Abstract
We studied the pattern of type 1 diabetes-associated autoantibodies during pregnancy and the transplacental transfer of these autoantibodies to the fetal circulation and searched for possible signs of prenatal induction of β-cell autoimmunity in newborn infants. The population comprised 208 mothers and their newborn infants. Seventy-four of the mothers (36%) had type 1 diabetes and 134 (64%) of the infants had an affected father or sibling. Blood samples were obtained from the mother at the end of the first trimester and at delivery, and from the cord blood of the newborn infant. Close to 40% of the mothers with type 1 diabetes had antibodies to islet cells (ICA), 55% to glutamic acid decarboxylase (GADA) and 54% to the IA-2 protein (IA-2A) in early pregnancy, whereas the corresponding frequencies in the nonaffected mothers were 5·2%, 5·2% and 3·0%. No significant changes could be seen in autoantibody levels during pregnancy, and there was a close correlation between the two maternal samples. One third of the infants of mothers with type 1 diabetes tested positive for ICA, 50% for GADA and 51% for IA-2A. Six percent of the infants of nondiabetic mothers had ICA, 2·2% GADA and none had IA-2A. None of the infants of the antibody negative mothers had antibodies in their cord blood. These observations indicate that the immunomodulatory effect of pregnancy on signs of β-cell autoimmunity is weak, but if diabetes-associated autoantibodies are present in the mother, most of them are transferred to the fetal circulation. Our data do not provide any support for fetal induction of β-cell autoimmunity.
Keywords: glutamic acid decarboxylase antibodies, IA-2 antibodies, islet cell antibodies, pregnancy, transplacental transfer
Introduction
There is clinical and experimental evidence that maternal immune responses during pregnancy are biased towards antibody-mediated and away from the cell-mediated. A majority of women with rheumatoid arthritis, a cell-mediated autoimmune disorder, experience a temporary remission of their symptoms during gestation [1]. Systemic lupus erythematosus (SLE), in which the principal pathology is mediated by excessive autoantibody production, tends to flare up during pregnancy [2]. Contradictory results have been reported on the effect of pregnancy on humoral immune responses; autoantibody levels have been reported to decrease [3], increase [4] or remain unchanged [5] depending on the autoimmune disorder studied.
Two of the primary hormones increasingly produced during pregnancy, i.e. corticosteroids and progesterone, are capable of polarizing the cytokine balance towards a Th2 dominance [6]. The shift towards antibody-mediated immune responses during pregnancy implies that the cell-mediated immune function and the Th1 cytokine production (e.g. IL-12, interferon-gamma) are suppressed, and humoral immunity and Th2 cytokine production (e.g. IL-4, IL-10) are enhanced [6,7]. The Th2 type cytokines down-regulate the Th1 type ones and may thereby protect the fetus from a harmful immune rejection [1].
A Th2 biased immune response leading to increased antibody production may be useful to the fetus, since antibodies are transferred across the placenta, providing the newborn infant with protective immunity against infectious agents before the endogenous immune system develops [1]. This active transport of antibodies to the fetus, which involves exclusively immunoglobulin G (IgG) antibodies, is well documented by 12 weeks of gestation and continues at a low but steady rate until 16–22 weeks, after which the rate increases, so that IgG concentrations in the fetus often exceed at birth those in the maternal circulation [8–10]. Most of the autoantibodies associated with type 1 diabetes are of the IgG class [11,12], and the newborn infants of affected mothers have been shown to have insulin antibodies (IA), islet cell antibodies (ICA) and other disease-associated antibodies in their circulation as a consequence of transplacental transfer from the maternal circulation [13–16]. The autoantibodies associated with type 1 diabetes act as predictive markers of β-cell destruction in the prediabetic period, and accordingly the presence of such autoantibodies in the cord blood from a newborn infant could be an indicator of prenatal β-cell damage if the mother tests negative for these autoantibodies.
