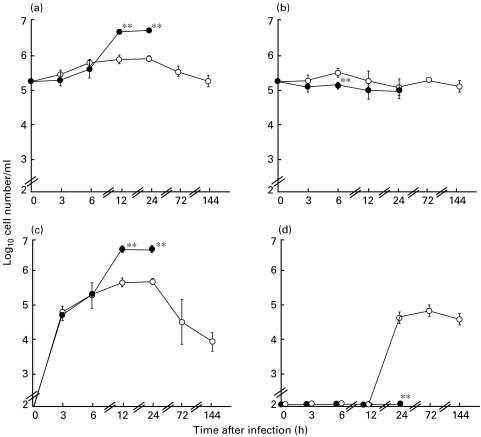
The numbers of alveolar macrophages in BAL from DEX-treated mice showed a slight but significant decrease at 6 h after challenge, compared with those from untreated mice (Fig. 2b). The levels of neutrophil influx in the BAL fluids from untreated mice increased progressively within 24 h, and declined at 72 h and 144 h after bacterial challenge (Fig. 2c). On the other hand, the levels of total cells and neutrophil influx in BAL fluids from DEX-treated mice also increased progressively up to 5 × 106 cells within 12 h, reaching a plateau at 24 h after infection (Figs 2a,c). A progressive increase in total cells and neutrophils appeared to be correlated with an increased density of bacteria in the lungs of DEX-treated mice. The levels of lymphocyte migration in BAL fluids increased rapidly between 12 h and 24 h, reaching a plateau at 24 h after a bacterial challenge in untreated mice (Fig. 2d). No lymphocyte migration was found in BAL fluids from the DEX-treated mice until 24 h.
Fig. 2.
Comparisons of the numbers of total cells (a), alveolar macrophages (b), neutrophils (c) and lymphocytes (d) in bronchoalveolar lavage (BAL) fluids between from untreated (open circles) and dexamethasone-treated mice (closed circles) after an intratracheal challenge of P. aeruginosa It-1 strain at a dose of 2·4 × 105 CFU/mouse. Data represent the mean ± s.d. of five animals. **P < 0·01 (versus untreated mice).
Kinetics of CXC chemokines and TNF-α in BAL fluids
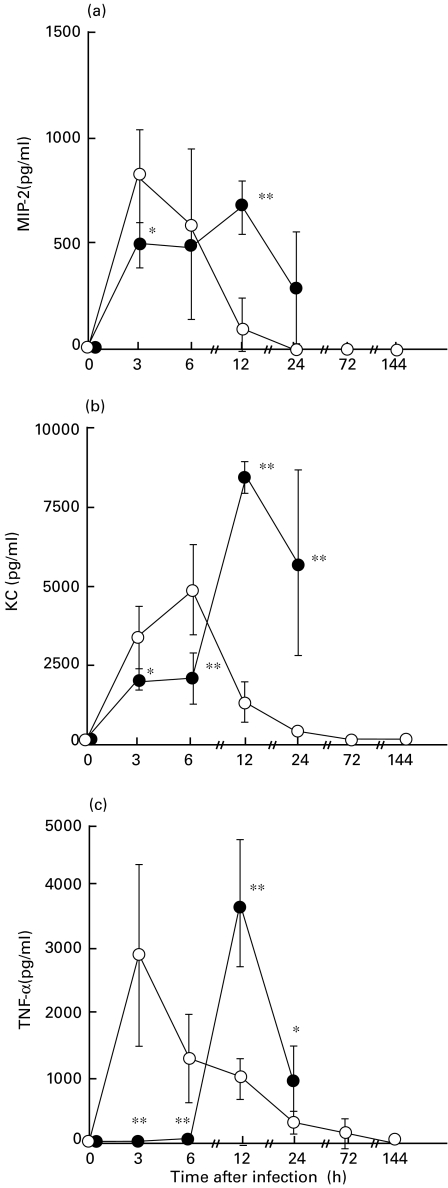
The levels of CXC chemokines in BAL fluids from DEX-treated mice were slightly but significantly lower than those in untreated mice only at 3 h postchallenge for MIP-2 (P < 0·05) and at 3 h (P < 0·05) and 6 h postchallenge (P < 0·01) for KC (Fig. 3a,b). The levels of TNF-α in BAL fluids from DEX-treated mice were completely suppressed, compared with those from untreated mice up to 6 h postchallenge (P < 0·01, Fig. 3c). In contrast, the levels of these CXC chemokines and TNF-α in BAL fluids from DEX-treated mice increased significantly as a function of bacterial densities at 12 h after infection, compared with those from untreated mice (P < 0·01), and attenuated at 24 h after infection. The levels of CXC chemokines and TNF-α in BAL fluids from untreated mice decreased progressively 3 h or 6 h after infection.
