Abstract
Although increased expression of mucosal addressin cell adhesion molecule-1 (MAdCAM-1) has been demonstrated in inflammatory sites of various diseases, its role in colitis remains unknown. In this study, we examined whether MAdCAM-1 is involved in the pathogenesis of granulomatous colitis induced by peptidoglycan-polysaccharide (PG-PS). Experimental colitis was induced by intramural injection of PG-PS to rat colon. After 3 weeks the colon was removed and the mucosal inflammation was assessed. The area of MAdCAM-1-positive venules and the subsets of infiltrating cells were determined in colonic mucosa by immunohistochemistry. In another experiment, monoclonal antibody against MAdCAM-1 was administered intraperitoneally to examine its attenuating effect on colitis. The intramural injection of PG-PS induced significant colonic inflammation with granuloma formation. The submucosa was drastically thickened with the infiltration of CD4 positive lymphocytes and ED-1 positive macrophages. Intense MAdCAM-1 expression was observed on endothelium of the submucosal venules in inflamed mucosa. Administration of anti-MAdCAM-1 antibody significantly attenuated the PG-PS-induced colonic damage and cell infiltration. Enhanced expression of MAdCAM-1 was demonstrated in venular endothelium of the inflamed colon in PG-PS-induced colitis. The attenuating effect of anti-MAdCAM-1 suggests the importance of the MAdCAM-1-dependent process in the formation of chronic granulomatous colitis.
Keywords: adhesion molecules, colitis, lymphocytes, PG-PS
Introduction
Recirculation of lymphocytes from blood to lymphoid tissue is generally accepted as a key phenomenon in immunological surveillance [1]. In general, naive lymphocytes have the capability to migrate from the blood into secondary lymphoid tissues, such as lymph nodes and Peyer's patches, by extravasating through the endothelium of specialized high endothelial venules [2]. Lymphocyte homing to Peyer's patches and mucosal sites is thought to be regulated mainly by α4β7-integrin on lymphocytes and its counter ligand, MAdCAM-1 [3]. MAdCAM-1 is an immunoglobulin superfamily adhesion molecule expressed exclusively on mucosal endothelium and blocking monoclonal antibodies for α4β7-integrin and MAdCAM-1 has been reported to inhibit normal homing of α4β7-positive lymphocytes to the small intestine, Peyer's patches and mesenteric lymph nodes [4].
It is becoming increasingly apparent that inflammation of the intestine and colon is associated with enhanced expression of adhesion molecules in both humans and experimental animals [5]. In human ulcerative colitis and Crohn's disease, the expression of MAdCAM-1 is up-regulated in factor VIII-positive vessels in inflamed colonic mucosa [6]. Souza et al. also demonstrated the increased expression of MAdCAM-1 and OX40 ligand in sites of mucosal inflammation in patients with active ulcerative colitis and Crohn's disease [7]. However, whether enhanced expression of MAdCAM-1 in inflamed intestinal mucosa plays a significant role in the development of inflammatory bowel diseases has not been demonstrated clearly.
PG-PS is a structural component of the cell walls of Gram-positive and Gram-negative bacteria that has well-described proinflammatory properties in vitro and in vivo [8]. After subserosal intestinal injection, PG-PS induces chronic relapsing local and systemic inflammation in susceptible Lewis rats [9]. The characteristic inflammatory hallmarks of this model are the development of transmural, granulomatous enterocolitis, arthritis and anaemia and this model shares several histological features with Crohn's disease. The mechanisms by which PG-PS induces chronic inflammation in experimental animals are not clear, but appear to be immunologically mediated [10]. Increased gene expression of TNF-α, IL-1, IL-6 and IFN-γ [11] and lack of chronic granulomatous disease in nude (athymic) rats [12] indicate the integral involvement of macrophages and T lymphocytes in PG-PS-induced inflammation.
We chose this model of experimental colitis because (1) the inflammation is chronic and granulomatous in nature, as in Crohn's disease, (2) because spontaneous reactivation of inflammation occurs and it activates the mucosal immune system, and (3) because similar bacteria cell wall polymers are found normally in the small intestine and colon. The aims of this study were (1) to examine the changes of expression of adhesion molecules and the inflammatory cell infiltration in the colonic mucosa of PG-PS-induced colitis and (2) to investigate whether treatment with anti-MAdCAM-1 antibody has a prophylactic effect on the development of PG-PS colitis.