The present work was aimed at investigating the pattern of diabetes-associated autoantibodies during pregnancy, and whether there would be any signs of immunomodulation of the Th1/Th2 balance of β-cell autoimmunity towards a Th2 profile in pregnant women. We wanted to assess further the transplacental transfer of these autoantibodies to the fetal circulation, and in addition we wished to search for any signs of prenatal induction of β-cell autoimmunity in the newborn infant.
Subjects and methods
The population comprised 208 mothers and their newborn infants from families with type 1 diabetes who had entered the second pilot study of the Trial to Reduce IDDM in the Genetically at Risk (TRIGR), which aims at evaluating the possible effect of elimination of cow's milk proteins in early infancy on the risk of the infant to progress to type 1 diabetes later [17]. The infants were born between March 1995 and September 1996 in 15 hospitals around Finland. Seventy-four (36%) of the 208 mothers had type 1 diabetes. One hundred and two of the 208 infants were born into a family with an affected father and 38 into one with an affected sibling. In two families the mother and one of the siblings had type 1 diabetes, in three families the father and one of the siblings were affected and in one family both parents were affected. Altogether there were 74 mother–infant pairs with maternal type 1 diabetes and 134 (64%) without. The mean age of the mothers at delivery was 31·0 + 5·4 (SD) years (range 18·9–46·7 years). The severity of type 1 diabetes in the pregnant women was classified according to White [18], 21 being in class B, 15 in class C, 28 in class D and 10 in class F. The mean duration of diabetes at delivery was 14·4 ± 7·2 years (range 2·0–29·0 years) The mean individual HbA1C levels throughout pregnancy varied from 4·6% to 8·0% (mean 6·3% ± 0·7), with a range of 4–6% in nondiabetic subjects [19]. Slightly more than half of the infants were boys (108/208; 52%). The mean gestational age was 39·1 ± 1·5 weeks, mean birth weight 3703 ± 627 g (range 1695–5600 g) and mean birth length 50·3 ± 2·3 cm (range 41–56 cm). Blood samples for the analysis of diabetes-associated autoantibodies were obtained from the mother at the end of first trimester and at delivery, and cord blood samples were taken from the newborn infant. Serum samples for the antibody assays were stored at − 20°C or − 70°C until analysed. All three samples from each mother–infant pair were analysed in the same assay. Written informed consent was obtained from the mother before enrolment. The study was approved by the Ethics Committees of the participating hospitals.
Islet cell antibodies (ICA)
ICA were determined by a standard indirect immunofluorescence assay on sections of frozen human group O pancreas [20] using goat antihuman IgG (Sigma, St Louis, MD, USA) for the detection of ICA. End-point dilution titres were examined for the ICA-positive samples, and the results were expressed in Juvenile Diabetes Foundation (JDF) units relative to an international reference standard. The detection limit was 2·5 JDF units. Our laboratory has participated in the International Workshops on Standardization of the ICA assay, in which its sensitivity was 100% and specificity 98% in the most relevant round.
Antibodies to the 65 kD isoform of glutamic acid decarboxylase (GADA)
GADA were analysed by a radioligand method as described previously [21,22]. The results were expressed in relative units (RU) representing the specific binding as a percentage of that obtained with a positive standard serum. The cut-off limit for GAD65 antibody positivity was 6·6 RU (99th percentile in 372 subjects without type 1 diabetes). The disease sensitivity of the present assay was found to be 76% and the disease specificity 99% based on the 1995 Multiple Autoantibody Workshop [23].
Antibodies to the protein tyrosine phosphatase related IA-2 molecule (IA-2A)
IA-2A were analysed with a radiobinding assay as described in detail elsewhere [24,25]. The results were expressed in relative units (RU) based on a standard curve run on each plate using the MultiCalcTM software program (PerkinElmer Life Sciences Wallac, Turku, Finland). The cut-off limit for IA−2A positivity was 0·43 RU, which represents the 99th percentile in 374 Finnish subjects without type 1 diabetes. The disease sensitivity of the assay was observed to be 62% and the specificity 97% based on 140 samples included in the Multiple Autoantibody Workshop [23].