Fig. 3.
Comparisons of concentrations of MIP-2 (a), KC (b) and TNF-α (c) in bronchoalveolar lavage (BAL) fluids between from untreated (open circles) and dexamethasone (DEX)-treated (closed circles) mice after an intratracheal challenge of P. aeruginosa It-1 strain at a dose of 2·4 × 105 CFU/mouse. Data represent the mean ± s.d. of five animals. *P < 0·05. **P < 0·01 (versus untreated mice).
INOS mRNA in lung tissue
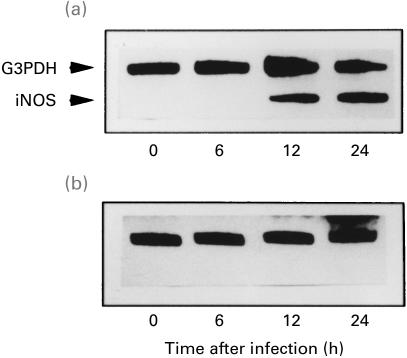
The complete suppression of iNOS mRNA expression in lung tissues from DEX-treated mice was observed (Fig. 4b), while iNOS mRNA expression was clearly detected in the lung tissues from untreated mice at 12 h and 24 h postinfection (Fig. 4a).
Fig. 4.
iNOS mRNA expression in lung tissues of untreated (a) and dexamethasone-treated (b) mice after an intratracheal challenge of P. aeruginosa It-1 strain at a dose of 2·4 × 105 CFU/mouse. RT-PCR was used for G3PDH mRNA in the lung tissue as the control. Representative results of four animals are shown.
Immunohistochemical staining for nitrotyrosine
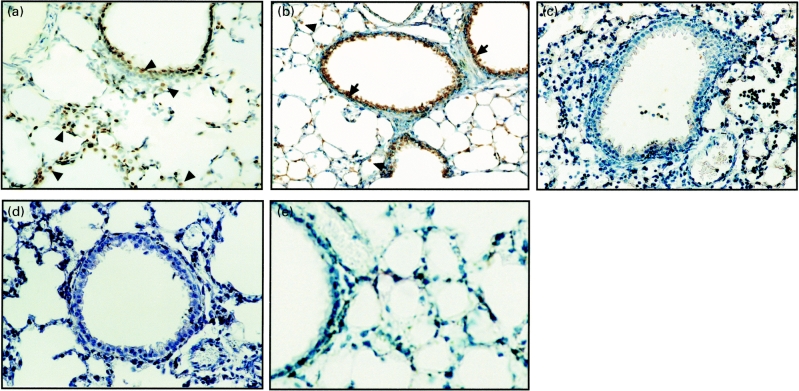
A strong immunoreactivity for nitrotyrosine was detected within alveolar phagocytic cells such as alveolar macrophages and neutrophils (Figs 5a,b) and bronchial epithelium (Fig. 5b) of untreated mice at 24 h postinfection. In contrast, the nitrotyrosine formation was not detected in the lung section of DEX-treated mice (Fig. 5c) nor for untreated mice immediately prior to infection (Fig. 5d). Lung tissue of untreated mice at 24 h postinfection was not stained with a control monoclonal antibody (data not shown).