Materials and methods
Reagents
The following substances were used in this study: PG-PS derived from Group A streptococci and obtained as a sterile, endotoxin-free solution (5·8 mg rhamnose/ml) (Lee Labs, Grayson, GA, USA). Blocking antibody against rat MAdCAM-1(OST-2) and non-blocking antibody against rat MAdCAM-1 (OST-20) were obtained as described previously [13]. Anti‐rat macrophage antibody (ED-1: mouse monoclonal IgG), anti-CD4 antibody (W3–25: mouse monoclonal IgG) and anti-CD8 antibody (OX-8: mouse monoclonal IgG) were purchased from Serotec Ltd, Kidlington, UK. Anti-ICAM-1 antibody (1A29: mouse monoclonal IgG) and anti-VCAM-1 antibody (MR106: mouse monoclonal IgG) were purchsed from PharMingen, San Diego, CA, USA.
Induction of colitis
Specific pathogen-free female Lewis rats weighing 200 g (Saitama Experimental Animal Supply Co., Saitama, Japan) were used. The care and use of laboratory animals were in accordance with the guidelines of the Animal Committee of National Defense Medical College (Saitama, Japan). A total of 44 rats were randomized into four major groups consisting of a control group (n = 8), a PG-PS-treated group (n = 12), a PG-PS + blocking antibody against MAdCAM-1 (OST-2) group (n = 12) and a PG-PS + non-blocking antibody against rat MAdCAM-1 (OST-20) group (n = 12). The animals were anaesthetized and their descending colons were exposed by laparotomy using the aseptic technique. Colitis was induced via 9–10 subserosal injections (20–25 µl/injection) of PG-PS (10 µg rhamnose/g body weight) into the distal colon (4 cm) using a 30G needle [14]. Control animals were treated identically using sterile saline solution. In the anti-MAdCAM-1 MoAb administration group, OST-2 was injected intraperitoneally every other day i.p with 2 mg/kg each after receiving PG‐PS until the rats were sacrificed. For negative controls, non-blocking antibody of MAdCAM-1 (OST20) was used.
Assessment of histological damage
Previous studies using this model have demonstrated maximal colonic inflammation at 3 weeks after injection of PG-PS [14]. Therefore, at 3 weeks after induction of colitis, the animals were anaesthetized and descending colons were excised and opened longitudinally. The descending colon was cut in half longitudinally and one of each segment was fixed in 10% buffered formalin and stained with H&E staining. The thickness of submucosa was measured throughout the descending colon and averaged in proportion with the length of muscularis mucosa using H&E sections because histological damage differed with the portion of the colon even in one animal.
Immunohistochemistry
Another part of removed colon was fixed in periodate-lysine-paraformaldehyde (PLP) and used for immunohistochemical study by the LSAB method. Colonic tissues were vertically embedded in OCT compound (Sakura Fineteck Inc., Tokyo, Japan) carefully and well-orientated 7 µm cryostat sections were used for immunohistochemistry. Primary antibodies used were antibodies against MAdCAM-1 (OST-2), rat macrophage, CD4, CD8, ICAM-1 and VCAM-1. They were visualized by streptavidin-FITC and examined by fluorescence microscope. The MAdCAM-1 positive vessels in lamina propria were calculated using image analyser and quantified as area of positively stained vessels per mm muscularis mucosa. The infiltrated cells were expressed as the number of CD4, CD8 or ED-1 positive cells per mm muscularis mucosa.
Statistical analysis
Results are expressed as mean ± s.d. Data were statistically analysed by Kruskal–Wallis and Scheffé's F-test (dry weight, thickeness of submucosa, infiltrating cells) or Mann–Whitney test (area of MAdCAM-1 positive vessels). P-values of 0·05 or less were considered to be statistically significant.