Statistical analysis
The data were evaluated statistically using the SPSS software (SPSS Inc., Chicago, IL, USA), by means of cross-tabulation and chi-square statistics or Fisher's exact probability test, the unpaired Student's t-test in the case of normally distributed variables, and the Mann–Whitney U-test and Wilcoxon test for paired samples in the case of an unequal distribution. Correlation analyses were performed with the Spearman rank correlation test (rs).
Results
Frequencies and levels of single antibodies
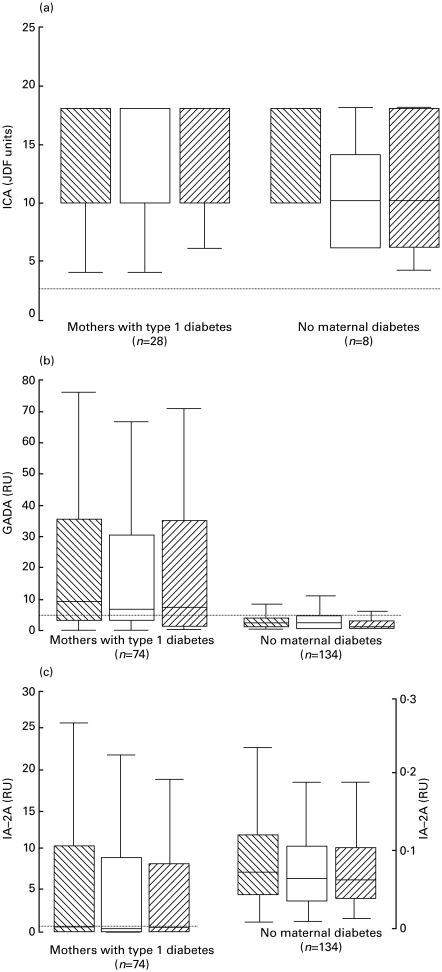
More than one-third of the mothers with type 1 diabetes (28/74; 38%) tested positive for ICA at the end of the first trimester, 55% (41/74) were positive for GADA and 54% (40/74) for IA-2A. Two of the mothers who were initially ICA-positive became negative during pregnancy, while three mothers became positive for GADA and eight initially positive ones turned negative, and one became positive for IA-2A and four negative. Seven out of the 134 mothers without type 1 diabetes (5·2%) had detectable ICA at the end of the first trimester, 5·2% (7/134) tested positive for GADA and 3·0% (4/134) for IA-2A. One unaffected mother became positive for ICA during pregnancy, two became positive for GADA and three initially positive ones became negative and the same occurred for IA−2A. No significant differences in ICA levels could be observed between the two maternal samples and the cord blood samples when all the maternal–infant pairs with detectable ICA on at least one occasion were included (Fig. 1a) and the same held true for GADA (Fig. 1b) and IA−2A (Fig. 1c) in all the mother–infant pairs. There was a relatively close correlation (rs = 0·79; P < 0·001) between the levels of ICA in the first and second maternal sample in the mothers with type 1 diabetes and a weaker one in those without (rs = 0·63; P = 0·05) when the women with detectable ICA on at least one of the two occasions were included (n = 28 and 8, respectively). There was also a close correlation between GADA levels (rs = 0·87; P < 0·001) and IA-2A levels (rs = 0·96; P < 0·001) in the samples from the affected mothers, whereas the correlations were weaker among the unaffected mothers (GADA: rs = 0·27; P = 0·002, IA−2A: rs = 0·48; P < 0·001).
Fig. 1.
ICA levels in ICA positive cases (a) and GADA (b) and IA−2A (c) levels in all cases in samples taken from the mother at the end of first trimester (▒) and at delivery (□), and from the infant's cord blood ( ). Each box plot represents the median (–) and the 25th and 75th percentiles. The error bars represent the smallest and largest observed values that are not outliers. The dotted line indicates the cutoff limit for antibody positivity.
). Each box plot represents the median (–) and the 25th and 75th percentiles. The error bars represent the smallest and largest observed values that are not outliers. The dotted line indicates the cutoff limit for antibody positivity.