Fig. 5.
Immunohistochemical localization of nitrotyrosine in lung tissues from untreated (a and b) and dexamethasone (DEX)-treated mice (c) at 24 h postchallenge of P. aeruginosa It-1 strain at a dose of 2·4 × 105 CFU/mouse. Alveolar phagocytic cells (a and b, arrowheads) and bronchial epithelium (b, arrows) were stained with an antinitrotyrosine monoclonal antibody in the lung tissues from untreated mice, while no immunoreactivity with the same monoclonal antibody was detected in the lung tissues from DEX-treated mice (c). The lung tissue of untreated mice immediately before infection (d) was not stained with the same monoclonal antibody (d). The lung tissues of mice that received SMT as described in the Materials and methods at 24 h postinfection were not stained with antinitrotyrosine monoclonal antibody (e). Original magnification; × 200 in a, b and c, × 400 in d and e.
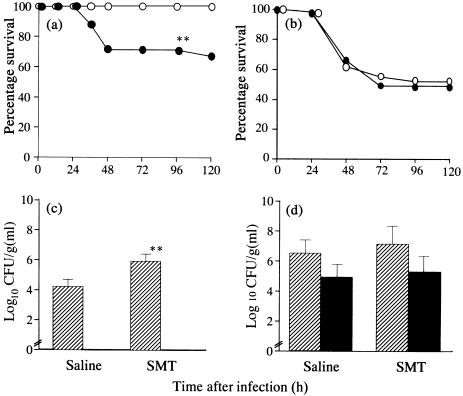
Effects of SMT on the survival and bacterial number
To determine whether iNOS is responsible for pulmonary antibacterial defence in DEX-treated mice, the effects of an intraperitoneal administration of SMT was examined relative to the survival of untreated and DEX-treated mice after an intratracheal challenge of P. aeruginosa. Treatment with SMT decreased the survival of untreated mice significantly (62·5%), compared with untreated mice that had received saline (100%; P < 0·01, Fig. 6a). In contrast, no significant difference was found for the survival of DEX-treated mice between the group receiving SMT (51·2%) and saline (52·3%; Fig. 6b). We examined further the effects of intraperitoneal administration of SMT on bacterial density in the lung tissues and blood of untreated and DEX-treated mice. A significant increase in bacterial density was found in lung tissues from untreated mice at 24 h postinfection by treatment with SMT, compared with those of the group that received saline in untreated mice (P < 0·01, Fig. 6c). No bacteraemia was found in either the group receiving SMT and saline in untreated mice at the same time point. In contrast, no significant difference was evident on the bacterial density in the lung tissues and blood between the group receiving SMT and saline (Fig. 6d).
Fig. 6.
Effects of S-methyl-isothiourea (SMT) treatment on the survival of untreated (a) and dexamethasone (DEX)-treated mice (b). All 24 untreated mice received SMT or saline and all 42 DEX-treated mice received SMT (closed circles) or saline (open circles), and evaluated for survival. Effects of SMT on the bacterial density in lung tissue (hatched bars) and blood (closed bars) of untreated (c) and DEX-treated mice (d) at 24 h postchallenge of P. aeruginosa It-1 strain. The challenge doses of 2·4 × 105 CFU/mouse for untreated mice and 2·4 × 104 CFU/mouse for DEX-treated mice were employed, respectively. Untreated mice and DEX-treated mice received SMT or saline as described in Materials and methods. Data represent the mean ± s.d. of five animals. **P < 0·01 (versus the group receiving saline, a, c).
Effects of SMT on TNF-α production and the formation of nitrotyrosine
The effects of SMT on the on the levels of TNF-α in BAL fluids as well as the formation of nitrotyrosine in lung tissue were evaluated further in the untreated mice. The administration of SMT had no effect on the levels of TNF-α in BAL fluids of mice (2428 ± 743 pg/ml at 3 h, 1472 ± 415 pg/ml at 6 h in mice receiving SMT) compared with those of mice receiving saline during the early phase of pneumonia, while nitrotyrosine formation was markedly inhibited in the lung sections of mice receiving SMT (Fig. 5e).
