Abstract
Hyperfunction of Th2 cells and aberrant glycosylation of IgA have been proposed independently as factors in the pathogenesis of IgA nephropathy (IgAN), the most common form of glomerulonephritis. To investigate the relationship between Th2 cytokines and IgA glycosylation in the genesis of IgAN, we induced IgAN in C3HeB and BALB/c mice by oral immunization and intranasal challenge with Sendai virus. Although both strains of mice developed microhaematuria and glomerular IgA immune deposits to similar degrees, only BALB/c mice developed significant renal insufficiency. More profound reductions of terminal galactosylation and sialylation occurred in Sendai virus-specific IgA from BALB/c versus C3HeB mice, and splenocytes from immunized BALB/c mice produced more Th2 and less Th1 cytokines compared to C3HeB mice when stimulated with antigen in vitro. Furthermore, the decreased glycosylation of IgA elicited by Th2 cytokines in vitro was blunted by the addition of IFN-γ. We conclude that increased production of Th2 cytokines can lead to abnormalities in IgA glycosylation, which in turn promote heightened phlogistic responses to IgA immune complexes lodging in the glomerulus. We suggest that a relative or absolute increase in Th2 cytokine production in response to mucosal infection is a significant pathogenic factor in human IgAN.
Keywords: infectious immunity-virus, in vivo animal models, mucosa
Introduction
Worldwide, IgA nephropathy (IgAN) is the most common form of glomerulonephritis [1–3]. Up to 60% of patients with IgAN develop episodes of haematuria and variable proteinuria in close temporal association with acute upper respiratory or gastrointestinal syndromes [1–3]. Circulating immune complexes (IC) containing IgA1, C3 and often IgG and/or IgM are detected frequently, and deposits of these proteins in the glomerular mesangium are implicated in pathogenesis. However, the antigen(s) in the circulating IC and mesangial deposits is/are unknown. Many clinical and experimental studies of diseases such as AIDS, hepatobiliary disease and mucosal neoplasia indicate that IgA-IC are not equally prone to deposit in glomeruli, or to alter glomerular function if they do deposit [1,2,4,5]. Presumably, the increased circulating IgA and IgA-IC in IgAN patients differ qualitatively from those in other diseases with similar increases.
Serum IgA1 from IgAN patients bears altered glycan side chains, with especially reduced terminal galactose in O-linked oligosaccharides [4–10]. Such alterations can profoundly affect the nephritogenicity of IgA-IC and/or their clearance from the circulation [2,4–11,]. After parenteral injection into rodents, macromolecular aggregates of IgA isolated from patients' serum, or immune complexes prepared with human or mouse IgA bearing enzymatically truncated oligosaccharides, deposit in glomeruli more rapidly than IC containing native, normally glycosylated IgA. When incorporated into IC, aberrantly glycosylated IgA is more efficient at complement activation than intact IgA. Finally, hypogalactosylated human IgA1 is bound by IgG antiglycosyl antibodies, promoting immune aggregation [5,12].
Abnormalities of the cellular immune response might also support pathogenesis (reviewed in [1,2]). Normal human B cells consistently hyperproduce IgA when co‐cultured with histocompatible T cells from IgAN patients, whereas B cells from IgAN patients co-cultured with histocompatible normal T cells produce normal amounts of IgA. Furthermore, blood mononuclear cells from patients and their relatives produce more IL-4 upon mitogen stimulation and express higher levels of mRNA encoding IL-4 and IL-5 compared to controls [13,14]. We demonstrated that stimulation of a murine B cell line in vitro with the Th2 cytokines IL-4 and IL-5 selectively alters the terminal glycosylation of the IgA synthesized, in addition to the known effects of these cytokines in promoting IgA secretion [15]. Aberrant cytokine responses may thus have significance for the pathogenesis of IgAN.
The present study examines the role of Th2-predominant cytokine responses in vivo in abnormal IgA glycosylation and/or glomerular dysfunction in IgAN. To reproduce the essential pathophysiological features of human IgAN in its most prevalent, synpharyngitic form, we modified our earlier murine model of IgAN [16] induced by Sendai virus, a respiratory pathogen [17]. We now demonstrate that increased production of Th2 cytokines can lead to abnormalities in IgA glycosylation that promote heightened glomerular deposition and phlogistic response to IgA-IC.
Materials and methods
Animals
Six-week-old, 20-g female BALB/c and C3HeB/FeJ mice were purchased from The Jackson Laboratory (Bar Harbor, ME, USA), housed in plastic microisolator cages, fed ad libitum with sterile Formulab Chow 5008 (Purina Mills, Richmond, IN, USA) and sterile acidified water, and used as approved by the Institutional Animal Care and Use Committee.
Immunization and challenge
Three groups of each strain of mice (four mice per group in each of two replicate experiments) were immunized intragastrically four times (days 0, 14, 28 and 30) with 0·5 ml of a suspension containing 1010 inactivated Sendai virions and 10 µg cholera toxin (a mucosal adjuvant), as reported previously [16,18–20]. On day 34, two groups from each strain were challenged intranasally with 108 infectious virions: one group was sacrificed 36 days and the other 38 days after the priming immunization (i.e. 2 or 4 days after intranasal challenge). The third immunized group of each strain, not challenged with virus, was sacrificed 36 days after priming. Age-matched non-immunized controls (n = 16 per strain per experiment), half challenged with infectious virus on day 34, were sacrificed 36 or 38 days after the immunized mice were primed. As additional controls, groups of 16 immunized mice of each strain were challenged intranasally 34 days after priming with 108 non-infectious, inactivated Sendai virions, and sacrificed 36 (n = 8) or 38 (n = 8) days postpriming.
Viral titres
Nasal lavage was performed by retrograde perfusion of 0·5 ml PBS into the isolated upper trachea, and collection of effluent from the nares. The virus titre for each mouse was calculated as the geometric mean of the number of plaques formed in a haemagglutination plaque assay [16,18–20], multiplied by the dilution factor.