One-third of the infants of the mothers with type 1 diabetes (25/74; 34%) tested positive for ICA, 50% (37/74) for GADA and 51% (38/74) for IA-2A in their cord blood, while 6·0% of the infants of unaffected mothers (8/134) had ICA, 2·2% (3/134) GADA and none IA-2A at birth. The ICA levels in cord blood correlated closely with those in the maternal sample taken at delivery in both pregnancies with (rs = 0·91; P < 0·001) and without (rs = 0·94; P < 0·001) type 1 diabetes, when those with detectable ICA in at least one of the samples were included (n = 28 and 8, respectively) and a significant correlation was also seen for GADA levels (rs = 0·87; P < 0·001) in the affected mother–infant pairs but not in the unaffected ones (rs = 0·10; P = 0·27). IA−2A levels correlated closely (rs = 0·97; P < 0·001) in the group comprising mother–infant pairs with type 1 diabetes, while a weaker association was found in the unaffected group (rs = 0·63; P < 0·001). There was a significant association (P < 0·001) between antibody positivity in the maternal sample taken at delivery and positivity in cord blood for all antibodies studied.
Frequency of antibody combinations
Eighteen of the mothers with type 1 diabetes tested positive for one antibody specificity in the sample taken at the end of first trimester, 23 had two antibodies and 15 had all three, as shown in Table 1. The corresponding numbers at the end of pregnancy were 19, 22 and 12, and those in the cord blood 18, 26 and 10. In the group of unaffected mothers one antibody was observed in the first sample in 12 cases and two antibodies in three. The numbers at delivery were 11 and three in the maternal samples and seven and two in the cord blood, and all three antibodies were not detected on any occasion. The single antibody specificity detected was more often ICA in the case of the unaffected mothers than among the mothers with type 1 diabetes [5/11 (46%) versus 0/18 (0%); P < 0·01], and if two antibodies were detected, these comprised ICA in combination with GADA or IA−2A in all three of the unaffected mothers and in 64% (14/22) of the mothers with type 1 diabetes.
Table 1.
Number of cases (%) testing positive for one, two or three antibodies in early pregnancy, at delivery and in cord blood in mother–infant pairs with type 1 diabetes and in unaffected mother–infant pairs
| ICA | GADA | IA−2A | ICA + GADA | ICA + IA−2A | GADA + IA−2A | ALL 3 AB | |
|---|---|---|---|---|---|---|---|
| Mothers with type 1 diabetes | |||||||
| Pregnancy | 0 (0) | 10 (14) | 8 (11) | 6 (8) | 7 (10) | 10 (14) | 15 (20) |
| Delivery | 0 (0) | 9 (12) | 10 (14) | 7 (10) | 7 (10) | 8 (11) | 12 (16) |
| Cord blood | 1 (1·4) | 9 (12) | 8 (11) | 6 (8) | 8 (11) | 12 (16) | 10 (14) |
| No maternal type 1 diabetes | |||||||
| Pregnancy | 4 (3) | 5 (4) | 3 (2) | 2 (1·5) | 1 (0·8) | 0 (0) | 0 (0) |
| Delivery | 5 (4) | 4 (3) | 2 (1·5) | 2 (1·5) | 1 (0·8) | 0 (0) | 0 (0) |
| Cord blood | 6 (4·5) | 1 (0·8) | 0 (0) | 2 (1·5) | 0 (0) | 0 (0) | 0 (0) |
Autoantibodies in the cord blood of infants with antibody-negative mothers
If the infant tested autoantibody-positive the corresponding autoantibodies were always found in maternal samples taken during pregnancy, i.e. none of the infants of antibody-negative mothers had a positive cord blood sample.