Discussion
In this study, we demonstrated that pretreatment with a high dose of DEX for 5 days led to an alteration in pulmonary defence susceptibility to infection with P. aeruginosa in mice. An increased bacterial density in the lung resulted subsequently in bacteraemia in DEX-treated mice. These data imply that a long-term treatment with a high dose of glucocorticoid may impair pulmonary defence. It is also important to note that a complete suppression of iNOS mRNA expression in the lung was documented in DEX-treated mice with fatal pneumonia (Fig. 4). We also found a complete suppression of TNF-α production in BAL fluids during the early phase of pneumonia (Fig. 3c). These in vivo findings can be explained by the fact that glucocorticoids are known to suppress various gene expressions which are subject to NF-κB-regulation, such as iNOS and TNF-α [15,16]. Furthermore, a recent study has demonstrated that DEX inhibits iNOS gene transcription in rat hepatocytes which had been stimulated with proinflammatory cytokines by increasing I-κB a expression and decreasing NF-κB activity [26].
Previous studies have reported that inhibitors of NO synthesis abolished alveolar macrophage- or neutrophil-mediated bactericidal activity [8,27], although the precise mechanism for how NO mediates the regulation of bactericidal activities remains unknown. One possible reason for the antibacterial effect of NO is the bactericidal action of reactive nitrogen species such as peroxynitrite (ONOO) which is produced as a result of the reaction of NO with 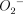 , even though NO itself is not a potent microbicidal molecular species [28–30]. Peroxynitrite, which is generated in lung tissue, can be assessed indirectly via the immunohistochemical detection of nitrotyrosine [31,32]. Umezawa et al. have demonstrated previously that both
, even though NO itself is not a potent microbicidal molecular species [28–30]. Peroxynitrite, which is generated in lung tissue, can be assessed indirectly via the immunohistochemical detection of nitrotyrosine [31,32]. Umezawa et al. have demonstrated previously that both 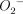 and NO are major effector molecules in the host defence against Salmonella typhimurium infection in mice [25]. The authors concluded that antimicrobial activity found in the infected liver might be mediated through the formation of peroxynitrite rather than
and NO are major effector molecules in the host defence against Salmonella typhimurium infection in mice [25]. The authors concluded that antimicrobial activity found in the infected liver might be mediated through the formation of peroxynitrite rather than 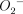 or NO per se. We found an intense immunoreactivity for nitrotyrosine within the bronchial epithelium as well as alveolar phagocytic cells of untreated mice after bacterial infection (Fig. 5a,b). These findings can be explained in part by the induction of iNOS expression in alveolar macrophages, neutrophils and bronchial epithelial cells as the result of bacterial infection [33–35]. We demonstrated further an association between the impairment of bacterial clearance and a lack of nitrotyrosine formation in the lungs of DEX-treated mice after infection, although an increased number of neutrophils was observed in BAL fluids from these mice. Decreased
or NO per se. We found an intense immunoreactivity for nitrotyrosine within the bronchial epithelium as well as alveolar phagocytic cells of untreated mice after bacterial infection (Fig. 5a,b). These findings can be explained in part by the induction of iNOS expression in alveolar macrophages, neutrophils and bronchial epithelial cells as the result of bacterial infection [33–35]. We demonstrated further an association between the impairment of bacterial clearance and a lack of nitrotyrosine formation in the lungs of DEX-treated mice after infection, although an increased number of neutrophils was observed in BAL fluids from these mice. Decreased 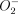 production in neutrophils, as well as a lack of NO production, may also contribute to a decrease in the level of nitrotyrosine formation in the lung tissue of Pseudomonas-infected animals after treatment with glucocorticoids [6]. These lines of evidence suggest strongly a pivotal role for peroxynitrite as a bactericidal molecule in the airways of infected mice. The findings herein also support the view that the administration of SMT, a selective inhibitor of NO synthase, had no effect on the survival of and the numbers of bacteria in the lung and blood of DEX-treated mice, while the administration of SMT suppressed the survival significantly and led to an increase in bacterial density in the lung of untreated mice after bacterial infection.