Results
Characterization of PG-PS-induced colitis
Subserosal injection of PG-PS into the distal colon produces a transmural, chronic granulomatous colitis manifested by colonic thickening and cell infiltration of the colonic interstitium, which consists mainly of mononuclear cells, 3 weeks after injection. PG-PS treatment induced a significant increase in the dry weight of descending colon (control: 6·47 ± 1·5 mg/mm versus PG-PS treatment: 11·0 ± 2·7 mg/mm) and histologically PG-PS also induced a significant increase in the submucosal thickness of the descending colon. Under higher magnification numerous venules were observed in the thickened submucosa besides the many infiltrated inflammatory cells. On the other hand, there were few lymphocytes in the proper muscularis or in the serosa. In order to elucidate the feature of infiltrating cells, an immunohistochemical study was performed. Figure 1 illustrates immunohistochemical staining of CD4-positive cells and ED-1 positive cells in the colonic mucosa of PG‐PS-treated rat. In colonic mucosa of sham operated rat, only a few CD4-positive cells infiltrated to the submucosa and lamina propria (Fig. 1a), but in PG‐PS-treated rat on day 21, a significant cell infiltration of CD4-positive cells were seen in the submucosa and lamina propria (Fig. 1b). In colonic mucosa of sham operated rats only a few ED-1positive cells infiltrated to the submucosa and lamina propria (Fig. 1c). On the other hand, in PG-PS-treated rat on day 21, many ED-1 positive cells infiltrated in the submucosa and lamina propria (Fig. 1d). CD8-positive cells were hardly seen in sham-operated rat and in PG‐PS-treated inflamed colonic mucosa they were observed only in parafollicular area of lymphoid follicles (data not shown).
Fig. 1.
Immunohistochemical staining of CD4-positive cells and ED-1 positive cells in the colonic mucosa. (a) CD4-positive cells of sham operated rat, showing only a few cells infiltrating the submucosa and lamina propria (× 100). (b) CD4-positive cells in PG‐PS-treated rat on day 21, showing a significant cell infiltration in the submucosa and lamina propria (× 100). (c) ED-1-positive cells of sham operated rat, showing only a few cells infiltrating the submucosa and lamina propria (× 100). (d) ED-1-positive cells in PG‐PS-treated rat on day 21, showing a significant cell infiltration in the submucosa and lamina propria (× 100).
Next we examined the expression of adhesion molecules on venular endothelium by immunohistochemistry in order to assess which adhesion molecules are involved in inflammatory cell migration into inflamed colonic mucosa. Figure 2 shows the immunohistochemical staining of MAdCAM-1 and its quantitative analysis on positive vessels. In colonic mucosa of control rats, constitutive expression of MAdCAM-1 was observed on the small vessels at the bases of crypts in the lamina propria of colonic mucosa. However, this expression was weak and sporadic (Fig. 2a). In inflamed colonic mucosa induced by PG-PS, a significant increase in MAdCAM-1 expression was demonstrated at various sites of vessels; namely, in PG-PS-treated rats MAdCAM-1 was observed at the vessels in the lamina propria and in the submucosal layer, and at the vessels just below the serosa (Fig. 2b). Most of the numerous vessels in the submucosa in PG-PS-treated animals showed more abundant cytoplasm than usually found in endothelial cells. Lymphocytes in the vessel wall were also observed (Fig. 2c). Although inflammation was transmural, there was no apparent expression of MAdCAM-1 near the serosa where PG-PS was originally injected. Figure 2d illustrates the quantitative analysis on MAdCAM-1 positive vessels in distal colon determined as the area of positively stained vessels per mm of muscularis mucosa. Asignificant increase in MAdCAM-1 expression was shown in this figure.
Fig. 2.
Immunohistochemical study of MAdCAM-1 expression and its quantitative analysis on positive vessels in the colonic mucosa. (A) MAdCAM-1 expression of non-treated rat was weak and sporadic on the vessels in the lamina propria (× 100). (B) MAdCAM-1 expression of PG‐PS-treated rat on day 21, demonstrating the increased MAdCAM-1 on the vessels mainly in the submucosal layer (× 100). (C) H&E-staining of distal colon of PG‐PS treated rat. Submucosal vessels (V) in PG-PS-treated animals appear to have more abundant cytoplasm than usually found. Lymphocytes in the vessel wall were also observed (black arrows) (× 400; A: arteriole). (d) Changes in MAdCAM-1 expression in the distal colon of PG‐PS-treated rat. Results are expressed as the mean ± s.d. *P < 0·05 compared with controls.