Serum antibody
IgA and IgG anti-Sendai virus antibody were determined by ELISA, as described previously [16,18–20]. Serum samples (50 µl/well) were serially diluted from 1/40 to 1/320 for IgA titres, and 1/1600–1/12800 for IgG titres in 0·1% BSA/PBS. Bound Ig was detected with alkaline phosphatase-conjugated goat antimouse IgA or -mouse IgG (both from Southern Biotechnology Associates, Birmingham, AL, USA). Antibody levels were normalized to absorbances generated by a standard antiserum sample at the same dilutions.
Assessment of IgA glycosylation
To assess the glycosylation of Sendai virus-specific IgA, samples purified from individual sera by sequential affinity chromatography [15] were incubated in plates coated with 10 µg/ml of inactivated Sendai virus. Measurement of glycosylation of total IgA employed capture by solid-phase antimouse (Fab′)2 antibody [15]. Detection employed biotinylated lectins (Vector Laboratories, Burlingame, CA, USA) and alkaline phosphatase-conjugated streptavidin. Sambucus nigra (SNA) lectin recognizes terminal sialic acid, whereas Ricinus communis agglutinin-I (RCA-I) binds to terminal N-linked galactose [15]. Optical density was normalized to the optical density developed with anti-IgA heavy chain, as detailed previously [15]. The degree of specific binding of each lectin to IgA, per unit protein mass, provides an estimate of terminal sugars on the IgA, in accord with oligosaccharide profile analysis and enzymatic oligosaccharide sequencing [15].
Lymphocyte isolation
Single cell suspensions were prepared by gently teasing spleens, removed aseptically from each mouse, in ice-cold HBSS, and pressing through a wire screen (60 mesh). After lysis of erythrocytes (0·15 m NH4Cl, 0·01 m KHCO3, 0·1 m Na2EDTA, pH 7·2 for 15 min at room temperature), cells were pelleted (500 g for 7 min), resuspended in RPMI 1640 medium and counted in a haemocytometer. After washing, the cells were resuspended at a concentration of 4 × 106 cells/ml in RPMI 1640, supplemented as detailed elsewhere [15]. Replicate cultures, two each with or without 50 µg/ml of inactivated virus [15,18,20], were centrifuged after 48 h. Supernatants were stored at − 70°C for measurement of cytokines.
Cytokine content in supernatants
The concentrations of IL-2, ‐ 4, and ‐ 5 and IFN-γ were determined in duplicate by sandwich ELISA on microwell polystyrene plates (Immunoplate MaxiSorp, Nunc, Roskilde, Denmark). To measure IFN-γ, a commercially available kit was used (Genzyme, Cambridge, MA, USA). Capture and detection antibodies specific for IL-2 (JES6–1A12 at 4 µg/ml and biotinylated polyclonal rabbit antibody at 1·25 µg/ml, both from R&D Systems, Minneapolis, MN, USA), IL-4 (BVD4–1D11 and biotinylated BVD6–24G2, both at 5 µg/ml, from Pharmingen, San Diego, CA, USA) or IL-5 (TRFK-4 at 5 µg/ml and biotinylated TRFK-5 at 4 µg/ml, both purified from supernatants of cells generously provided by Dr R. Coffman, DNAX, Palo Alto, CA, USA) were employed. Supernatants were diluted appropriately (1 : 5) for IL-2 and IFN-γ, or undiluted in the case of IL-4 and IL-5, as determined in preliminary experiments. Unstimulated samples were assayed undiluted for all cytokines. Plates were developed with a 1 : 1000 dilution of streptavidin-alkaline phosphatase (Zymed, San Francisco, CA, USA) in 0·1% BSA/PBS, followed by chromogenic substrate solution. Cytokine concentrations in unknown samples were determined by interpolation of optical density into the appropriate reference standard curve obtained with recombinant mouse IFN-γ, IL-2, IL-4 and IL-5 (all from Genzyme), corrected for any dilution factor, and expressed as ng/ml for IL-2 and IFN-γ, and as pg/ml for IL-4 and IL-5.
Assessment of renal function
Urine was collected from individual mice housed in metabolic cages with free access to water but no food during the 16–24 h prior to sacrifice. The volume was measured by micropipette. Dipsticks (Multistix® 10 SG, Bayer Corporation, Diagnostics Division, Elkhart, IN, USA) and direct microscopic examination of urinary sediments were employed to assess urine pH, specific gravity, proteinuria and haematuria [16,21,22]. Serum creatinine concentration was measured by scaling a commercial assay kit (Sigma) for a 20-µl sample volume [22]. Standard curves with commercial standards of creatinine in microvolume samples indicated excellent linearity (all r2 > 0·97) and interassay reproducibility (± 3%), at a resolution better than 0·15 mg/dL.
Renal histology
Fluorescein-labelled antibodies specific for the heavy chains of mouse IgA, IgG or IgM, or mouse C3 (NL Cappel, Cochraneville, PA, USA; diluted 1 : 40 in PBS) were applied to cryostat sections (2 µm) of mouse kidney for 30 min at room temperature [16,21,22]. Virus antigen deposits were detected in parallel sections by indirect immunofluorescence, using a rabbit anti-Sendai virus antiserum and FITC-goat antirabbit IgG (not cross-reactive with mouse Ig) [16,19,20]. Coded sections were examined in an epifluorescence microscope (E. Leitz, Inc., Rockleigh, NJ, USA) and scored from 0 to 4 + as detailed elsewhere, with appropriate controls for specificity [16,21,22]. Parallel kidney samples were embedded in paraffin, sectioned at 4 µm, stained with haematoxylin–eosin and periodic acid-Schiff and examined by light microscopy.
Effects of selected cytokines on IgA synthesis in vitro
Subclones of CH12LX mouse B cells expressing surface IgM (IgM+, IgA−) or surface IgA (IgM−, IgA+), both generous gifts of Dr G. Haughton, University of North Carolina at Chapel Hill, were cultured as detailed previously [15]. Some cells were stimulated with IFN-γ (50 ng/ml), a mixture of IL-4 (10 ng/ml) and IL-5 (25 U/ml), or all three cytokines (all purchased from Genzyme). The concentration and glycosylation pattern of IgA purified from culture supernatants were quantified by the methods detailed above, as previously described [15].
