Abstract
Serum levels of interleukin-7 (IL-7), a non-redundant cytokine that plays a crucial role in lymphopoiesis, are known to be elevated in HIV-1-infected subjects. To examine further the association between levels of IL-7, CD4+ cell counts and viraemia, we analysed these parameters in a large cohort of HIV-1 patients along with serum levels of 90K, a marker of disease severity but with no established involvement in lymphopoiesis. While IL-7 levels were only found to correlate with CD4+ cell counts, 90K levels presented strong correlations with both CD4+ cell numbers and with plasma viral loads (VLs). These correlations were maintained in patients naive to treatment with antiretrovirals (n = 38) but were abolished when the analysis was restricted to the group receiving highly active antiretroviral therapy (HAART, n = 82). Moreover, although 90K levels were significantly reduced in patients on HAART, IL-7 levels continued to be elevated despite successful treatment. The influence of HAART on the variations in these serum parameters was further assessed in a longitudinal study on 32 subjects. The HAART-induced decrease in VLs and increase in CD4+ counts were found to correlate with a reduced serum level of 90K and IL-7, respectively. Nevertheless, following a median period of 33 months of immunological and virological successful HAART, serum levels of IL-7 continued to be significantly elevated compared with those detected in healthy controls. These findings suggest that immunotherapy with IL-7, aimed to replenish T-cell stock in HAART-treated subjects, may have a limited impact on the process of immune reconstitution.
Keywords: IL-7, 90K (Mac−2 BP), HIV-1, lymphopoiesis
INTRODUCTION
Following an asymptomatic period, HIV infection is characterized by a dramatic perturbation of T-cell homeostasis with increased T-cell destruction and decreased T-cell production. This leads to defects in cell-mediated immunity and to the development of AIDS in most patients [1]. Hence, disease progression has been evaluated by determining the changes in viral load (VL) and in the number of circulating CD4+ T cells. However, the need for other prognostic markers to accurately and rapidly monitor disease stage became apparent and has fuelled numerous studies [2–5]. On the other hand, the advent of highly active antiretroviral therapy (HAART) has considerably modified the outcome of HIV infection in developed countries, driving it from a fatal disease to a chronic infection. Nevertheless, despite suppression of viral replication, the number of CD4+ cells does not frequently return to normal level and is not notably increased in a fraction of patients [6]. In this context, markers allowing prediction or even assessment of the extent of immune recovery would be valuable.
Along with other candidates, the potential use of serum 90K and IL-7 levels as prognostic markers has been investigated before the HAART era. The 90K or Mac-2-binding protein is a 90 kDa protein secreted by different cell types, including peripheral blood mononuclear cells [7]. In HIV-1 patients, it has been shown that there is a significant correlation between 90K levels and CD4+ cell counts [8], and that elevated 90K levels and low numbers of CD4+ cells were associated with a higher incidence of AIDS [8,9]. Moreover, serum 90K level was demonstrated to be an independent predictor of the risk for disease progression even in HIV-infected patients whose CD4+ cell counts have not fallen [10]. In addition, increased levels of this molecule have been found in different diseases which are not targeting the T-cell compartment, including chronic hepatitis C [11], cirrhosis [12] and several malignancies [12,13]. On the other hand, a recent study on HIV-infected subjects has demonstrated that high levels of IL-7 in plasma were associated with low CD4+ cell counts, high viraemia and disease progression [14], thereby suggesting its possible use as a marker of disease severity. In addition, studies on the regulation of lymphopoiesis have highlighted the central role of IL-7 in T-cell development, regeneration and function in both humans and mice [15]. As an example, mice in which the IL-7 gene has been disabled show reduction in lymphoid tissue cellularity, a marked decrease in the number of thymocytes, and lymphopenia [16]. In addition, injection of IL-7 accelerates lymphocyte reconstitution in mice with cyclophosphamide- or irradiation-induced leukopenia [17,18].
Serum levels of IL-7 and 90K have mainly been studied as prognostic markers in untreated HIV-seropositive populations. Hence, in the current HAART setting, the importance of these two parameters needs to be re-evaluated. Therefore, we set out to examine the levels of IL-7 and 90K in an HIV-seropositive cohort, and also in untreated and HAART-treated groups. The levels of these two markers were then compared with those of a control, healthy population. Moreover, to better define the impact of HAART, we conducted a longitudinal study on 32 patients examining the levels of these two molecules before and after treatment. Our results show that HIV-positive patients have higher serum levels of IL-7 and 90K compared with healthy controls, and that IL-7 is correlated to CD4+ cell counts whereas 90K is correlated to CD4+ cell counts and to VL. These correlations are also observed in the untreated group, but HAART abolishes them. However, HAART only leads to a near normalization of 90K levels without notably decreasing IL-7 secretion. Nevertheless, results from the longitudinal study of HAART-treated patients show that changes in IL-7 and 90K are correlated to changes in CD4+ cell counts and VL, respectively. Furthermore, despite an efficacious and long-term HAART, levels of both IL-7 and 90K do not decrease to normal values.