In colonic mucosa of control rats, expression of either ICAM-1or VCAM-1 was hardly seen (data not shown). Figure 3a shows the immunohistochemical staining of ICAM-1 in the inflamed colonic mucosa induced by PG-PS. Even though strong ICAM-1 expression on infiltrating cells was apparent, it was not apparent on the vessels. Figure 3b shows the immunohistochemical staining of VCAM-1 in the inflamed colonic mucosa induced by PG-PS. Like ICAM-1, strong VCAM-1 expression was observed only on infiltrating cells, but it was not apparent on the vessels.
Fig. 3.
Immunohistochemical staining of ICAM-1 and VCAM-1 in the colonic mucosa of PG‐PS treated rat. (A) ICAM-1 expression was not apparent on the vessels of the submucosa, but positive on infiltrating cells. (x 200). (B) VCAM-1 expression was not apparent on the vessels of the submucosa (x 200).
Effect of anti-MAdCAM-1 mAb on PG-PS-induced colitis
Because involvement of MAdCAM-1 is apparent in development of PG-PS induced colitis, we next examined possible role of MAdCAM-1 in pathogenesis of colitis by blocking MAdCAM-1. The attenuating effect of anti-MAdCAM-1 MoAb which functionally blocks MAdCAM-1 on PG-PS-induced colitis was investigated. Table 1 compares colonic dry weight and thickness of submucosa at 3 weeks after the induction of colitis among each groups. Antibody which blocks MAdCAM-1 (OST-2) significantly attenuated the PG-PS-induced increase in colonic weight and submucosal thickness compared with the control group or treatment group with non-blocking antibody against MAdCAM-1(OST-20).
Table 1.
Effect of anti-MAdCAM-1 administration on PG-PS-induced increase in weight and submucosal thickness of descending colon
| Control (n = 8) | PG-PS alone (n = 12) | PG-PS + OST-2 (n = 12) | PG-PS + OST-20 (n = 12) | |
|---|---|---|---|---|
| Dry weight/length (mg/mm) | 6·47 ±1·5 | 11·0 ± 2·7* | 7·06 ± 1·4†‡ | 9·51 ± 1·9* |
| Thickness of submucosa (μm) | 29·5 ± 0·9 | 97 ± 15* | 57·1 ±27†‡ | 92·8 ± 18* |
Colonic dry weight was quantified at 3 weeks after the induction of colitis. Rats were treated with OST-2 (blocking antibody of MAdCAM-1 2 mg/kg every other day) or OST-20 (non-blocking antibody of MAdCAM-1) for control antibody. Results are expressed as the mean ± s.d.
P < 0.05 compared with controls.
P < 0.05 compared with PG-PS alone.
P < 0.05 compared with PG-PS + OST-20.
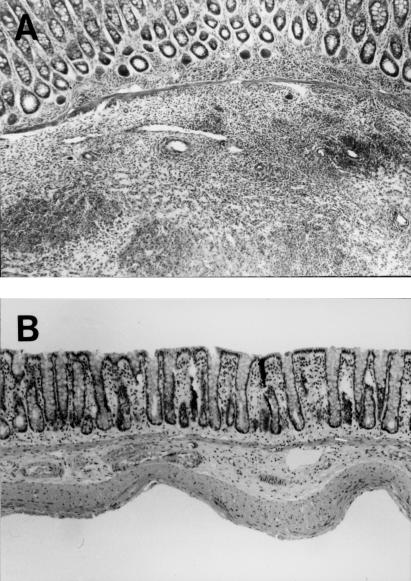
Figure 4a and b compares H&E-stained sections of PG-PS colitis tissues of blocking antibody (OST-2)-treated and non-blocking antibody (OST-20)-treated groups as controls. As shown in this figure, the degree of inflammation and the thickness of submucosa were attenuated significantly by continuous treatment with OST-2, compared with the positive control group (PGPS + OST-20). Interestingly, even mucosal thickening and the number of cell infiltration were decreased in OST 2-treated rats, a significant number of high endothelial-like vessels still remained in the submucosa. We also examined the effect of MoAb treatment on number of cells infiltrating to the colonic mucosa in PG-PS-induced colitis. Figure 5 displays the numbers of CD4-, CD8- and ED-1-positive cells in the colonic mucosa of PG-PS induced colitis on day 21 and the attenuating effects of anti-MAdCAM-1 MoAb on the number of infiltrating cells. Infiltration of CD4-positive cells was inhibited significantly by blocking MoAb (OST-2) treatment compared with the positive control group (OST-20). Anti-MAdCAM-1 MoAb OST-2 slightly inhibited the increased ED-1-positive cell infiltration induced by PG-PS, although it was not significant.