Statistical analysis
Quantitative data were compared among the groups by anova, and multiple inferences were drawn by Dunnett's t-tests. Count data were analysed by the chi-squared test, with Fisher's correction for small numbers always applied. The 5% confidence limits were adopted as the criteria for statistical significance.
Results
Viral titres in nasal washings after nasal virus challenge
Oral immunization resulted in partial protection from infection upon intranasal challenge with infectious virus. Viral titres in immunized mice did not differ significantly across strain, and were reduced by 1·2–1·9 logs (94–98%) relative to titres recovered from syngeneic naive control mice challenged with the same dose of virus (Table 1). No virus was recovered from naive or immunized mice challenged with inactivated virus, or from unchallenged mice. Non-immune BALB/c mice shed significantly more virus than non-immune C3HeB mice (Table 1), although the two strains are equally susceptible to fatal infection [17].
Table 1.
Viral titres in nasal washings of mice challenged with infectious Sendai virus
| Virus titre in nasal wash * | ||||
|---|---|---|---|---|
| Immunization status | No. of mice (each strain) | Day of sacrifice post-challenge | C3HeB | BALB/c |
| Immunized | 8 | 2 | 2·4 ± 1·5‡ | 3·3 ± 1·5‡ |
| Immunized | 8 | 4 | 2·2 ± 1·0‡ | 2·8 ± 0·7‡ |
| Non-immune | 8 | 2 | 3·6 ± 0·5§ | 5·1 ± 0·2 |
| Non-immune | 8 | 4 | 3·4 ± 0·4§ | 4·7 ± 0·4 |
Data are mean log pfu/ml lavage ± SD; no virus (i.e. titre < 1·4 in our assay) was detected in immunized (n = 8 per strain) or non-immune (n = 6 per strain) mice challenged with inactivated virus, or not challenged with intranasal virus.
Different (F = 10·4, all t ≥ 2·2, all P < 0·05) from syngeneic nonimmune mice challenged with infectious virus and sacrificed on the same day post-challenge.
Different (all t ≥ 3·4, all P < 0·01) from the same treatment group in the BALB/c strain.
Serum antibody
Immunized BALB/c mice showed significantly higher levels of IgA anti-Sendai virus than immunized C3HeB mice (Fig. 1), whether or not the mice were challenged. However, all immunized mice, both C3HeB and BALB/c, developed similar levels of IgG Sendai-specific antibodies (not shown). Age-matched control (non-immunized) mice showed no detectable anti-Sendai virus antibody (defined as baseline). Although antiviral IgA antibody levels in immunized C3HeB mice increased significantly 4 days after challenge, the IgG levels were not statistically significantly elevated up to 4 days after intranasal challenge. Challenge of immunized BALB/c mice did not significantly affect IgG or IgA antibody levels.
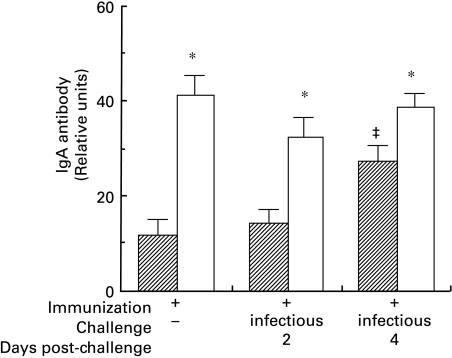
Fig. 1.
ELISA levels of IgA antibodies in sera of immunized C3HeB and BALB/c mice are expressed in units relative to a standard murine anti-Sendai virus antiserum. All values from immunized mice differed (F = 7·9, all P < 0·001) from non-immune mice of the same strain, which generated the background optical density (zero units, not shown). The IgA antibody levels in BALB/c mice were significantly higher than in C3HeB mice (* denotes t > 2·0, P < 0·05 between strains within treatment groups). In C3HeB mice, IgA levels increased significantly by 4 days post-challenge (‡ denotes t > 2·5, P < 0·05 versus other values within the strain), but the IgG levels did not differ significantly from immunized syngeneic mice that were not challenged (not shown).  , C3HeB; □, BALB/c.
, C3HeB; □, BALB/c.
Antigen-specific cytokine production
Splenocytes from immunized C3HeB mice produced significantly more IFN-γ in response to viral antigen than cells from immunized BALB/c mice (Fig. 2). Splenocytes from immunized but unchallenged C3HeB or BALB/c mice produced comparable low levels of IL-2 (not shown). Intranasal challenge of immunized C3HeB mice with infectious virus resulted in progressively heightened IFN-γ production (Fig. 2) but similarly elevated IL-2, whether sacrificed 2 (28-fold) or 4 (34-fold) days after challenge (t > 5·5, P < 0·001, data not shown); such responses to challenge were not seen in immunized BALB/c mice. No IFN-γ or IL-2 was produced by cells from non-immune mice, or from immunized mice of either strain in the absence of antigen stimulation in vitro (data not shown).
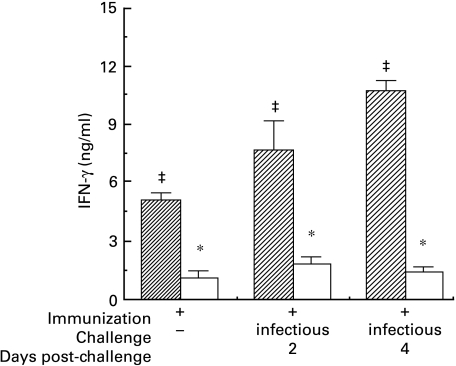
Fig. 2.