PATIENTS, MATERIALS AND METHODS
Patients
A cohort of 131 HIV-positive adult patients with a documented infection period ranging from 1 to 16 years was enrolled at the Hôpital Dron in Tourcoing, France; 38 of them were naive to treatment with antiretrovirals and 82 had been receiving HAART for over 6 months. Eleven of the patients were receiving treatment with reverse transcriptase inhibitors but without protease inhibitors. A physical examination and haematological profile, including leucocyte, monocyte, lymphocyte, CD4+ and CD8+ cell counts, were assessed at each visit. Twenty age- and sex-matched healthy adult individuals were included in this study as controls. Venous blood was collected for either plasma or serum preparation and stored at − 70°C until HIV-1 RNA levels or cytokine assays were carried out. This study was approved by the local ethics committee (Lille, France).
HIV-1 RNA assay
HIV-1 RNA was measured in plasma samples using a PCR (Amplicor Monitor HIV-1; Hoffman-La-Roche, Basel, Switzerland) according to the manufacturer's instructions. The limit of detection of this assay is 200 RNA copies/ml and when levels below this cut-off were found, an arbitrary number of 199 copies/ml was attributed to the tested samples.
Cytokine assays
Quantification in serum samples of IL-7 and 90K levels was done using commercially available ELISA kits and according to the manufacturers' instructions. Free IL-7, and IL-7 bound to carrier protein or to soluble IL-7 receptor, were measured using the Quantikine HS human IL-7 kit (R & D Systems, Abingdon, UK), and 90K/Mac-2 binding protein using the Bender MedSystems kit (MedSystems Diagnostics GmbH, Vienna, Austria).
Statistical analysis
Pearson product moment correlation coefficients or Spearman rank-order correlation coefficients (for n = 30) were calculated to evaluate associations between any two continuous variables. Multivariate analysis was performed after log10 transformation of VL. Predictor variables, including log10VL, leucocyte, monocyte, lymphocyte, CD4+ and CD8+ cell counts, were considered by two different methods to perform regression. Firstly, we used the best subset regression method to determine which independent variables best contributed to the prediction of IL-7 or 90K levels. Thereafter, we used the multiple linear regression method to fit the equation model that most closely described the actual data. Differences between subgroups were tested using the Mann–Whitney U Rank test. Statistical analyses of the differences in IL-7 or 90K levels observed before and after HAART onset were carried out using the Wilcoxon Signed-Rank test. P-values < 0·05 were considered statistically significant. All statistical analyses were performed using SigmaStat statistical software version 2·0 (SPSS Inc., Chicago, IL, USA).
Flow cytometry
The percentage of circulating naive (CD45RA+CD62L+CD4+) T cells was determined by flow cytometry (FACSCalibur) using the Lymphocyte Immuno Kit (Pharmingen, BD Biosciences, Life Science Research Europe, Heidelberg, Germany) and blood samples collected on EDTA from 42 HIV-infected subjects (17 naive to treatment and 25 on HAART). Live cells were gated on their forward and side light scatter characteristics, and percentage of positive cells as well as mean fluorescence intensity were recorded and analysed using CellQuest software (Becton Dickinson).
RESULTS
Cross-sectional analysis
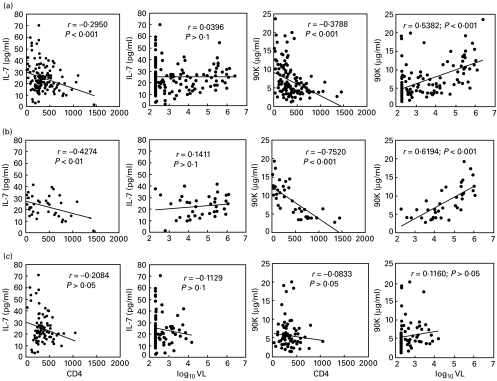
For this study, we enrolled 20 uninfected healthy controls and 131 HIV-positive patients of whom 38 were naive to treatment and 82 were under HAART for > 6 months (range, 6–44 months). The characteristics of the populations are summarized in Table 1. Due to the large variation in VL among patients, median values were selected to better reflect the level of viraemia. Of note, the untreated but infected group comprised patients about to undergo HAART and asymptomatic patients and thus, the median VL was higher in this group than in the HAART-treated one. We first compared the serum levels of IL-7 and of 90K in the HIV-seropositive cohort and in the control, uninfected population. As shown in Table 1, there was a higher level of both proteins in the infected population. These differences were also maintained in the untreated HIV-positive population. Moreover, even after HAART, HIV-seropositive patients still demonstrated nearly threefold higher levels of IL-7 compared with the healthy population and, although 90K levels were lower in this group compared with the untreated one, they did not return to normal levels. We then analysed the potential association of IL-7 and 90K levels with biological parameters relevant to HIV pathology such as VL, leucocyte, lymphocyte and monocyte counts. In the whole HIV cohort, multivariate analysis showed that only CD4+ cell counts were associated with IL-7 serum levels (P < 0·001, R2 = 0·087) whereas 90K levels were associated with CD4+ cell counts and VL (P = 0·002 and P < 0·001, respectively, R2 = 0·34). Furthermore, as shown by univariate analysis, there was a strong negative correlation between the number of CD4+ lymphocytes and the serum level of both IL-7 and 90K (Fig. 1a). Interestingly, we determined the number of naive CD4+ T cells by flow cytometry according to expression of the cell surface markers CD4+, CD45RA+ and CD62L+ in a subset of the infected population (n = 42) and failed to detect any correlation between 90K or IL-7 levels in this T-cell compartment (not shown). On the other hand, in the overall infected population, there was a strong correlation between the levels of 90K and VL (Fig. 1a) whereas IL-7 levels were not significantly correlated to this parameter. In addition, the only other significant correlation found was between IL-7 levels and total lymphocyte counts (r = − 0·251, P < 0·01). The same analyses were performed in the untreated and HAART-treated groups. In the untreated group, multivariate analysis showed that IL-7 was associated with CD4+ cell counts (P = 0·007, R2 = 0·183), and 90K was associated with CD4+ cell counts and with VL (P = 0·003 and P = 0·035, respectively, R2 = 0·618). Univariate analysis showed that CD4+ cell counts negatively correlated with IL-7 and 90K levels whereas VL correlated positively with 90K levels but not with those of IL-7 (Fig. 1b). In contrast, in the treated group, multivariate analysis did not detect any association between either IL-7 or 90K levels and CD4+ cell counts and/or VL. However, in this group IL-7 was associated with total lymphocyte counts and 90K with leucocyte counts (r = − 0·241, P = 0·031 and r = 0·246, P = 0·026, respectively). Accordingly, we did not find any statistically significant correlation between IL-7 or 90K levels and CD4+ cell counts or VL in this group (Fig. 1c). In conclusion, HIV-positive patients have higher IL-7 and 90K levels compared with healthy controls, and HAART does not appear to correct these elevated levels to normal values despite a generally low plasma VL.