Discussion
Previous studies on diabetes-associated antibodies during pregnancy have focused mainly on insulin antibodies and antibody complexes and their effects on the pregnancy or the outcome for the infant [14,26,27]. We had the opportunity to explore the levels and frequencies of ICA, GADA and IA−2A with samples taken in the first and last trimester of the pregnancy in a fairly large cohort comprising 208 mothers, about one-third being affected by type 1 diabetes. In this context we did not analyse insulin antibodies, since in insulin-treated women these antibodies represent a humoral immune response to exogenous insulin and are accordingly not true autoantibodies. About one-third of the mothers with type 1 diabetes tested positive for ICA and approximately half had GADA and IA−2A in the two samples taken during pregnancy. These results confirm our previous findings of ICA positivity in 37% of blood samples obtained from 20 mothers with type 1 diabetes at the end of the first trimester and GADA positivity in 42% [15], and they are also in accordance with our observation that diabetes-associated autoantibodies persist for a long time after clinical diagnosis [28]. Ziegler et al. [29] reported 20% of mothers with type 1 diabetes to test positive for ICA at delivery, this lower frequency most probably due to the higher detection limit used in their assay (> 20 JDF-units). In another study, 13% of the affected mothers were found to be positive for ICA, 31% for GADA and 35% for IA−2A, but one-third of that population comprised mothers with gestational diabetes, which most probably contributed to the lower antibody frequencies observed [30]. Approximately 5% of the unaffected mothers had detectable ICA and GADA and 3% IA−2A at the end of the first trimester in the present series.
Seroconversion from autoantibody positivity to negativity and vice versa occurred during pregnancy only in cases with low initial titres and with autoantibody levels close to the cut-off limit. There was a tendency of lower levels of all the detected autoantibodies in samples taken at the time of the delivery compared to the samples taken at the end of the first trimester, but none of the differences were statistically significant. Our observations indicate that even though diabetes-associated autoantibodies can be detected in up to half of pregnancies of mothers with type 1 diabetes and in less than 10% of normal pregnancies, pregnancy itself does not have any strong modulating effect on the prevalence and titres of these autoantibodies. Normal pregnancy leads to enhanced humoral responses due to a shift in the Th1/Th2 cytokine balance favouring responses of Th2 type, but it does not seem to enhance the production of diabetes-associated autoantibodies.
All three antibody specificities were detected in cord blood at largely the same frequencies as in the maternal circulation. Roll et al. [30] detected ICA in 13% of infants of mothers with diabetes, GADA in 37% and IA−2A in 35%. As mentioned earlier, however, one-third of those mothers had gestational diabetes which probably reduced the observed prevalences. The frequency of ICA positivity seen here in the infants of the unaffected mothers represents a somewhat higher prevalence than we found recently when studying 1002 cord blood samples from infants of non-affected families (ICA 2·4%), whereas the present frequencies of GADA and IA−2A are closer to those observed in the general population (1·8% and 0·4%, respectively) [31]. On the other hand, 36 of the present unaffected mothers (27%) had a daughter or a son with type 1 diabetes, and were accordingly first-degree relatives who would be expected to have a higher frequency of diabetes-associated autoantibodies than in the general population. There was a significant correlation between the ICA levels in the infants and those in the mothers with type 1 diabetes, and also relatively close correlations between the levels of both GADA and IA−2A, but the correlations were weaker in the unaffected mother–infant pairs, which could be related to the fact that most of the readings were close to the detection limit. It can thus be concluded that if autoantibodies were present in the mother at the time of the delivery, most of them were transferred to the fetal circulation.
We did not detect any autoantibodies in the infants of autoantibody-negative mothers. This is in conflict with the study by Lindberg et al. [32], who reported the occurrence of at least one diabetes-associated antibody in cord blood in 17% of children (14/81) who subsequently developed type 1 diabetes by the age of 15 years, and the authors suggested that prepubertal diabetes may often be a consequence of intrauterinely induced autoimmunity. In that series 10% of the infants still had, however, an affected father after the exclusion of the infants of mothers with type 1 diabetes, and in addition the authors did not give the number of the affected siblings. First-degree relatives are at greater risk of progressing to clinical diabetes and would therefore be expected to have a higher frequency of disease-associated autoantibodies. Moreover, the authors did not observe the antibody-positive children to assess their postnatal antibody pattern before the diagnosis of type 1 diabetes. Ivarsson et al. [33] found that ICA and GADA positivity during pregnancy may predict diabetes in the mother but not in the offspring. The infants of unaffected mothers in the present series do not represent the normal population, as they have an affected father or sibling. The risk of such an infant of contracting type 1 diabetes during childhood is approximately 6%. No antibodies were detected in the infants of the 120 non-diabetic mothers testing negative for autoantibodies at delivery. If the autoimmune events leading to type 1 diabetes start during fetal life we would expect to find autoantibodies in 6%, i.e. seven of these infants. Our observations support the view that β-cell autoimmunity is, in most cases, induced in early infancy rather than during pregnancy [17].