Secretion of IFN-γ by splenocytes from immunized C3HeB and BALB/c mice over 48 h in vitro in the presence of inactivated Sendai virus, as determined by ELISA. No cytokine (data not shown) was detected in supernatant from splenocytes from non-immune mice of either strain in the presence of inactivated virus, or from immunized mice in the absence of virus (F = 27·7, all P < 0·001). Production of IFN-γ by immunized but unchallenged BALB/c mice was less than that from comparable C3HeB mice (* denotes t > 2·5, P < 0·05 between strains in the same treatment group). After challenge in vivo with virus, the production of IFN-γ by cells from immunized C3HeB mice increased (‡ denotes t > 2·0, P < 0·05 versus other values within the same strain), whereas no increase was observed for BALB/c mice. This increase further distinguishes the cytokine response of C3HeB from BALB/c mice (t > 5·5, P < 0·001 for interstrain comparisons between immunized and challenged mice).  , C3HeB; □, BALB/c.
, C3HeB; □, BALB/c.
Splenocytes from immunized BALB/c mice produced significant IL-4 (Fig. 3) and IL-5 (not shown) in the presence of antigen in vitro, but this response was not evident in cells from non-immune controls or in cells from immune mice cultured in the absence of viral antigen (data not shown). Challenge of immunized BALB/c mice elicited a transient increase in IL-4 production (Fig. 3), and a delayed or reciprocal flux in IL-5 (not shown). In contrast, splenocytes from immunized (or non-immune) C3HeB mice did not produce detectable Th2 cytokines in response to antigen stimulation in vitro, regardless of whether the mice were challenged intranasally prior to sacrifice. Overall, lymphocytes from immunized BALB/c mice produced significantly more Th2 cytokines (IL-4 and IL-5) but significantly less Th1 cytokines (IFN-γ and IL-2) than those from immunized C3HeB mice.
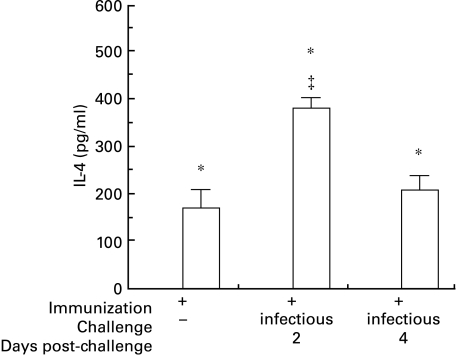
Fig. 3.
Production of IL-4 was detected only in supernatants from splenocytes of immunized BALB/c mice cultured in the presence of inactivated virus, and not in cells from naive BALB/c mice (not shown) or immunized C3HeB mice (* denotes F = 26·8, t > 5·5, P < 0·001 between strains in the same treatment group). Among immunized BALB/c mice challenged with virus, those sacrificed 2 days after challenge produced more IL-4 than those sacrificed on the same day with no challenge, or sacrificed 4 days after intranasal viral challenge (‡ denotes t > 2·9, P < 0·01 versus the other treatment groups within the same strain).  , C3HeB; □, BALB/c.
, C3HeB; □, BALB/c.
IgA glycosylation
To determine whether the cytokine profiles correlated with glycosylation patterns of IgA in vivo, we compared the IgA purified from C3HeB to that from BALB/c mice. Total serum IgA from immunized BALB/c mice, and to a lesser extent IgA from immunized C3HeB mice, contained significantly less terminal sialic acid and terminal galactose than serum IgA from nonimmune syngeneic controls (data not shown). Terminal glycosylation of total IgA increased after intranasal challenge within 2 days in immunized C3HeB mice; in immune BALB/c mice, sialylation but not galactosylation normalized by 4 days post-challenge (data not shown). The interstrain differences in terminal glycosylation of Sendai virus‐specific IgA antibody were more pronounced. Indeed, 2 days after intranasal challenge, specific IgA produced by immunized BALB/c mice contained significantly less terminal sialic acid (Fig. 4a) and galactose (Fig. 4b) per unit IgA than the IgA antibody from C3HeB mice. In both strains, terminal glycosylation with both sialic acid and galactose increased after in vivo challenge, but the elevations were more pronounced and occurred earlier (d 2 post-challenge) in the C3HeB strain. The differences between the two strains decreased by day 4 after challenge.
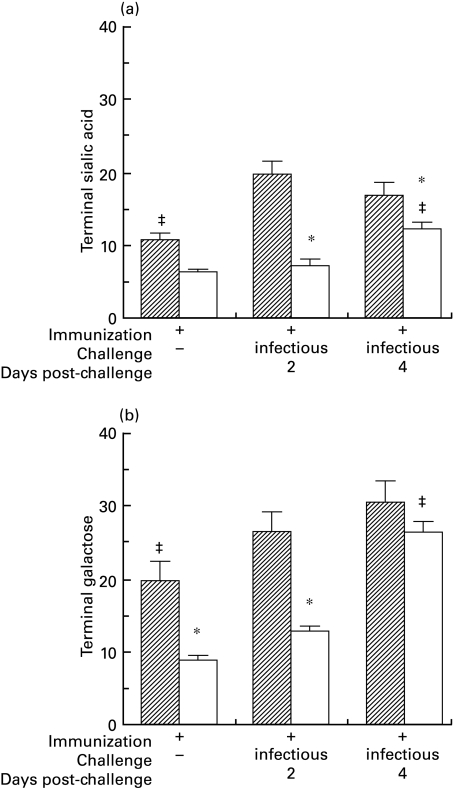
Fig. 4.
Terminal sialic acid (a) and galactose (b) on Sendai virus-specific IgA purified from the serum of immunized mice was determined by binding of SNA lectin (a) or RCA-I lectin (b) to IgA captured by solid-phase antigen. Two days after intranasal challenge, virus-specific IgA from immunized BALB/c mice contained significantly less terminal sialic acid (a) and terminal galactose (b) per unit IgA, compared to virus-specific IgA from immunized C3HeB mice (* denotes F = 9·9, t > 2·6, P < 0·05 for interstrain comparisons within the same treatment group). The levels of terminal sugars increased in response to intranasal challenge, but the increases were quicker and more intense in C3HeB than in BALB/c mice (‡ denotes t > 2·1, P < 0·05 versus other treatment groups in the same strain).  , C3HeB; □, BALB/c.