Table 1.
Cohort characteristics
| IL-7 | 90K | Viral load | CD4 | CD8 | Lymphocyte | Age | ||
|---|---|---|---|---|---|---|---|---|
| Cohort | n | (pg/ml) | (µg/ml) | (copies/ml) | (cells/µl) | (cells/µl) | (cells/µl) | (years) |
| Healthy | 20 | 8·6 ± 1·4† | 3·7 ± 0·4 | – | ND | ND | ND | 35 ± 4 |
| controls | (0·8–25·7)‡ | (1·3–6·53) | (26–48) | |||||
| All HIV+ | 131 | 24·6 ± 1·1* | 7·1 ± 0·4* | 646§ | 368 ± 22 | 851 ± 37 | 1632 ± 57 | 39 ± 1 |
| patients | (1·47–70·3) | (1·3–23·6) | (199–2454 709) | (4–1457) | (57–2410) | (151–3405) | (21–69) | |
| Untreated-HIV+ | 38 | 22·5 ± 1·5* | 8·4 ± 0·7* | 33 195 | 445 ± 61 | 846 ± 75 | 1690 ± 126 | 37 ± 2 |
| patients | (1·4–41·7) | (2·8–19·2) | (199–1178 588) | 445(4–1457) | (57–2410) | (151–3405) | 2(21–64) | |
| HAART-treatedHIV+ | 82 | 24·6 ± 1·5* | 5·8 ± 0·4*¶ | 199§ | 359 ± 19 | 856 ± 46 | 1642 ± 67 | 41 ± 1¶ |
| HIV+ patients | (3·2–70·3) | (1·3–20) | (199–16 203) | (30–1050) | (255–1962) | (544–3129) | (25–69) |
ND, not done; HAART, highly active antiretroviral therapy.
Mean ± s.e.m.
Range.
Median.
Significantly different values versus healthy controls (P < 0·01, Mann Whithey U rank test).
Significantly different values versus untreated-HIV + patients (P < 0·05, Mann Whithey U rank test).
Fig. 1.
Correlation between IL-7 or 90K serum levels, and CD4+ cell counts or viral loads (VL), in (a) HIV-infected patients (n = 131), (b) untreated HIV-infected patients (n = 38) and (c) HIV-infected patients treated with highly active antiretroviral therapy (HAART) for at least 6 months (n = 82). Each significant correlation was determined using Pearson's correlation coefficient (r) and P < 0·05. The continuous line represents the linear regression.
Intra-group comparisons
In order to determine the extent of association between IL-7 or 90K and CD4+ cell counts or VL, we divided the different infected populations according to CD4+ cell counts or VL and compared the levels of IL-7 and 90K in the subgroups. The untreated cohort was divided into two subgroups defined by the median VL: those with high (≥ 33 195 copies/ml) and those with low (< 33 195) VL. Of note, the median VL was close to 30 000 copies/ml, the accepted cut-off point in France above which HAART is initiated. Incidentally, the high VL subgroup also had significantly lower CD4+ and CD8+ cell counts, indicating a more advanced disease stage. Moreover, an identical stratification was obtained whether median CD4+ counts or median VL was taken into account. In both subgroups, the circulating levels of IL-7 and 90K were significantly higher than in the control population (Table 2). However, IL-7 levels were not different between the two subgroups, in contrast to 90K levels which were significantly higher in the subgroup with high viraemia, thus suggesting that high 90K levels were linked to more advanced disease. In the high viraemia subgroup, we did not find any significant correlation between IL-7 or 90K levels and CD4+ cell counts or VL. These correlations were as follows: IL-7 and CD4+ counts, r = − 0·583 and P = 0·129; IL-7 and VL, r = 0·344 and P = 0·159; 90K and CD4+ counts, r = − 0·312 and P = 0·201; 90K and VL, r = 0·45 and P = 0·059. In contrast, in the low viraemia subgroup, IL-7 and 90K levels were found to be correlated only with CD4+ counts (r = − 0·583, P = 0·011 and r = − 0·56, P = 0·0154, respectively) whereas correlations between these markers and VL were not significant (r = − 0·148, P = 0·552 and r = 0·099, P = 0·686, respectively). Next, we carried out these types of analysis on the HAART-treated group, which was divided according to the clinical criteria used to assess treatment efficacy. At first, this cohort was divided into two subgroups defined by VL: those with undetectable viraemia (i.e. < 200 copies/ml) and those with VL above the detection threshold. Of note, the two subgroups had similar CD4+ and CD8+ cell counts. As shown in Table 2, there was no statistical difference in IL-7 levels between these subgroups, although the low VL subgroup had significantly lower levels of circulating 90K. The only significant correlation found was between IL-7 levels and CD4+ counts in the low viraemia subgroup (r = − 0·374, P = 0·011). Moreover, when the HAART-treated group was divided into high (≥ 300/µl) and low CD4+ cell counts, we found no difference in either IL-7 or 90K levels between the two subgroups, although the levels of both proteins tended to be lower in the high CD4+ cell count subgroup. In addition, we found no significant correlation between IL-7 or 90K, and CD4+ counts or VL, in any of the subgroups (P > 0·05). Of note, the lowest levels of 90K were detected in the HAART-treated population with both virological and immunological successes (i.e. < 200 copies/ml and CD4+ counts ≥ 300/µl, not shown), and these levels were not significantly different from those of the healthy population. Thus, successful HAART restores 90K to near normal levels but does not significantly affect IL-7.