The present observations indicate that pregnancy does not modulate the circulating levels of diabetes-associated autoantibodies to any significant extent, and accordingly pregnancy does not appear to induce a shift in the signs of β-cell autoimmunity towards an enhanced Th2 response. Evidence of fetal induction of β-cell autoimmunity could not be observed.
Acknowledgments
This research was supported by the European Commission DGXII, Contract no. BMH4-CT96–0233, the Finnish Medical Foundation, the Sigrid Jusélius Foundation, the Juvenile Diabetes Foundation International (grant 192612, 195003), the Liv and Hälsa Foundation, the Alma and K.A. Snellman Foundation, the Novo Nordisk Foundation and the Paediatric Research Foundation, Finland. We thank Marja Salonen and Tarja Tenkula for their excellent collaboration, and Sirpa Anttila, Susanna Heikkilä, Päivi Salmijärvi and Riitta Päkkilä for their skilful technical assistance.
The Finnish Trial to Reduce IDDM in the Genetically at Risk (TRIGR) Study Group is composed of the following members:
Principal investigator: H.K. Åkerblom.
Local investigators: V. Eskola, H. Haavisto, R. Jokisalo, A.-L. Järvenpää, U. Kaski, J. Komulainen, P. Korpela, M-L. Käär, P. Lautala, A.-M. Hämäläinen, K. Niemi, A. Nuuja, M. Renlund, M. Salo, T. Uotila, G. Wetterstrand.
Special investigators: J. Ilonen, J. Karjalainen, P. Klemetti, M. Knip, P.K. Kulmala, J. Paronen, A. Reunanen, T. Saukkonen, E. Savilahti, K. Savola, K. Teramo, O. Vaarala, S.M. Virtanen.
References
- 1.Wegmann TG, Lin H, Guilbert L, Mosmann TR. Bidirectional cytokine interactions in the maternal-fetal relationship: is successful pregnancy a Th2 phenomenon? Immunol Today. 1993;14:353–5. doi: 10.1016/0167-5699(93)90235-D. [DOI] [PubMed] [Google Scholar]
- 2.Varner MW. Autoimmune disorders and pregnancy. Semin Perinatol. 1991;15:238–50. [PubMed] [Google Scholar]
- 3.D'Armiento M, Slabe H, Betrano G, Scruccia M, Pachi A. Decrease of thyroid antibodies during pregnancy. J Endocrinol Invest. 1980;4:437–41. doi: 10.1007/BF03349385. [DOI] [PubMed] [Google Scholar]
- 4.Levy DL. Fetal neonatal involvement in maternal autoimmune diseases. Obstet Gynecol. 1982;72:596–602. doi: 10.1097/00006254-198202000-00025. [DOI] [PubMed] [Google Scholar]
- 5.El-Roey A, Shoenfeld Y. Autoimmune diseases in pregnancy. Am J Reprod Immunol Microbiol. 1985;9:25–32. doi: 10.1111/j.1600-0897.1985.tb00337.x. [DOI] [PubMed] [Google Scholar]
- 6.Wilder Rl. Hormones, pregnancy, and autoimmune diseases. Ann NY Acad Sci. 1998;840:45–50. doi: 10.1111/j.1749-6632.1998.tb09547.x. [DOI] [PubMed] [Google Scholar]
- 7.Reinhard G, Noll A, Schlebusch H, Mallman P, Ruecker AV. Shifts in the Th1/Th2 balance during human pregmancy correlate with apoptotic changes. Biochem Biophys Res Com. 1998;245:933–8. doi: 10.1006/bbrc.1998.8549. 10.1006/bbrc.1998.8549. [DOI] [PubMed] [Google Scholar]
- 8.Toivanen P, Mäntyjärvi R, Hirvonen T. Maternal antibodies in human foetal sera at different stages of gestation. Immunology. 1968;15:395–403. [PMC free article] [PubMed] [Google Scholar]