, C3HeB; □, BALB/c.
Effects of cytokines on the glycosylation of IgA secreted by CH12LX cells
In order to determine whether IFN-γ plays a role in IgA glycosylation, we measured the effects of cytokines upon murine B cells in vitro. Stimulation of membrane IgM+ CH12LX B cells by IL-4 plus IL-5 evoked significant proliferation, accompanied by a 6–10-fold increase in IgA secretion (data not shown) [23]. Relative to unstimulated cells, the IgA produced after IL-4 plus IL-5 stimulation had diminished sialic acid (30%) and terminal galactose (27%) (Fig. 5a), as reported previously [15]. We now report that incubation of IgM+ CH12LX cells with IFN-γ alone did not alter cell proliferation or IgA production (not shown), or the terminal glycosylation of IgA (Fig. 5a). On the other hand, when added together with IL-4 and IL-5, IFN-γ abrogated the proliferation of and increased IgA production by IgM+ CH12LX cells (not shown) and the decreases in IgA glycosylation seen in response to IL-4 plus IL-5 alone (Fig. 5a).
Fig. 5.
Terminal sialic acid (SNA binding) and terminal galactose (RCA binding) on IgA secreted in vitro by IgM+ CH12LX cells (a) or IgA+ CH12LX cells (b) was determined by lectin binding. When added to preswitch (IgM+) cells (a), the mixture of IL-4 plus IL-5 reduced both terminal sugars by ∼30% (* denotes F = 5·0, t > 2·0, P < 0·05 versus unstimulated cells), whereas IFN-γ had no effect. The addition of IFN-γ to the IL-4 plus IL-5 mixture abrogated their effect. Although IL-4 plus IL-5 acted on post-switch (IgA+) cells (b) to reduce terminal sugars by ∼20% (* denotes F = 15·8, t > 2·0, P < 0·05 versus unstimulated cells), IFN-γ alone elicited an 18% increase in terminal sialic acid, and a minor (5·6%) increase in terminal galactose. Addition of IFN-γ to IL-4 plus IL-5 reversed the effect of IL-4 plus IL-5, increasing terminal sialic acid and galactose by 24% and 45%, respectively (‡ denotes significantly higher terminal sugar relative to unstimulated cells or cells stimulated by the IL-4 plus IL-5 mixture). ▪, Sialic acid; □, galactose.
Stimulation of membrane IgA+ CH12LX cells with IL-4 plus IL-5 increased cell proliferation and IgA production (not shown) [23], but decreased terminal sialic acid (20%) and terminal galactose (16%) on the secreted IgA (Fig. 5b), as reported earlier [15]. In new observations, the addition of IFN-γ alone had no effect on cell proliferation or IgA synthesis (not shown), but increased terminal sialic acid (18%) and terminal galactose (5·6%) on the secreted IgA compared to IgA from unstimulated IgA+ CH12LX cells (Fig. 5b). Addition of IFN-γ together with IL-4 and IL-5 abrogated the increases in B cell proliferation and IgA production in response to IL-4 plus IL-5 alone, and increased terminal sialic acid (by 24%) and terminal galactose (by 45%) relative to IgA produced by unstimulated cells (Fig. 5b). The effect of all three cytokines in concert on IgA glycosylation by IgA+ B cells was opposite that of the Th2 cytokines without IFN-γ, and augmented relative to IFN-γ alone. In summary, although IFN-γ does not influence IgA production, it opposes the effects of IL-4 plus IL-5 on synthesis and glycosylation of IgA.
Renal function
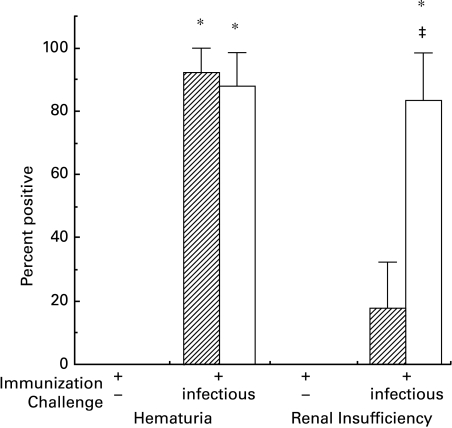
Microscopic haematuria was detected in ≥ 84% of immunized C3HeB and BALB/c mice 2 (Fig. 6) or 4 (not shown) days after intranasal viral challenge. Haematuria was absent among immunized mice left unchallenged, and rare (< 15%) among immunized mice challenged with inactivated virus or non-immune mice regardless of challenge.
Fig. 6.
Haematuria and acute renal insufficiency (measured by serum creatinine) in immunized C3HeB and BALB/c mice. By direct microscopic examination of the urinary sediment, haematuria was detected in nearly all immunized C3HeB or BALB/c mice by 2 days after intranasal challenge with infectious virus, but not in syngeneic immunized controls not challenged intranasally (* denotes χ2 = 7·1, P < 0·01 versus syngeneic controls) or challenged with inactivated (non-infectious) virions (data not shown). Intranasal challenge of naive mice with infectious virus did not elicit haematuria in either strain (not shown), despite high levels of virus in nasal washings (see Table 1). Most (≥ 72%) immunized BALB/c mice challenged with infectious virus, but none of the BALB/c controls, had elevated serum creatinine levels, indicative of acute renal insufficiency (* denotes χ2 = 5·7, P < 0·02 compared to all the BALB/c control groups). Mice of the C3HeB strain rarely (< 17%) exhibited evidence of acute renal insufficiency, even if immunized and challenged with infectious virus (‡ denotes χ2 = 5·7, P < 0·02 between strains in the same treatment group).  , C3HeB; □, BALB/c.
, C3HeB; □, BALB/c.