Table 2.
Intra-group comparisons
| IL-7 | 90K | Viral load | CD4 | CD8 | Age | HAART | ||
|---|---|---|---|---|---|---|---|---|
| Cohort | n | (pg/ml) | (µg/ml) | (copies/ml) | (cells/µl) | (cells/µl) | (years) | (months) |
| Untreated-HIV+ patients | ||||||||
| VL < 33 195 | 18 | 21·0 ± 2·5† | 5·2 ± 0·4 | 7315§ | 760 ± 70 | 1047 ± 119 | 35 ± 2 | – |
| (1·47–39·6)‡ | (2·8–8·6) | (199–26 938) | (438–1457) | (356–2410) | (21–55) | – | ||
| (P > 0·05)¶ | (P < 0·001) | (P < 0·001) | (P < 0·001) | (P = 0·020) | (P > 0·05) | – | ||
| VL ≥ 33 195 | 18 | 24·5 ± 1·9 | 11·7 ± 0·8 | 276 081 | 139 ± 21 | 677 ± 83 | 39 ± 3 | – |
| (11·9–41·7) | (6·3–19·2) | (39 452–1178 588) | (4–303) | (57–1400) | (25–64) | – | ||
| HAART-treated | ||||||||
| HIV+ patients | ||||||||
| Undetectable VL (< 200) | 46 | 26·0 ± 2·0 | 5·2 ± 0·5 | 199 | 382 ± 28 | 806 ± 62 | 41 ± 2 | 23·4 ± 2 |
| ((3·2–59·8) | (1·3–19) | (199–199) | (83–1050) | (255–1962) | (25–69) | (6–44) | ||
| (P > 0·05) | (P = 0·036) | (P < 0·001) | (P > 0·05) | (P > 0·05) | (P > 0·05) | (P > 0·05) | ||
| Detectable VL (≥ 200) | 36 | 22·9 ± 2·2 | 6·5 ± 0·6 | 915 | 329 ±23 | 919 ± 68 | 40 ± 1 | 21·8 ± 2 |
| (4·1–70·3) | (1·8–20) | (210–16 203) | (30–808) | (305–1841) | (25–52) | (6–42) | ||
| HAART-treated HIV+ patients | ||||||||
| CD4 < 300 | 38 | 26·6 ± 2·9 | 6·2 ± 0·6 | 199 | 225 ± 11 | 867± 72 | 43 ± 2 | 26·7 ± 2 |
| (3·2–70·3) | (1·8–19) | (199–16 203) | (30–291) | (255–1962) | (27–69) | (6–43) | ||
| (P > 0·05) | (P > 0·05) | (P > 0·05) | (P < 0·001) | (P > 0·05) | (P > 0·05) | (P = 0·006) | ||
| CD4 ≥ 300 | 44 | 23·0 ± 1·2 | 5·4 ± 0·6 | 199 | 474 ± 22 | 845 ± 60 | 39 ± 1 | 19·0 ± 2 |
| (9·5–42) | (1·3–20) | (199–6965) | (312–1050) | (305–1937) | (25–58) | (6–44) | ||
HAART, highly active antiretroviral therapy; VL, viral load.
Mean ± s.e.m.
Range.
Median.
Mann–Whitney U rank test.