Fig. 4.
(A) H&E staining of distal colon 21 days after continuous treatment with OST‐20 (non-blocking antibody of MAdCAM-1), demonstrating marked cell infiltration with submucosal thickening (× 100). (B) H&E staining of distal colon of PG-PS-treated mice 21 days after continuous treatment with OST‐2 (blocking antibody of MAdCAM-1), demonstrating the decreased infiltrating cells and attenuation of submucosal thickening (× 100).
Fig. 5.
The number of infiltrating cells and their subpopulations in the distal colonic mucosa of control rats (▪), PG-PS-treated rats (□), PG-PS-treated rats with continuous treatment with OST‐2 (blocking antibody of MAdCAM-1) (2 mg/kg every other day) (dotted bar) and PG-PS-treated rats with continuous treatment with OST‐20 (non-blocking antibody of MAdCAM-1) (hatched bar) on day 21. Results are expressed as the mean ± s.d. *P < 0·05 compared with the control. †P < 0·05 compared with PG-PS alone. ‡P < 0·05 compared PG-PS + OST-20.
Discussion
In this study we induced transmural, chronic granulomatous colitis in distal colon of Lewis rats 3 weeks after PG-PS injection. Strong MAdCAM-1 expression was observed on the endothelium of the increased venules in the inflamed submucosa accompanying with significant cell infiltration. In this model of colitis, we also demonstrated that treatment with anti-MAdCAM-1 MoAb ameliorated the colitis, based on histological assessment of mucosal inflammatory changes. Taken together, our results suggest the possibility that MAdCAM-1 is an important ligand recruiting lymphocytes toward the inflamed colonic tissues in rats. The increased expression of MAdCAM-1 has been reported in humans and experimental colitis [6,15,16]. The prophylactic effects of anti-MAdCAM-1 antibody on experimental colitis have also been reported in several animal models [16,17]. Our present study showed that MAdCAM-1-dependent lymphocyte migration to the inflamed mucosa also plays a key role in chronic granulomatous colitis in the nature of Crohn's disease.
It is known that PG-PS injection induces a biphasic inflammatory response. In the acute phase, many inflammatory cells, consisting mainly of macrophages, can migrate in response to a sterile bacterial cell wall at the serosal site [14]. Elevated TNF-α and IL-1 levels in the acute phase are thought to be produced chiefly by activated macrophages [18]. One may speculate that subsequent interaction between macrophages and lymphocytes further augments the chronic inflammation of colonic mucosa with crossregulation of Th1/Th2 responses [19]. It was previously reported that expression of MAdCAM-1 is increased by administration of TNF-α in the murine colon in vivo [15], and by TNF-α or IL-1 in endothelial cells in vitro [20]. Based on these observations, we consider that marked expression of MAdCAM-1 in submucosa and mucosa in PG-PS-induced colitis is induced by enhanced TNF-α and IL-1 production from macrophages which was activated initially with PG-PS.
It is interesting that PG-PS-treated rats that received anti-MAdCAM-1 antibody still showed a number of vessels with abundunt cytoplasm, even when inflammation was ameliorated. This finding strongly suggest that anti-MAdCAM-1 MoAb treatment may not have affected the initial activation of macrophages induced by PG-PS or the subsequent cytokine-induced MAdCAM-1 expression itself, but rather suppressed the consequent spontaneous reactivation phase of granulomatous colitis involving T cell recruitment. Macrophages could migrate into inflamed colon via MAdCAM-1 positive vessels. Yang et al. reported that VLA-4 bearing monocytes and VLA4 + LPAM-1 + macrophages can bind to MAdCAM-1 and have the potential to migrate into chronically inflamed tissues [21]. However, in this study the increased number of ED-1 positive macrophages in PG-PS colitis was not significantly attenuated by anti-MAdCAM-1 treatment, suggesting that MAdCAM-1-independent mechanisms are important for the macrophage infiltration.