An acute reduction in urine volume in the immunized BALB/c mice challenged with infectious virus (414 ± 214 µl/24 h vs. 1929 ± 310 µl/24 h in all syngeneic controls, all P < 0·01) suggested acute renal insufficiency. Indeed, the mean serum creatinine concentration in immunized BALB/c mice challenged intranasally with infectious virus (0·58 ± 0·2 mg/dL) was significantly higher (all t > 2·2, all P < 0·05) than that in any of the syngeneic controls (0·21 ± 0·1–0·24 ± 0·1 mg/dL), and serum creatinine levels in excess of the 95% confidence limits of the control groups occurred in the majority of immunized BALB/c mice 2 days (86%, Fig. 6) and 4 days (72%, not shown) after challenge with infectious virus. Mean urine volume and mean serum creatinine did not differ among the groups of C3HeB mice. There was no significant proteinuria in any group in either strain.
Renal microscopy
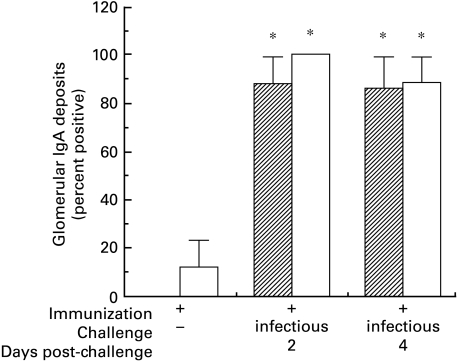
Moderately intense granular mesangial deposits of IgA (Fig. 7) and IgG (not shown) were detected in ≥ 75% of immunized C3HeB or BALB/c mice sacrificed 2 and 4 days after intranasal challenge with infectious virus. These deposits, accompanied by less intense IgM and C3 (not shown) in the same distribution, occasionally extended into glomerular capillary walls, and resembled the deposits in patients with IgAN. Glomerular immune deposits were rarely (< 15%) observed in mice of either strain that were immunized with Sendai virus but left unchallenged (Fig. 7) or challenged with inactivated virus, or in nonimmune mice given infectious virus (data not shown). Weak but definite deposits of Sendai virus antigen were detected in the majority (≥ 76%) of immunized mice challenged with infectious virus, but in only a few (≤ 17%) mice in any of the control groups, regardless of strain. There was no difference between C3HeB and BALB/c mice in the intensity of immunofluorescence staining for any of the reactants tested. By routine light microscopy, glomeruli and extraglomerular renal tissue appeared normal in all mice, regardless of immunization status or intranasal viral challenge.
Fig. 7.
Glomerular deposits of IgA in immunized C3HeB and BALB/c mice. Prominent granular mesangial deposits were detected by immunofluorescence in nearly all immunized C3HeB or BALB/c mice 2 and 4 days after intranasal challenge with infectious virus. Weak deposits were detected in only a few immunized mice challenged with inactivated virus or not challenged at all (* denotes χ2 = 5·2, P < 0·02 versus all the various syngeneic controls). In mice scored positive, essentially all glomeruli were uniformly stained, and there were no significant differences between immunized mice in the two strains after challenge with infectious virus. Deposits of IgG, IgM, Sendai virus antigen and complement C3 (not shown) were also detected. Sendai virus antigen was detected only rarely in glomeruli of non-immune mice challenged with infectious virus.  00023;, C3HeB; □, BALB/c.
00023;, C3HeB; □, BALB/c.
Discussion
Despite the apparent importance of mucosal infection for the origin and exacerbation of most cases of IgAN, and the development of several animal models, there are only limited data on the role of pathogens in the genesis of this disease [1–3]. Here, we examined how mucosal immune responses can interact with mucosal pathogens to elicit IgAN, and addressed several issues related to pathogenesis in a mouse model. From the results, we suggest conditions which favour the generation and deposition of IgA immune complexes that lead to significant glomerular dysfunction.
The onset of synpharyngitic glomerulonephritis requires the simultaneous presence of a pathogen as a source of antigen and an antibody response. Oral immunization with Sendai virus provides only partial protection from respiratory infection, despite strong intestinal and serum IgA antibody responses [18,19]. Although immunization of one mucosal surface can promote the seeding of other secretory surfaces with antigen-specific B cells, such trafficking develops over many hours or days and is enhanced by antigenic challenge at the remote site [24]. Apparently, a limited low-level infection can develop during this temporal window, providing a source of antigen in an immunized host, giving rise to glomerular immune deposits of viral antigen and predominantly IgA antibody.
The functional consequences of such IC deposited or formed in glomeruli include, at least in the BALB/c strain, acute renal insufficiency similar to that which occurs in approximately 10% of patients with IgAN. We believe that the major difference in renal function between the BALB/c compared to C3HeB mice, which did not develop renal failure, is related to differences in glycosylation of the IgA antibodies specific for Sendai virus. Complexes of specific antigen with aberrantly glycosylated murine IgA deposit more rapidly in the glomeruli of experimental mice and activate the alternative pathway of complement in human serum more effectively than those with normally glycosylated IgA (reviewed in [2,5,9,11]). Paradoxically, these differences between the IgA glycoforms were not reflected in the semiquantitative immunofluorescence scores in BALB/c compared to C3HeB mice, perhaps becasue the differences in deposition were too small to detect by this method. However, aberrantly glycosylated IgA also binds better to cultured human mesangial cells, and to components of the extracellular mesangial matrix [2,10,25,26]. Such enhanced binding, and/or more rapid complement activation, could increase intraglomerular production of a variety of mediators that are considered pathophysiologically significant in nephritis [1,2,25–28,]. For example, we reported that intraglomerular synthesis of vasoactive eicosanoids leads to diminished glomerular filtration, the critical determinant of renal function, in a rat model of IgAN [21]. The lack of histological abnormalities in either of the mouse strains, even though immune deposits were present by immunofluorescence, is consistent with the histological appearance in patients with IgAN, even when acute renal insufficiency develops [1,3].