production in neutrophils, as well as a lack of NO production, may also contribute to a decrease in the level of nitrotyrosine formation in the lung tissue of Pseudomonas-infected animals after treatment with glucocorticoids [6]. These lines of evidence suggest strongly a pivotal role for peroxynitrite as a bactericidal molecule in the airways of infected mice. The findings herein also support the view that the administration of SMT, a selective inhibitor of NO synthase, had no effect on the survival of and the numbers of bacteria in the lung and blood of DEX-treated mice, while the administration of SMT suppressed the survival significantly and led to an increase in bacterial density in the lung of untreated mice after bacterial infection.
Previous studies have reported that the intrapulmonary production of TNF-α has a protective role in Gram-negative bacterial pneumonias using the neutralization of endogenous TNF-α [7,19,36] or a TNF agonist peptide [37]. A recent study has reported a 19-fold and a threefold decrease in the efficiency of P. aeruginosa clearance from lung relative to the wild mice for TNF-α and iNOS-deficient mice, respectively [9]. These published findings suggest that a significant suppression of intrapulmonary TNF-α levels may be another factor in the impairment of antibacterial pulmonary defence in DEX-treated infected mice in this study. We also found obvious differences of the effects between DEX and SMT on the survival and bacterial densities in the lungs of mice (Figs 1,6). These data can be explained by the fact that the administration of DEX completely suppressed peroxynitrite and TNF-α production during the early phase of pneumonia in the lung, while treatment with SMT markedly inhibited peroxynitrite production, but not TNF-α production (Fig. 5e).
MIP-2 has been shown to be important for pulmonary defence in a murine pneumonia model due to K. pneumoniae [10]. In addition, the overexpression of KC in the lung resulted in increased bacterial clearance and survival in a murine model due to the same pathogen [11]. The levels of neutrophil influx were found to be closely correlated to the production of MIP-2 and KC in BAL fluids in both untreated and DEX-treated mice in the present study. We found a slight but significant decrease in levels of MIP-2 and KC in BAL fluids from DEX-treated mice within 6 h after bacterial infection, compared with those from untreated mice, although the levels of these CXC chemokines in BAL fluids from DEX-treated mice increased in parallel with the increased bacterial load at 12 h after infection. These results indicate that the production of these CXC chemokines may not affect the DEX-induced impairment of pulmonary defence. On the other hand, murine MIP-2 appears to be regulated by NF-κB, because inhibitors of NF-κB completely or partially blocked MIP-2 mRNA expression in an Orientia tsutsugamushi-infected murine macrophage cell line [38]. The administration of DEX, however, suppressed MIP-2 production weakly in the airways during the early phase of infection, while a marked inhibition of iNOS gene expression and TNF-α production was observed in our pneumonia model. These data may suggest the involvement of transcription factors other than NF-κB in the induction of the MIP-2 gene.
In summary, a complete suppression of iNOS gene expression associated with the formation of nitrotyrosine and TNF-α production in the lung during the early phase of pneumonia is clearly associated with the progression of fatal pneumonia due to P. aeruginosa in DEX-treated mice. Our data suggest that a lack of generation of peroxynitrite as a bactericidal molecule in the lung as well as the suppression of TNF-α production in the early phase of pneumonia are responsible for the impairment of pulmonary antibacterial defence in immunocompromised hosts who are receiving high-doses of glucocorticoid.
Acknowledgments
This study was carried out at the Animal Research Center for Infectious Tropical Diseases, Institute of Tropical Medicine, Nagasaki University. We are grateful to Miss Y. Terai and Miss M. Yanase for the excellent technical support, and Emeritus Professor K. Matsumoto, Aino Memorial Hospital, for his helpful comments and encouragement throughout the work.