Longitudinal study
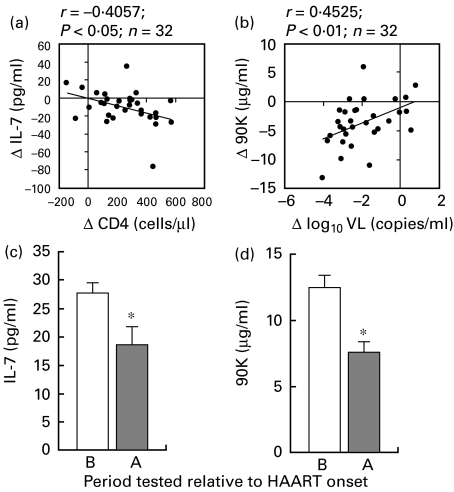
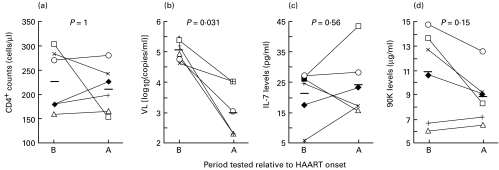
We next investigated the influence of HAART on the correlation between IL-7 or 90K levels and CD4+ cell counts in a longitudinal study. To this aim, 32 patients were enrolled at HAART onset, 19 of whom were naive to any treatment and 13 of whom were taking antiretrovirals devoid of protease inhibitors, and were followed for 4–45 months (32 months median follow-up). At the time of enrolment, the patients presented low CD4+ cell counts (150 cells/µl ± 17, mean ± s.e.m.) along with high viraemia (262 670 median copies/ml) and at the end of the study, there was a significant increase in CD4+ cell counts (398 cells/µl ± 34, P < 0·001) and decrease in viraemia (1087 median copies/ml, P < 0·001) subsequent to HAART. We analysed the variations in IL-7 or 90K levels, and the variations in CD4+ cell counts or VL, during the course of this study. We found that the variation in IL-7 levels was only correlated to the variation in CD4+ cell counts (r = − 0·4057, Fig. 2a), whereas there was a positive correlation between the variation in 90K levels and that of VL (r = 0·4525, Fig. 2b). To ascertain the effect of HAART, we first focused our analysis on those patients who responded most to treatment. Thus, we studied the variation in IL-7 and 90K levels in a subgroup of 19 patients defined by an increase in CD4+ cells of over 100 cells/µl and a decrease in the VL of 2log10 copies/ml. At the end of this study, these patients had been treated with HAART for a median length of time of 33 months; the mean increase in CD4+ cell counts was 286 cells/µl (± 29, s.e.m.) and the mean decrease in VL was 3log10 copies/ml (± 0·1, s.e.m.). As shown in Fig. 2c and 2d, there was a significant decrease in both IL-7 and 90K levels at the end of the study compared with HAART onset. However, these levels were still significantly higher than those in healthy controls (P < 0·05). We next examined the variation of IL-7 and 90K levels in six patients treated for a median length of time of 20 months and in whom CD4+ cell counts did not increase > 50 cells/µl despite a good suppression of viral replication (Fig. 3). Although not statistically significant, there was a trend towards decreased 90K levels while IL-7 levels remained almost unchanged. Thus, in the absence of a considerable increase in CD4+ cell number, no measurable decrease in IL-7 levels could be detected.
Fig. 2.
Longitudinal study on the changes in serum IL-7 and 90K levels before and after highly active antiretroviral therapy (HAART) onset. Correlation (a) between changes (Δ) in IL-7 serum levels and changes in CD4+ cell counts, and (b) between changes in 90K serum levels and changes in log10 viral load (VL). Thirty-two HIV-infected patients were followed for a median period of 32 months after initiation of HAART. IL-7 and 90K levels were determined before and after HAART onset. Significant correlations were determined using Pearson's correlation coefficient (r) and P < 0·05. The continuous line represents the linear regression. Effect of an efficacious HAART on (c) IL-7 levels and (d) 90K levels. IL-7 and 90K levels were determined before (B) and after (A) HAART onset in 19 of the 32 patients whose CD4+ counts increased > 100 cells/µl and VL decreased > 2log10 copies/ml. Bars represent mean ± s.e.m., and * indicates P < 0·05 using Wilcoxon Signed-Rank test.
Fig. 3.
Changes in (a) CD4+ cell counts, (b) viral load (VL), (c) IL-7 and (d) 90K levels before (B) and after (A) treatment with highly active antiretroviral therapy (HAART). Patients were selected as those who had not had increases in CD4+ cell numbers above 50 cells/µl following a median period of 20 months of HAART, and despite a significant control of viral replication. P-values were determined using Wilcoxon Signed-Rank test and are shown above each graph (n =6).