ICAM-1 and VCAM-1 are other adhesion molecules which may play a role in sites of inflammation in colon [22,23]. However, in our model significant expression of neither ICAM-1 nor VCAM-1 was recognized in the vascular endothelium of colonic mucosa of control rats or inflamed colonic mucosa of PG-PS colitis. These results suggest that MAdCAM-1 plays a more important role in the induction of PG-PS-induced colitis than ICAM-1 and VCAM-1. Recently Soriano et al. reported that VCAM-1, but not ICAM-1 or MAdCAM-1 immunoblockade, ameliorated DSS-induced murine colitis [24]. The lack of therapeutic effect of anti-MAdCAM-1 MoAb in their study is surprising in light of our present and previous studies [17], in which we found a prophylactic effect of MAdCAM-1 antibody in DSS-induced murine colitis. In the study by Soriano et al. colitis was relatively acutely (for 4 days) induced and the blocking effect was assessed at day 10, while in our previous report, it was induced more slowly (for 21 days) and the blocking effect was observed in animals that received antibody in the late-phase (days 7–13) but not the early-phase group (days 0–6). Considering these results together, we speculate that the relative role of the different endothelial cell adhesion molecules in experimental colitis may depend on the type of chronic inflammation, and that MAdCAM-1 plays more important roles at a late time point or in the chronic phase.
Acknowledgments
This study was supported in part by Grants from the National Defense Medical College of Japan.
References
- 1.Butcher EC, Picker LJ. Lymphocyte homing and homeostasis. Science. 1996;272:60–6. doi: 10.1126/science.272.5258.60. [DOI] [PubMed] [Google Scholar]
- 2.Girard JP, Springer TA. High endothelial venules (HEVs): specialized endothelium for lymphocyte migration. Immunol Today. 1995;16:449–57. doi: 10.1016/0167-5699(95)80023-9. [DOI] [PubMed] [Google Scholar]
- 3.Berlin C, Berg EL, Briskin MJ, et al. α4β7 integrin mediates lymphocyte binding to the mucosal vascular addressin MAdCAM-1. Cell. 1993;74:185. doi: 10.1016/0092-8674(93)90305-a. [DOI] [PubMed] [Google Scholar]
- 4.Hamann A, Andrew DP, Jablonski-Westrich D, Holzmann B, Butcher EC. Role of α4-integrins in lymphocyte homing to mucosal tissues in vivo. J Immunol. 1994;152:3282–93. [PubMed] [Google Scholar]
- 5.Miura S, Tsuzuki Y, Kurose I, et al. Endotoxin stimulates lymphocyte–endothelial interactions in rat intestinal Peyer's patches and villus mucosa. Am J Physiol. 1996;271:G282–92. doi: 10.1152/ajpgi.1996.271.2.G282. [DOI] [PubMed] [Google Scholar]
- 6.Briskin M, Winsor-Hines D, Shyjan A, et al. Human mucosal addressin cell adhesion molecule-1 is preferentially expressed in intestinal tract and associated lymphoid tissue. Am J Pathol. 1997;151:97–110. [PMC free article] [PubMed] [Google Scholar]
- 7.Souza HS, Elia CC, Spencer J, MacDonald TT. Expression of lymphocyte–endothelial receptor–ligand pairs, alpha4beta7/MAdCAM-1 and OX40/OX40 ligand in the colon and jejunum of patients with inflammatory bowel disease. Gut. 1999;45:856–63. doi: 10.1136/gut.45.6.856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schwab JH. Phlogistic properties of peptidoglycan-polysaccharide polymers from cell walls of pathogenic and normal-flora bacteria which colonize humans. Infect Immun. 1993;61:4535–9. doi: 10.1128/iai.61.11.4535-4539.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sartor RB, DeLa Cadena RA, Green KD, et al. Selective kallikrein–kinin system activation in inbred rats differentially susceptible to granulomatous enterocolitis. Gastroenterology. 1996;110:1467–81. doi: 10.1053/gast.1996.v110.pm8613052. [DOI] [PubMed] [Google Scholar]