References
- 1.Bryan CS, Reynold KL. Bacteremic nosocomial pneumonia. Am Rev Respir Dis. 1984;129:668–71. doi: 10.1164/arrd.1984.129.5.668. [DOI] [PubMed] [Google Scholar]
- 2.Diasio RB, LoBuglo AF. Immunomodulators: immunosuppressive agents and immunostimulants. In: Hardman JG, Limbird LE, editors. The pharmacological basis of therapeutics. New York: McGraw-Hill; 1996. pp. 1291–308. [Google Scholar]
- 3.Fireman P. Combination of inhaled corticosteroids plus other medications in the management of moderate to severe persistent asthma. Allergy Asthma Proc. 2000;21:315–22. doi: 10.2500/108854100778248232. [DOI] [PubMed] [Google Scholar]
- 4.Flaherty KR, Toews GB, Lynch JP, III, et al. Steroids in idopathic pulmonary ficrosis. a prospective assessment of adverse reactions, response to therapy, and survival. Am J Med. 2001;110:326–8. doi: 10.1016/s0002-9343(00)00711-7. [DOI] [PubMed] [Google Scholar]
- 5.Pallillo JE, Fauci AS. Mechanism of glucocorticoid action on immune process. Ann Rev Pharmacol Toxicol. 1979;19:179–201. doi: 10.1146/annurev.pa.19.040179.001143. [DOI] [PubMed] [Google Scholar]
- 6.Fukushima K, Ando M, Suga M, Araki S. Impaired function of polymorphonuclear leukocytes exuded into bronchoalveolar spaces infected with Pseudomonas aeruginosa in steroid-treated rabbits. Exp Lung Res. 1987;13:141–55. doi: 10.3109/01902148709064315. [DOI] [PubMed] [Google Scholar]
- 7.Gosselin DD, Esantis J, Boule M, Skamene E, Matouk C, Radzioch D. Role of tumor necrosis factor alpha in innate resistance to mouse pulmonary infection with Pseudomonas aeruginosa. Infect Immun. 1995;63:3272–8. doi: 10.1128/iai.63.9.3272-3278.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tsai WC, Strieter RM, Zisman DA, et al. Nitric oxide is required for protective innate immunity against Klebsiella pneumoniae. Infect Immun. 1997;65:1870–5. doi: 10.1128/iai.65.5.1870-1875.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yu H, Nasr SZ, Deretic V. Innate lung defence and compromised Pseudomonas aeruginosa clearance in the malnourished mouse model of respiratroy infections in cystic fibrosis. Infect Immun. 2000;68:2142–7. doi: 10.1128/iai.68.4.2142-2147.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Greenberger MJ, Strieter RM, Kunkel SL, et al. Neutralization of macrophage inflammatory protein-2 attenuates neutrophil recruitment and bacterial clearance in murine Klebsiella pneumoniae. J Infect Dis. 1996;173:159–65. doi: 10.1093/infdis/173.1.159. [DOI] [PubMed] [Google Scholar]
- 11.Tsai WC, Strieter RM, Wilkowski JM, et al. Lung-specific transgenic expression of KC enhances resistance to Klebsiella pneumoniae in mice. J Immunol. 1998;161:2435–40. [PubMed] [Google Scholar]
- 12.Mukaida N, Morita M, Ishikawa Y, et al. Novel mechanism of glucorticoid-mediated gene repression. Nuclear factor-κB is target for glucocorticoid-mediated interleukin-8 gene repression. J Biol Chem. 1994;269:13289–95. [PubMed] [Google Scholar]
- 13.Matsusaka T, Fujikawa K, Nishio Y, et al. Transcription factors NF-IL 6 and NF-κB synergistically activate transcription of the inflammatory cytokines, interleukin 6 and interleukin 8. Proc Natl Acad Sci USA. 1993;90:10193–7. doi: 10.1073/pnas.90.21.10193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mukaida N, Okamoto S, Ishikawa Y, Matsushima K. Molecular mechanism of interleukin-8 gene expression. J Leukoc Biol. 1994;56:554–8. [PubMed] [Google Scholar]