DISCUSSION
As the non-redundant cytokine IL-7 is involved in lymphopoiesis, it may play a crucial role in diseases that are characterized by depletion of the T-cell pool, such as HIV infection. Recently, an association between higher levels of IL-7 and low CD4+ cell counts, high viraemia and disease progression has been described [14]. To examine this association further, we determined the levels of this cytokine in the HIV-infected population and those of 90K, a marker of disease severity not involved in lymphopoiesis [8]. Our results show that IL-7 and 90K levels are correlated with CD4+ cell counts, and only 90K levels are correlated with VL. Furthermore, our study on HAART-treated patients demonstrates that even in those patients responding best to treatment, IL-7 levels do not return to normal values. In this study, we extend to a HAART-treated group the previously-reported finding that 90K levels are elevated in the HIV-infected population [8,9]. Although the use of HAART leads to decreased 90K levels compared with the untreated population, it does not seem to be sufficiently efficient to normalize the level of this serum marker. The 90K protein has been suggested to play a role in innate immunity through its capacity to stimulate natural killer cells [19], and to induce the production of IL-1 and IL-6 by human monocytes [20]. This is corroborated by the fact that mice in which the gene coding for the 90K homologue has been deleted are more susceptible to the lethal effect of the bacterial cell-wall component endotoxin [21]. In addition, this protein is produced constitutively by peripheral blood mononuclear cells in humans and by macrophages in mice. Thus, higher 90K levels in HIV infection may well reflect the activation of the innate arm of the immune system. This would provide an explanation for the marked decrease in 90K levels observed in patients successfully treated by HAART, as progressive deactivation of the innate immune system has been observed in this type of patient [22]. On the other hand, our findings demonstrated that IL-7 levels are higher in the HIV-infected population compared with healthy controls. It should be noted that the healthy controls in the present study had higher IL-7 serum levels than those reported by the ELISA kit manufacturer (8·6 pg/ml versus 2·2 pg/ml). At the present time, the reason for this difference is not clear. Nevertheless, the healthy population matched the HIV-positive cohort with respect to age, gender and geographical location. Furthermore, both HAART-treated and untreated groups presented higher serum IL-7 levels compared with controls. However, there was no difference in IL-7 levels between these two groups of HIV-infected patients. This could be explained by the absence of a significant difference in CD4+ cell counts between the HAART-treated and untreated groups, further confirming that the strongest predictor of IL-7 levels is CD4+ count. Recently, a group reported that HIV-infected children have higher IL-7 levels than their healthy counterparts and that non-infected children who have been exposed to HIV in utero present intermediate levels of IL-7 as well as intermediate CD4+ cell counts [23]. In addition, increased levels of circulating IL-7 have already been observed in a number of pathologies related to a defect in lymphocyte homeostasis, such as adult Hodgkin's lymphoma [24]. Importantly, children with marked lymphopenia due either to severe combined immunodeficiency or acute lymphoblastic leukaemia present high circulating levels of IL-7, whereas those with acute non-lymphoblastic leukaemia have normal IL-7 levels [25]. Nevertheless, following bone marrow transplant, these levels drop as the number of total lymphocytes increases [25]. Thus, as already pointed out [14], the high IL-7 levels detected in HIV-infected patients may be due to a compensatory mechanism aimed at restoring the normal lymphocyte homeostasis.
Univariate analysis demonstrated that in the overall HIV-infected population and in the untreated group, 90K levels were only correlated with VL whereas IL-7 levels were negatively correlated with CD4+ cell counts but not with VL. This latter result is at variance with a previous report claiming that IL-7 levels were also correlated with VL [14]. The lack of correlation between IL-7 and VL in our cohort could be explained by the high number of patients with undetectable viraemia (about 35% of the cohort compared with 19% [14]). However, this hypothesis has to be ruled out as (i) there is a significant correlation between 90K levels and VL in our cohort, and (ii) there is no correlation between IL-7 and VL even in the untreated group. Furthermore, in patients under HAART we do not find correlation between any of the parameters assayed. The lack of correlation between 90K levels and either CD4+ cell counts or VL in HAART-treated patients reinforces the hypothesis that 90K is a marker of infection, as tight control of viral replication results in decreased activation of the immune system. However, it is not clear at present why we do not observe any correlation between IL-7 and CD4+ cell counts in this group, as even elimination of the 10 patients with odd values in either of the two parameters did not modify the outcome of the analysis.
Stratification of the untreated and HAART-treated populations according to VL showed that 90K levels were significantly lower in the low viraemia subgroup of both populations, thus confirming the relationship between VL and 90K levels. With respect to IL-7 levels, we did not find any significant difference whatever the stratified groups considered. This may be due to small sample size along with high variation in IL-7 levels among patients.
The longitudinal study provided further insight into the relationship between IL-7 or 90K and CD4+ cell counts or VL. We found a negative correlation between the changes in IL-7 levels and the changes in CD4+ cell counts, and a positive correlation between changes in 90K levels and changes in VL. In addition, patients had lower IL-7 and 90K levels after initiation of a successful HAART. This is in contradiction to results from the intra-group comparison study, and may be due to the fact that longitudinal studies are less sensitive to the well known inter-individual variation in cytokine production than cross-sectional studies. More importantly, the hypothesis of an association between IL-7 levels and CD4+ cell counts is reinforced by our finding that in HAART-treated patients whose CD4+ cell counts have not increased despite a marked decrease in viral replication, IL-7 levels remain elevated.
Infection with HIV-1 leads to depletion of the CD4+ T-cell pool. Although the exact mechanisms governing T-cell production are complex and subject to controversy, recent studies have proposed that HIV-1 infection inhibits T-cell production. Indeed, CD4+ cells from untreated HIV-positive patients have lower half-lives than, but their production rate is identical to those of healthy controls, thus suggesting that T-cell homeostasis is perturbed [26,27]. Also, suppression of viral replication following HAART often results in increased T-cell production [26–28]. However, this increase is short-lived, as higher rates of T-cell production are no longer observed in patients under HAART for over a year [27]. Thus, these and our results suggest that following suppression of HIV replication under HAART, the high IL-7 serum levels may trigger the increase in T-cell production. This subsequently leads to a decrease in IL-7 levels due either to down-regulation of its production, or to a higher number of available receptors. This hypothesis is substantiated by the recent finding that IL-7 increases both thymic-dependent and -independent T-cell regeneration [29]. Nevertheless, in long-term HAART-treated patients, IL-7 levels remain high compared with healthy controls and CD4+ cell expansion has stopped. Among other hypotheses, infection with HIV could result in impaired function of the virus receptor CD4, which has been shown to play a role in T helper-cell survival and homeostasis in mice [30]. Alternatively, it may be possible that IL-7 acts on a particular, yet unidentified, subset of CD4+ lymphocytes. Depletion of this subset during infection would provide an explanation for the lack of increase in CD4+ cells observed in some patients, despite good virological control and high IL-7 levels. It may also explain why children exposed to HIV in utero have lower CD4+ cell counts and higher levels of this cytokine several years after exposure compared with healthy controls [23]. Also, it has been shown that lymphocytes from HIV-infected individuals are less responsive to IL-2 and IL-7 and therefore, this could contribute to the immune dysfunction observed in these patients [31]. Besides, IL-7 immunotherapy has recently been proposed as a way of restoring the immune response in HIV patients, as this cytokine restores immunity in athymic T cell-depleted mice when administered along with a limited number of T cells [32]. However, compared with mice, lymphopoiesis in humans may be a more complex process involving several factors. In addition, our findings that HAART-treated patients continue to present high IL-7 serum levels despite clear and measurable clinical benefits do not argue in favour of such an approach.