- 10.Schwab JH, Toffaletti DL, Brown RR. Effects of streptococcal components on immunity. In: Kline TW, Sventivanyl A, editors. Immunomodulation by bacteria and their products. New York: Plenum; 1981. pp. 49–57. [Google Scholar]
- 11.Herfarth HH, Mohanty SP, Rath HC, Tonkonogy S, Sartor RB. Interleukin 10 suppresses experimental chronic, granulomatous inflammation induced by bacterial cell wall polymers. Gut. 1996;39:836–45. doi: 10.1136/gut.39.6.836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Allen JB, Malone DG, Wahl SM, Calandra GB, Wilder RL. Role of the thymus in streptococcal cell wall-induced arthritis and hepatic granuloma formation. Comparative studies of pathology and cell wall distribution in athymic and euthymic rats. J Clin Invest. 1985;76:1042–56. doi: 10.1172/JCI112057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Iizuka T, Tanaka T, Suematsu M, et al. Stage-specific expression of mucosal addressin cell adhesion molecule-1 during embryogenesis in rats. J Immunol. 2000;164:2463–71. doi: 10.4049/jimmunol.164.5.2463. [DOI] [PubMed] [Google Scholar]
- 14.Yamada T, Sartor RB, Marshall S, Specian RD, Grisham MB. Mucosal injury and inflammation in a model of chronic granulomatous colitis in rats. Gastroenterology. 1993;104:759–71. doi: 10.1016/0016-5085(93)91011-6. [DOI] [PubMed] [Google Scholar]
- 15.Connor EM, Eppihimer MJ, Morise Z, Granger DN, Grisham MB. Expression of mucosal addressin cell adhesion molecule-1 (MAdCAM-1) in acute and chronic inflammation. J Leukoc Biol. 1999;65:349–55. doi: 10.1002/jlb.65.3.349. [DOI] [PubMed] [Google Scholar]
- 16.Picarella D, Hurlbut P, Rottman J, Shi X, Butcher EC, Ringler DJ. Monoclonal antibodies specific for β7 integrin and mucosal addressin cell adhesion molecule-1 (MAdCAM-1) reduce inflammation in the colon of scid mice reconstituted with CD45RBhigh CD4+ T cells. J Immunol. 1997;158:2099–106. [PubMed] [Google Scholar]
- 17.Kato S, Hokari R, Matsuzaki K, et al. Amelioration of murine experimental colitis by inhibition of mucosal addressin cell adhesion molecule-1. J Pharmacol Exp Ther. 2000;295:183–89. [PubMed] [Google Scholar]
- 18.Sartor RB. Cytokines in intestinal inflammation: pathophysiological and clinical considerations. Gastroenterology. 1994;106:533–9. doi: 10.1016/0016-5085(94)90614-9. [DOI] [PubMed] [Google Scholar]
- 19.Mosmann TR, Moore KW. The role of IL-10 in crossregulation of TH1 and TH2 responses. Immunol Today. 1991;12:A49–53. doi: 10.1016/S0167-5699(05)80015-5. [DOI] [PubMed] [Google Scholar]
- 20.Sikorski EE, Hallmann R, Berg EL, Butcher EC. The Peyer's patch high endothelial receptor for lymphocytes, the mucosal vascular addressin, is induced on a murine endothelial cell line by tumor necrosis factor-alpha and IL-1. J Immunol. 1993;151:5239–50. [PubMed] [Google Scholar]
- 21.Yang Y, Harrison JE, Print CG, et al. Interaction of monocytoid cells with the mucosal addressin MAdCAM-1 via the integrins VLA-4 and LPAM-1. Immunol Cell Biol. 1996;74:383–93. doi: 10.1038/icb.1996.67. [DOI] [PubMed] [Google Scholar]
- 22.Sans M, Panes J, Ardite E, et al. VCAM-1 and ICAM-1 mediate leukocyte-endothelial cell adhesion in rat experimental colitis. Gastroenterolog. y. 1999;116:874–83. doi: 10.1016/s0016-5085(99)70070-3. [DOI] [PubMed] [Google Scholar]
- 23.Hesterberg PE, Winsor-Hines D, Briskin MJ, et al. Rapid resolution of chronic colitis in the cotton-top tamarin with an antibody to a gut-homing integrin alpha 4 beta 7. Gastroenterology. 1996;111:1373–80. doi: 10.1053/gast.1996.v111.pm8898653. [DOI] [PubMed] [Google Scholar]
- 24.Soriano A, Salas A, Salas A, et al. VCAM-1, but not ICAM-1 or MAdCAM-1, immunoblockade ameliorates DSS-induced colitis in mice. Lab Invest. 2000;80:1541–51. doi: 10.1038/labinvest.3780164. [DOI] [PubMed] [Google Scholar]