The differences in IgA glycosylation between the two mouse strains probably arise from the higher Th2 : Th1 cytokine ratio in the BALB/c relative to the C3HeB mice, consonant with our earlier observations in vitro [15]. Furthermore, the more pronounced effect on the glycosylation of specific IgA anti-Sendai virus antibody relative to total IgA suggests that cognate interactions between antigen-specific T and B cells are functionally significant. Of note, the addition of IFN-γ to cultures of B lymphocytes abrogated or even reversed the effects of IL-4 plus IL-5 on the synthesis and glycosylation of IgA, even though IFN-γ alone had no effect on Ig secretion and only minor effects on IgA glycosylation by post-switch B cells, and no effect on pre‐switch cells. Overall, the evidence suggests strongly that the balance among Th cell-derived cytokines closely regulates the glycosylation of IgA.
The cytokine profile and consequent aberrations in IgA glycosylation that we observed in the IgAN-prone BALB/c mice appear to be relevant to human IgAN. Compared to healthy controls or patients with other forms of glomerulonephritis, circulating lymphocytes from patients with clinically active IgAN produce twice the IL-4 in response to mitogenic stimulation [13]. IgAN patients with more severe renal dysfunction are more likely to hyperproduce Th2 cytokines, and synthesize more IL-4 compared to patients with more mild disease [13]. During remission, IgAN patients have more normal IL-4 responses and produce higher than normal levels of IFN-γ [13]. Although others detected increased levels of mRNA encoding IL-4, IL-5 and IFN-γ in blood mononuclear cells from IgAN patients relative to controls [14], patients in exacerbation were not stratified from those in remission. Similarly, IgA in patients' serum, and especially that in the circulating macromolecular IgA complexes that apparently form glomerular deposits, often bears truncated O-linked and N-linked oligosaccharides, containing diminished sialic acid and galactose [4–6,8,9]. Moreover, the truncated oligosaccharides can be antigenic targets of IgG isoagglutinins, and therefore the aberrantly glycosylated IgA can become bound (as antigen) in IC [4,5,12]. These observations in patients support the concept that T cell cytokine polarity is linked to a more nephritogenic pattern of IgA glycosylation, but do not establish a causal link between these abnormalities and disease.
In summary, local mucosal exposure of partially immune mice to virus may cause a limited infection that leads to IgAN. An analogous situation may arise if serological variants of a virus, or distinct but serologically related viruses, sequentially infect a patient. Indeed, reinfection of humans with paramyxoviruses in the presence of viral antibodies has been documented [29,30]. Furthermore, the associations among Th2 cytokine bias, aberrant IgA glycosylation and more severe glomerular dysfunction suggest that the synthesis of aberrantly glycosylated IgA antibody underlies renal disease in IgAN, and that abnormally polarized or poorly regulated T cell responses to common mucosal infections are integral to the pathogenesis of human IgAN.
Acknowledgments
This work was supported by NIH grants P01 AI 36359, R01 AI 26449, R01 AI 40701 and T32 AI 07427. We thank Sara Cechner for excellent secretarial support.
References
- 1.Emancipator SN. IgA nephropathy and Henoch–Schönlein syndrome. In: Jennette JC, et al., editors. Heptinstall's pathology of the kidney. New York: Lippincott-Raven; 1998. pp. 479–540. [Google Scholar]
- 2.Emancipator SN, Mestecky J, Lamm ME. IgA nephropathy and related diseases. In: Ogra PL, et al., editors. Mucosal immunology. New York: Academic Press; 1999. pp. 1365–80. [Google Scholar]
- 3.D'Amico G. Natural history of idiopathic IgA nephropathy: role of clinical and histological prognostic factors. Am J Kidney Dis. 2000;36:227–37. doi: 10.1053/ajkd.2000.8966. [DOI] [PubMed] [Google Scholar]
- 4.Tomana M, Matousovic K, Julian BA, Radl J, Konecny K, Mestecky J. Galactose-deficient IgA1 in sera of IgA nephropathy patients is present in complexes with IgG. Kidney Int. 1997;52:509–16. doi: 10.1038/ki.1997.361. [DOI] [PubMed] [Google Scholar]
- 5.Tomana M, Novak J, Julian BA, Matousovic K, Konecny K, Mestecky J. Circulating immune complexes in IgA nephropathy consist of IgA1 with galactose-deficient hinge region and antiglycan antibodies. J Clin Invest. 1999;104:73–81. doi: 10.1172/JCI5535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Allen AC, Harper SJ, Feehally J. Galactosylation of N- and O- linked carbohydrate moieties of IgA1 and IgG in IgA nephropathy. Clin Exp Immunol. 1995;100:470–4. doi: 10.1111/j.1365-2249.1995.tb03724.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Leung JC, Poon PY, Lai KN. Increased sialylation of polymeric immunoglobulin A1: mechanism of selective glomerular deposition in immunoglobulin A nephropathy? J Lab Clin Med. 1999;133:152–60. doi: 10.1016/s0022-2143(99)90008-2. [DOI] [PubMed] [Google Scholar]
- 8.Baharaki D, Dueymes M, Perrichot R, et al. Aberrant glycosylation of IgA from patients with IgA nephropathy. Glycoconj J. 1996;13:505–11. doi: 10.1007/BF00731436. [DOI] [PubMed] [Google Scholar]
- 9.Hiki Y, Kokubo T, Iwase H, et al. Underglycosylation of IgA1 hinge plays a certain role for its glomerular deposition in IgA nephropathy. J Am Soc Nephrol. 1999;10:760–9. doi: 10.1681/ASN.V104760. [DOI] [PubMed] [Google Scholar]