- 15.Blackwell TS, Christman JW. The role of nuclear factor-κB in cytokine gene regulation. Am J Respir Cell Mol Biol. 1997;17:3–9. doi: 10.1165/ajrcmb.17.1.f132. [DOI] [PubMed] [Google Scholar]
- 16.Taylor BS, de Vera ME, Ganster RW, et al. Multiple NF-kappa B enhancer elements regulate cytokine induction of human inducible nitric oxide synthase gene. J Biol Chem. 1998;273:15148–56. doi: 10.1074/jbc.273.24.15148. [DOI] [PubMed] [Google Scholar]
- 17.Trede NS, Tsytsykova AV, Chatila T, Goldfeld AE, Geha RS. Transcriptional activation of the human TNF-α promoter by superantigen in human monocytic cells: role of NF-κB. J Immunol. 1995;155:902–8. [PubMed] [Google Scholar]
- 18.Pennington JE, Ehrie MG. Pathogenesis of Pseudomonas aeruginosa pneumonia during immunosuppression. J Infect Dis. 1978;137:764–74. doi: 10.1093/infdis/137.6.764. [DOI] [PubMed] [Google Scholar]
- 19.Sonoda F, Oishi K, Iwagaki A, Matsumoto K. Endogenous tumor necrosis factor (TNF) α mediates neutrophil accumulation at the mid-phase of a murine model of Pseudomonas aeruginosa pneumonia. Microbiol Immunol. 1997;41:601–8. doi: 10.1111/j.1348-0421.1997.tb01898.x. [DOI] [PubMed] [Google Scholar]
- 20.Rosselet A, Feihl F, Markert M, Gnaegi A, Perret C, Liaudet L. Selective iNOS inhibition is superior to norepinephrine in the treatment of rat endotoxin shock. Am J Respir Crit Care Med. 1998;157:162–70. doi: 10.1164/ajrccm.157.1.9701017. [DOI] [PubMed] [Google Scholar]
- 21.Southan GJ, Szabo C, Thiemermann C. Isothioureas: potent inhibitors of nitric oxide synthases with variable isoform selectively. Br J Pharmacol. 1995;114:510–6. doi: 10.1111/j.1476-5381.1995.tb13256.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sonoda Y, Mukaida N, Wang JB, et al. Physiologic regulation of postovulatory neutrophil migration into vagina in mice by a C-X-C chemokine(s) J Immunol. 1998;160:6159–65. [PubMed] [Google Scholar]
- 23.Fujioka N, Mukaida N, Harada A, et al. Preparation of specific antibodies against murine IL-1 ra and the establishment of IL-1 ra as an endogenous regulator of bacterial-induced fulminant hepatitis in mice. J Leukoc Biol. 1995;58:90–8. doi: 10.1002/jlb.58.1.90. [DOI] [PubMed] [Google Scholar]
- 24.Amano H, Oishi K, Sonoda F, et al. Role of cytokine-induced neutrophil chemoattarctant-2 (CINC-2) α in a rat model of chronic bronchopulmonary infections with Pseudomonas aeruginosa. Cytokine. 2000;12:1662–8. doi: 10.1006/cyto.2000.0771. 10.1006/cyto.2000.0771. [DOI] [PubMed] [Google Scholar]
- 25.Umezawa K, Akaike T, Fujii S, et al. Induction of nirtic oxide synthesis and xanthine oxidase and their roles in the antimicrobial mechanism against Salmonella typhimurium infection in mice. Infect Immun. 1997;65:2931–40. doi: 10.1128/iai.65.7.2932-2940.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.De Vera ME, Taylor BS, Wang Q, Shapiro RA, Billiar TR, Geller DA. Dexamethasone suppresses iNOS gene expression by up-regulating I-κBa and inhibiting NF-κB. Am J Physiol. 1997;273:G1290–6. doi: 10.1152/ajpgi.1997.273.6.G1290. [DOI] [PubMed] [Google Scholar]