Acknowledgments
This work was supported by a research grant from the Association Stop SIDA, France. We are grateful to Professor A. Landay for critical revue of this manuscript.
References
- 1.Andrews CA, Koup RA. The immunopathology of HIV infection. J Antimicrob Chemother. 1996;37(Suppl.):13–25. doi: 10.1093/jac/37.suppl_b.13. [DOI] [PubMed] [Google Scholar]
- 2.Gattegno L, Bentata-Peyssare M, Gronowski S, Chaouche K, Ferriere F. Elevated concentrations of circulating intercellular adhesion molecule 1 (ICAM-1) and of vascular cell adhesion molecule 1 (VCAM-1) in HIV-1 infection. Cell Adhes Commun. 1995;3:179–85. doi: 10.3109/15419069509081285. [DOI] [PubMed] [Google Scholar]
- 3.Casoli C, Lisa A, Magnani G, et al. Prognostic value of adenosine deaminase compared to other markers for progression to acquired immunodeficiency syndrome among intravenous drug users. J Med Virol. 1995;45:203–10. doi: 10.1002/jmv.1890450216. [DOI] [PubMed] [Google Scholar]
- 4.Churchill DR. Prognostic markers and surrogate markers of clinical progression in HIV infection. Int J STD AIDS. 1997;8:552–6. doi: 10.1258/0956462971920776. 557. [DOI] [PubMed] [Google Scholar]
- 5.Zeller JM, McCain NL, Swanson B. Immunological and virological markers of HIV-disease progression. J Assoc Nurses AIDS Care. 1996;7:15–27. doi: 10.1016/S1055-3290(96)80034-3. [DOI] [PubMed] [Google Scholar]
- 6.Grabar S, Moing VL, Goujard C, et al. Clinical outcome of patients with HIV-1 infection according to immunologic and virologic response after 6 months of highly active antiretroviral therapy. Ann Intern Med. 2000;133:401–10. doi: 10.7326/0003-4819-133-6-200009190-00007. [DOI] [PubMed] [Google Scholar]
- 7.Fusco O, Querzoli P, Nenci I, et al. 90K (MAC−2 BP) gene expression in breast cancer and evidence for the production of 90K by peripheral-blood mononuclear cells. Int J Cancer. 1998;79:23–6. doi: 10.1002/(sici)1097-0215(19980220)79:1<23::aid-ijc5>3.0.co;2-y. 10.1002/(sici)1097-0215(19980220)79:1<23::aid-ijc5>3.3.co;2-n. [DOI] [PubMed] [Google Scholar]
- 8.Briggs NC, Natoli C, Tinari N, D'Egidio M, Goedert JJ, Iacobelli S. A 90-kDa protein serum marker for the prediction of progression to AIDS in a cohort of HIV-1+ homosexual men. AIDS Res Hum Retroviruses. 1993;9:811–6. doi: 10.1089/aid.1993.9.811. [DOI] [PubMed] [Google Scholar]
- 9.Natoli C, Dianzani F, Mazzotta F, et al. 90K protein: a new predictor marker of disease progression in human immunodeficiency virus infection. J Acquir Immune Defic Syndr. 1993;6:370–5. [PubMed] [Google Scholar]
- 10.Iacobelli S, Ullrich A, Tinari N, et al. The 90K tumor-associated antigen and clinical progression in human immunodeficiency virus infection. J Acquir Immune Defic Syndr Hum Retrovirol. 1995;10:450–6. doi: 10.1097/00042560-199512000-00008. [DOI] [PubMed] [Google Scholar]
- 11.Artini M, Natoli C, Tinari N, et al. Elevated serum levels of 90K/MAC−2 BP predict unresponsiveness to alpha-interferon therapy in chronic HCV hepatitis patients. J Hepatol. 1996;25:212–7. doi: 10.1016/s0168-8278(96)80076-6. [DOI] [PubMed] [Google Scholar]
- 12.Correale M, Giannuzzi V, Iacovazzi PA, et al. Serum 90K/MAC−2 BP glycoprotein levels in hepatocellular carcinoma and cirrhosis. Anticancer Res. 1999;19:3469–72. [PubMed] [Google Scholar]
- 13.Iacobelli S, Arno E, Sismondi P, et al. Measurement of a breast cancer associated antigen detected by monoclonal antibody SP-2 in sera of cancer patients. Breast Cancer Res Treat. 1988;11:19–30. doi: 10.1007/BF01807554. [DOI] [PubMed] [Google Scholar]
- 14.Napolitano LA, Grant RM, Deeks SG, et al. Increased production of IL-7 accompanies HIV-1-mediated T-cell depletion: implications for T-cell homeostasis. Nat Med. 2001(7):73–9. doi: 10.1038/83381. [DOI] [PubMed] [Google Scholar]
- 15.Hofmeister R, Khaled AR, Benbernou N, Rajnavolgyi E, Muegge K, Durum SK. Interleukin-7: physiological roles and mechanisms of action. Cytokine Growth Factor Rev. 1999;10:41–60. doi: 10.1016/s1359-6101(98)00025-2. 10.1016/s1359-6101(98)00025-2. [DOI] [PubMed] [Google Scholar]