- 9.Pitcher-Wilmott RW, Hindocha P, Wood CBS. The placental transfer of IgG subclasses in human pregnancy. Clin Exp Immunol. 1980;41:303–8. [PMC free article] [PubMed] [Google Scholar]
- 10.Billington WD. The normal fetomaternal immune relationship. Baillière's Clin Obstet Gynaecol. 1992;6:417–38. doi: 10.1016/s0950-3552(05)80004-5. [DOI] [PubMed] [Google Scholar]
- 11.Omar MAK, Srikanta S, Eisenbarth GS. Human islet cell antibodies: immunoglobulin class and subclass distribution defined by monoclonal antibodies. Diabetes Res. 1987;4:155–7. [PubMed] [Google Scholar]
- 12.Lernmark Å. Molecular biology of IDDM. Diabetologia. 1994;37:73–81. doi: 10.1007/BF00400829. [DOI] [PubMed] [Google Scholar]
- 13.Tingle AJ, Lim G, Wright VJ, Dimmick JE, Hunt JA. Transplacental passage of islet cell antibody in infants of diabetic mothers. Pediatr Res. 1979;13:1323–5. doi: 10.1203/00006450-197912000-00004. [DOI] [PubMed] [Google Scholar]
- 14.Di Mario U, Fallucca F, Gargiulo P, et al. Insulin–anti-insulin complexes in diabetic women and their neonates. Diabetologia. 1984;27:83–6. doi: 10.1007/BF00275654. [DOI] [PubMed] [Google Scholar]
- 15.Martikainen A, Saukkonen T, Kulmala PK, et al. Disease-associated antibodies in offspring of mothers with IDDM. Diabetes. 1996;45:1706–10. doi: 10.2337/diab.45.12.1706. [DOI] [PubMed] [Google Scholar]
- 16.Ziegler AG, Hummel M, Schenker M, Bonifacio E. Autoantibody apppearance and risk for development of childhood diabetes in offspring of parents with type 1 diabetes: the 2-year analysis of the German BABYDIAB Study. Diabetes. 1999;48:460–8. doi: 10.2337/diabetes.48.3.460. [DOI] [PubMed] [Google Scholar]
- 17.Åkerblom HK, Savilahti E, Saukkonen T, et al. The case for elimination of cow's milk in early infancy in the prevention of Type 1 diabetes: the Finnish experience. Diabetes Metab Rev. 1993;9:269–78. doi: 10.1002/dmr.5610090407. [DOI] [PubMed] [Google Scholar]
- 18.White P. Pregnancy and diabetes, medical aspects. Med Clin N Am. 1965;49:1015. doi: 10.1016/s0025-7125(16)33292-8. [DOI] [PubMed] [Google Scholar]
- 19.Stenman U-H, Pesonen K, Ylinen K, Huhtala M-L, Teramo K. Rapid chromatographic quantitation of glycosylated haemoglobins. J Chromatogr. 1984;297:327–32. doi: 10.1016/s0021-9673(01)89052-x. [DOI] [PubMed] [Google Scholar]
- 20.Bottazzo GF, Florin-Christensen A, Doniach D. Islet-cell antibodies in diabetes mellitus with autoimmune polyendocrine deficiencies. Lancet. 1974;ii:1279–82. doi: 10.1016/s0140-6736(74)90140-8. [DOI] [PubMed] [Google Scholar]
- 21.Petersen JS, Hejnaes KR, Moody A, et al. Detection of GAD65 antibodies in diabetes and other autoimmune diseases using a simple radioligand assay. Diabetes. 1994;43:459–67. doi: 10.2337/diab.43.3.459. [DOI] [PubMed] [Google Scholar]
- 22.Sabbah E, Kulmala P, Veijola R, et al. Glutamic acid decarboxylase antibodies in relation to other autoantibodies and genetic risk markers in children with newly diagnosed insulin-dependent diabetes. J Clin Endocrinol Metab. 1996;81:2455–9. doi: 10.1210/jcem.81.7.8675560. [DOI] [PubMed] [Google Scholar]