- 27.Fierro IM, Nascimento-DeSilva V, Arruda MA, et al. Induction of NOS in rat PMN in vivo and in vitro: modulation by tyrosine kinase and involvement in bactericidal activity. J Leukoc Biol. 1999;65:508–14. doi: 10.1002/jlb.65.4.508. [DOI] [PubMed] [Google Scholar]
- 28.Bruneli L, Crow JP, Beckman JS. The comparative toxicity of nitric oxide and peroxynitrite to Escherichia coli. Arch Biochem Biophys. 1995;316:327–34. doi: 10.1006/abbi.1995.1044. 10.1006/abbi.1995.1044. [DOI] [PubMed] [Google Scholar]
- 29.Zhu L, Gunn C, Beckman JS. Bactericidal activity of peroxynitrite. Arch Biochem Biophys. 1992;298:452–7. doi: 10.1016/0003-9861(92)90434-x. [DOI] [PubMed] [Google Scholar]
- 30.Yoshida K, Akaike T, Doi T, et al. Pronounced enhancement of NO-dependent antimicrobial action by an NO-oxydizing agent, imidazolineoxyl N-oxide. Infect Immun. 1993;61:3552–5. doi: 10.1128/iai.61.8.3552-3555.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Akaike T, Noguchi Y, Ijiri S, et al. Pathogenesis of influenza-induced pneumonia: involvement of both nitric oxide and oxygen radicals. Proc Natl Acad Sci USA. 1996;93:2448–53. doi: 10.1073/pnas.93.6.2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kooy NW, Royall JA, Ye YE, Kelly DR, Beckman JS. Evidence for in vivo peroxynitrite production in human acute lung injury. Am J Respir Crit Care Med. 1995;151:1250–4. doi: 10.1164/ajrccm/151.4.1250. [DOI] [PubMed] [Google Scholar]
- 33.Yoo HS, Rutherford MS, Maheswaran SK, Srinand S, Ames TR. Induction of nitric oxide by bovine alveolar macrophages in response to Pasteurella haemollytica A1. Microb Pathog. 1996;20:361–75. doi: 10.1006/mpat.1996.0034. [DOI] [PubMed] [Google Scholar]
- 34.Wheeler MA, Smith SD, Garcia-Cardena G, Nathan CF, Weiss RM, Sessa WC. Bacterial infection induces inducible nitric oxide synthase in human neutrophils. J Clin Invest. 1997;99:110–6. doi: 10.1172/JCI119121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Meng QH, Polak JM, Edgar AJ, et al. Neutrophils enhance expression of inducible nitric oxide synthase in human normal but not cystic fibrosis bronchial epithelial cells. J Pathol. 2000;190:126–32. doi: 10.1002/(SICI)1096-9896(200002)190:2<126::AID-PATH500>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 36.Laichalk LL, Bucknell KA, Huffnagle GB, et al. Intrapulmonary delivery of tumor necrosis factor agonist peptide augments host defence in murine Gram-negative bacterial pneumonia. Infect Immun. 1998;66:2822–6. doi: 10.1128/iai.66.6.2822-2826.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Laichalk LL, Kunkel SL, Strieter RM, Danforth JM, Bailie MB, Standiford TJ. Tumor necrosis factor mediates lung antibacterial host defence in murine Klebsiella pneumoniae. Infect Immun. 1996;64:5211–8. doi: 10.1128/iai.64.12.5211-5218.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cho N, Seong S, Huh M, et al. Expression of chemokine genes in murine macrophages infected with Orientia tsutsugamushi. Infect Immun. 2000;68:594–602. doi: 10.1128/iai.68.2.594-602.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]