- 10.Kokubo T, Hiki Y, Iwase H, et al. Protective role of IgA1 glycans against IgA1 self-aggregation and adhesion to extracellular matrix proteins. J Am Soc Nephrol. 1998;9:2048–54. doi: 10.1681/ASN.V9112048. [DOI] [PubMed] [Google Scholar]
- 11.Rifai A, Fadden K, Morrison SL, Chintalacharuvu KR. The N-glycans determine the differential blood clearance and hepatic uptake of human immunoglobulin (Ig) A1 and IgA2 isotypes. J Exp Med. 2000;191:2171–82. doi: 10.1084/jem.191.12.2171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Iwase H, Yokozeki Y, Hiki Y, et al. Human serum immunoglobulin G3 subclass bound preferentially to asialo-, agalactoimmunoglobulin A1/Sepharose. Biochem Biophys Res Commun. 1999;264:424–9. doi: 10.1006/bbrc.1999.1369. 10.1006/bbrc.1999.1369. [DOI] [PubMed] [Google Scholar]
- 13.Scivittaro V, Gesualdo L, Ranieri E, Marfella C, Schwen SA, Emancipator SN. Profiles of immunoregulatory cytokine production in vitro in patients with IgA nephropathy and their kindred. Clin Exp Immunol. 1994;96:311–6. doi: 10.1111/j.1365-2249.1994.tb06559.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lai KN, Leung JCK, Li PKT, Lui SF. Cytokine production by peripheral blood mononuclear cells in IgA nephropathy. Clin Exp Immunol. 1991;85:240–5. doi: 10.1111/j.1365-2249.1991.tb05712.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chintalacharuvu SR, Emancipator SN. The glycosylation of IgA produced by murine B cells is altered by Th2 cytokines. J Immunol. 1997;159:2327–33. [PubMed] [Google Scholar]
- 16.Jessen RH, Emancipator SN, Jacobs GH, Nedrud JG. Experimental IgA-IgG nephropathy induced by a viral respiratory pathogen: Dependence on antigen form and immune status. Lab Invest. 1992;67:379–86. [PubMed] [Google Scholar]
- 17.Parker JC, Whiteman MD, Richter CB. Susceptibility of inbred and outbred mouse strains to Sendai virus and prevalence of infection in laboratory rodents. Infect Immun. 1978;19:123–30. doi: 10.1128/iai.19.1.123-130.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nedrud JG, Liang X, Hague N, Lamm ME. Combined oral/nasal immunization protects mice from Sendai virus infection. J Immunol. 1987;139:3484–92. [PubMed] [Google Scholar]
- 19.Liang X, Lamm ME, Nedrud JG. Oral administration of cholera toxin-Sendai virus conjugate potentiates gut and respiratory immunity against Sendai virus. J Immunol. 1988;141:1495–501. [PubMed] [Google Scholar]
- 20.Emancipator SN. Animal models of IgA nephropathy. In: Coligan JE, et al., editors. Current protocols in immunology. New York: John Wiley & Sons; 1999. Unit15.1. [DOI] [PubMed] [Google Scholar]
- 21.Gesualdo L, Emancipator SN, Kesselheim C, Lamm ME. Glomerular hemodynamics and eicosanoid synthesis in a rat model of IgA nephropathy. Kidney Int. 1992;42:106–14. doi: 10.1038/ki.1992.268. [DOI] [PubMed] [Google Scholar]
- 22.Gesualdo L, Ricanati S, Hassan MO, Emancipator SN, Lamm ME. Enzymolysis of glomerular immune deposits in vivo with dextranase/protease ameliorates proteinuria, hematuria, and mesangial proliferation in murine experimental IgA nephropathy. J Clin Invest. 1990;86:715–22. doi: 10.1172/JCI114767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kunimoto DY, Harriman GR, Strober W. Regulation of IgA differentiation in CH12LX B cells by lymphokines. IL-4 induces membrane IgM-positive CH12LX cells to express membrane IgA and IL-5 induces membrane IgA-positive CH12LX cells to secrete IgA. J Immunol. 1988;141:713–20. [PubMed] [Google Scholar]
- 24.Weisz-Carrington P, Grimes SR, Jr, Lamm ME. Gut-associated lymphoid tissue as source of an IgA immune response in respiratory tissues after oral immunization and intrabronchial challenge. Cell Immunol. 1987;106:132–8. doi: 10.1016/0008-8749(87)90156-0. [DOI] [PubMed] [Google Scholar]
- 25.Gomez-Guerrero C, Lopez-Armada MJ, Gonzalez E, Egido J. Soluble IgA and IgG aggregates are catabolized by cultured rat MC and induce production of TNF-α and IL-6, and proliferation. J Immunol. 1994;153:5247–55. [PubMed] [Google Scholar]
- 26.Duque N, Gomez-Guerrero C, Egido J. Interaction of IgA with Fcα receptors of human mesangial cells activates transcription factor nuclear factor-κB and induces expression and synthesis of monocyte chemoattractant protein-1, IL-8, and IFN-inducible protein 10. J Immunol. 1997;159:3474–82. [PubMed] [Google Scholar]
- 27.Launay P, Grossetete B, Arcos-Fajardo M, et al. Fcα receptor (CD89) mediates the development of immunoglobulin A (IgA) nephropathy (Berger's disease). Evidence for pathogenic soluble receptor-IgA complexes in patients and CD89 transgenic mice. J Exp Med. 2000;191:1999–2009. doi: 10.1084/jem.191.11.1999. [published erratum appears in J Exp Med 2000; 192(2): following 309]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Barratt J, Greer MR, Pawluczyk IZ, et al. Identification of a novel Fcα receptor expressed by human mesangial cells. Kidney Int. 2000;57:1936–48. doi: 10.1046/j.1523-1755.2000.00043.x. [DOI] [PubMed] [Google Scholar]
- 29.Chanock RM, Parrott RH, Johnson KM, Kapikian AZ, Bell JA. Myxoviruses: parainfluenza. Am Rev Resp Dis. 1963;88:152–66. doi: 10.1164/arrd.1963.88.3P2.152. [DOI] [PubMed] [Google Scholar]
- 30.van Wyke Coelingh KL, Winter CC, Tierney EL, et al. Antibody responses of humans and nonhuman primates to individual antigenic sites of the hemagglutinin-neuraminidase and fusion glycoproteins after primary infection or reinfection with parainfluenza type 3 virus. J Virol. 1990;64:3833–43. doi: 10.1128/jvi.64.8.3833-3843.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]