- 23.Verge CF, Stenger D, Bonifacio E, et al. Combined use of autoantibodies (IA-2 autoantibody, GAD autoantibody, insulin autoantibody, cytoplasmic islet cell antibodies) in type 1 diabetes. Combinatorial islet cell autoantibody workshop. Diabetes. 1998;47:1857–66. doi: 10.2337/diabetes.47.12.1857. [DOI] [PubMed] [Google Scholar]
- 24.Bonifacio E, Genovese S, Bragi S, et al. Islet autoantibody markers in IDDM. risk assessment strategies yeilding high sensitivity. Diabetologia. 1995;38:816–22. doi: 10.1007/s001250050358. 10.1007/s001250050358. [DOI] [PubMed] [Google Scholar]
- 25.Savola K, Bonifacio E, Sabbah E, et al. IA−2 antibodies—a sensitive marker of IDDM with clinical onset in childhood and adolescence. Diabetologia. 1998;41:424–9. doi: 10.1007/s001250050925. [DOI] [PubMed] [Google Scholar]
- 26.Leiper JM, Fineberg SE, Lunan CB, MacCuish AC. Insulin antibodies in the maternal and foetal circulation of pregnant diabetic women treated with human insulin or recombinant DNA origin. Diabetes Res. 1984;1:75–81. [PubMed] [Google Scholar]
- 27.Mylvaganam R, Stowers JM, Steel JM, Wallace J, MacHendry JC, Wright AD. Insulin immunogenity in pregnancy: maternal and fetal studies. Diabetologia. 1983;24:19–25. doi: 10.1007/BF00275942. [DOI] [PubMed] [Google Scholar]
- 28.Savola K, Sabbah E, Kulmala P, Vähäsalo P, Ilonen J, Knip M. Autoantibodies associated with Type 1 diabetes mellitus persist after diagnosis in children. Diabetologia. 1998;41:1293–7. doi: 10.1007/s001250051067. [DOI] [PubMed] [Google Scholar]
- 29.Ziegler A-G, Hillebrand B, Rabl W, et al. On the appearance of islet associated autoimmunity in offspring of diabetic mothers: a prospective study from birth. Diabetologia. 1993;36:402–8. doi: 10.1007/BF00402275. [DOI] [PubMed] [Google Scholar]
- 30.Roll U, Christie MR, Fuchtenbusch M, Payton MA, Hawkes CJ, Ziegler A-G. Perinatal autoimmunity in offspring of diabetic parents. The German multicenter BABY-DIAB study: detection of humoral immune responces to islet antigens in early childhood. Diabetes. 1996;45:967–73. doi: 10.2337/diab.45.7.967. [DOI] [PubMed] [Google Scholar]
- 31.Martikainen A, Savola K, Kulmala P, Ilonen J, Simell O, Knip M, the DIPP Study Group IDDM-associated antibodies in cord blood samples from the general population. Eur J Endocrinol. 1997;136(Suppl.1):11. [Google Scholar]
- 32.Lindberg B, Ivarsson S-A, Landin-Olsson M, Sundkvist G, Svanberg G, Lernmark Å. Islet autoantibodies in cord blood from children who developed type 1 (insulin-dependent) diabetes mellitus before 15 years of age. Diabetologia. 1999;42:181–7. doi: 10.1007/s001250051137. 10.1007/s001250051137. [DOI] [PubMed] [Google Scholar]
- 33.Ivarsson S-A, Ackerfors M, Carlsson A, et al. Glutamate decarboxylase antibodies in non-diabetic pregnancy precedes insulin-dependent diabetes in the mother but not necessarily in the offspring. Autoimmunity. 1997;26:261–9. doi: 10.3109/08916939709008032. [DOI] [PubMed] [Google Scholar]