- 16.von Freeden-Jeffry U, Vieira P, Lucian LA, McNeil T, Burdach SE, Murray R. Lymphopenia in interleukin (IL)-7 gene-deleted mice identifies IL-7 as a nonredundant cytokine. J Exp Med. 1995;181:1519–26. doi: 10.1084/jem.181.4.1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Morrissey PJ, Conlon P, Braddy S, Williams DE, Namen AE, Mochizuki DY. Administration of IL-7 to mice with cyclophosphamide-induced lymphopenia accelerates lymphocyte repopulation. J Immunol. 1991;146:1547–52. [PubMed] [Google Scholar]
- 18.Faltynek CR, Wang S, Miller D, et al. Administration of human recombinant IL-7 to normal and irradiated mice increases the numbers of lymphocytes and some immature cells of the myeloid lineage. J Immunol. 1992;149:1276–82. [PubMed] [Google Scholar]
- 19.Ullrich A, Sures I, D'Egidio M, et al. The secreted tumor-associated antigen 90K is a potent immune stimulator. J Biol Chem. 1994;269:18401–7. [PubMed] [Google Scholar]
- 20.Powell TJ, Schreck R, McCall M, et al. A tumor-derived protein which provides T-cell costimulation through accessory cell activation. J Immunother Emphasis Tumor Immunol. 1995;17:209–21. doi: 10.1097/00002371-199505000-00003. [DOI] [PubMed] [Google Scholar]
- 21.Trahey M, Weissman IL. Cyclophilin C-associated protein: a normal secreted glycoprotein that down-modulates endotoxin and proinflammatory responses in vivo. Proc Natl Acad Sci USA. 1999;96:3006–11. doi: 10.1073/pnas.96.6.3006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cooper DA. Immunological effects of antiretroviral therapy. Antivir Ther. 1998;3:19–23. [PubMed] [Google Scholar]
- 23.Clerici M, Saresella M, Colombo F, et al. T-lymphocyte maturation abnormalities in uninfected newborns and children with vertical exposure to HIV. Blood. 2000;96:3866–71. [PubMed] [Google Scholar]
- 24.Trumper L, Jung W, Dahl G, Diehl V, Gause A, Pfreundschuh M. Interleukin-7, interleukin-8, soluble TNF receptor, and p53 protein levels are elevated in the serum of patients with Hodgkin's disease. Ann Oncol. 1994;5:93–6. doi: 10.1093/annonc/5.suppl_1.s93. [DOI] [PubMed] [Google Scholar]
- 25.Bolotin E, Annett G, Parkman R, Weinberg K. Serum levels of IL-7 in bone marrow transplant recipients: relationship to clinical characteristics and lymphocyte count. Bone Marrow Transplant. 1999;23:783–8. doi: 10.1038/sj.bmt.1701655. [DOI] [PubMed] [Google Scholar]
- 26.Hellerstein M, Hanley MB, Cesar D, et al. Directly measured kinetics of circulating T lymphocytes in normal and HIV-1-infected humans. Nat Med. 1999;5:83–9. doi: 10.1038/4772. 10.1038/4772. [DOI] [PubMed] [Google Scholar]
- 27.McCune JM, Hanley MB, Cesar D, et al. Factors influencing T-cell turnover in HIV-1-seropositive patients. J Clin Invest. 2000;105:R1–8. doi: 10.1172/JCI8647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fleury S, de Boer RJ, Rizzardi GP, et al. Limited CD4+ T-cell renewal in early HIV-1 infection: effect of highly active antiretroviral therapy. Nat Med. 1998;4:794–801. doi: 10.1038/nm0798-794. [DOI] [PubMed] [Google Scholar]
- 29.Mackall CL, Fry TJ, Bare C, Morgan P, Galbraith A, Gress RE. IL-7 increases both thymic-dependent and thymic-independent T-cell regeneration after bone marrow transplantation. Blood. 2001;97:1491–7. doi: 10.1182/blood.v97.5.1491. [DOI] [PubMed] [Google Scholar]
- 30.Strong J, Wang Q, Killeen N. Impaired survival of T helper cells in the absence of CD4. Proc Natl Acad Sci USA. 2001;98:2566–71. doi: 10.1073/pnas.051329698. 10.1073/pnas.051329698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vingerhoets J, Bisalinkumi E, Penne G, et al. Altered receptor expression and decreased sensitivity of T-cells to the stimulatory cytokines IL-2, IL-7 and IL-12 in HIV infection. Immunol Lett. 1998;61:53–61. doi: 10.1016/s0165-2478(97)00162-4. 10.1016/s0165-2478(97)00162-4. [DOI] [PubMed] [Google Scholar]
- 32.Fry TJ, Christensen BL, Komschlies KL, Gress RE, Mackall CL. Interleukin-7 restores immunity in athymic T-cell-depleted hosts. Blood. 2001;97:1525–33. doi: 10.1182/blood.v97.6.1525. [DOI] [PubMed] [Google Scholar]