Abstract
In order to elucidate the immunological properties of anti-U1-ribonucleoprotein (RNP) antibody, one of the autoantibodies detected in patients with connective tissue diseases (CTDs), we tested the endothelial cell-binding by anti-U1-RNP antibodies and epitopes on human pulmonary artery endothelial cells (HPAECs) to which the autoantibody bound. IgG fractions positive for anti-U1-RNP from patients with CTDs bound to the HPAECs. Furthermore, intact and F(ab′)2 IgG anti-U1-RNP purified by affinity chromatography also bound to endothelial cells. The binding activity of IgG fractions positive for anti-U1-RNP to the endothelial cells could be effectively absorbed by U1-RNP-Sepharose. An immunoblotting assay of purified IgG anti-U1-RNP antibodies showed that these antibodies could bind to various membrane proteins of NP40-treated HPAECs such as 68, 48, 43, 38, 33, 29, 28 and 24 kDa. Some bands, 68, 33, 28 and 24 kDa, seemed to correspond to components of U1-RNP, i.e. 68 kDa, A, B′ and C peptides, respectively. We confirmed that the anti-U1-RNP antibody from patients with CTDs can directly recognize a variety of antigens on the endothelial surface of the pulmonary artery, including the components of U1-RNP or other unknown polypeptides. These results suggest that binding to pulmonary artery endothelial cells of this autoantibody may be one of the triggers of endothelial cell inflammation in CTDs.
Keywords: anti-U1-ribonucleoprotein antibody, endothelial all-binding, connective tissue disease
Introduction
Autoantibodies against U1-ribonucleoprotein (RNP) have been reported to be present characteristically in sera from patients with mixed connective tissue disease (MCTD) [1]. Although anti-U1-RNP antibodies are also detected in the sera of other connective tissue disease (CTD) patients [2], their roles in the pathogenesis of CTD are virtually unknown. As well as autoantibodies against U1-RNP, autoantibodies against negatively-charged molecules such as cardiolipin and DNA have been suggested to be associated with pulmonary hypertension in patients with CTD [3–6].
In a previous study, we showed that autoantibodies against U1-RNP, cardiolipin and dsDNA increased the amounts of cytokines IL-1α, IL-1β, IL-6 and TNF-α released by, or associated with peripheral blood monocytes obtained from patients with CTD [7], and that they up-regulated the expression of adhesion and molecules, including intercellular adhesion molecule-1 (ICAM-1), E-selectin and Class II molecules on human pulmonary artery endothelial cells (HPAECs) [8]. These findings suggest that these autoantibodies may cause endothelial cell derangement and lead to proliferative vasculopathy. A possible mechanism by which anti-U1-RNP antibodies enhance the expression of adhesion and major histocompatibility complex (MHC) molecules is by binding to their target epitope, or to cross-reactive molecules present on the surface of endothelial cells. It has been suggested that endogenous autoantigens are present on MHC Class II molecules and are recognized by the corresponding T cells, which are tolerant under normal circumstances [9].
Anti-endothelial cell antibodies develop in many diseases, including systemic lupus erythematosus [10–16], systemic vasculitis [17], systemic sclerosis [18–21], autoimmune vasculitis [22], Wegener's granulomatosis [23], diabetes mellitus [24], scleroderma [25], Behçet's disease [26], rheumatoid arthritis [27–29], anti-phospholipid syndrome [30] and thrombotic thrombocytopenic purpura [31]. Although there are few reports indicating the kind of IgG that has anti-endothelial cell activity, Chan et al. reported that human anti-DNA antibodies bound to endothelial cells from human umbilical vein, both indirectly via immunoglobulin-bound DNA and directly through cross-reactivity [11].
We undertook the present study to clarify the activity of endothelial cell-binding by anti-U1-RNP antibodies in patients with connective tissue diseases and epitopes on HPAECs to which the autoantibody could bind.
Materials and methods
Patients and sera
We tested sera that were strongly positive to anti-U1-RNP antibody from 17 of 350 patients with CTD. These 17 patients had systemic lupus erythematosus (n = 5), Sjögren's syndrome (n = 4), mixed connective tissue disease (n = 5), systemic sclerosis (n = 2) or rheumatoid arthritis (n = 1). Sera from 10 normal healthy volunteers were also tested.
IgG fraction
The IgG fraction was isolated from sera by Protein G Sepharose chromatography (Pharmacia Fine Chemicals, Hounslow, UK). Serum (5 ml) was loaded onto a 1 ml Protein G Sepharose column. After extensive washing with 0·15 m Tris-HCl buffer (pH 7·6) (TB), IgG fractions were eluted from the column with 0·1 m glycine–HCl buffer (pH 2·7) and immediately dialysed against phosphate-buffered saline (PBS) containing 1 mm EDTA and 100 µm phenylmethylsulphonyl fluoride (PMSF) (PBS–EDTA–PMSF).
Purified anti-U1-RNP antibodies and anti-U1-RNP-depleted IgG
Extractable nuclear antigen (ENA) was prepared from rabbit thymus acetone powder (Pel-Freez Biologicals, Rogers, AR, USA) by ammonium sulphate fractionation [32], dialysed against PBS–EDTA–PMSF, and passed through an IgG-Sepharose column to remove any substances bound non-specifically to IgG. Then the ENA preparation was applied to a column of BrCN-activated Sepharose (Pharmacia) to which was bound the IgG fraction of serum (from a patient with mixed CTD) containing a high titre of the anti-U1-RNP antibody only (Anti-RNP-Sepharose). The bound U1-RNP fraction was eluted from the column with 3 m guanidine–HCl buffer (pH 4·8), dialysed extensively with EDTA–PMSF–PBS solution, then dialysed against coupling buffer (100 mm NaHCO3 [pH 8·3], containing 500 mm NaCl). The fraction was then coupled with BrCN-activated Sepharose (RNP-Sepharose) according to the manufacturer's instructions. Anti-U1-RNP-depleted IgG was prepared by passing about 50 mg IgG fraction positive for anti-U1-RNP in EDTA–PMSF–PBS through a 3 ml U1-RNP-Sepharose column that had been pre-equilibrated with EDTA–PMSF–PBS, and collecting the flow-through fraction. Purified anti-U1-RNP antibody preparations (purified anti-U1-RNP) were eluted with 3 m guanidine–HCl buffer (pH 4·8). Both anti-U1-RNP-depleted IgG and purified anti-U1-RNP antibodies were dialysed against PBS, concentrated to 1 mg/ml by ultrafiltration (Centricon 10; Amicon, Beverly, MA, USA) and sterilized by passing through a nitrocellulose filter (0·22 µm, Millipore Co. Ltd, Bedford, MA, USA).
Culture of endothelial cells from pulmonary artery
Non-immortalized, cryopreserved HPAECs from four passages were purchased from Clonetics Co., Ltd, CA, USA. HPAECs were cultured in 25 cm2 tissue culture flasks (Nunc, Roskilde, Denmark) in EGM-UV medium containing FCS (2% v/v), epithelial growth factor (10 ng/ml), hydrocortisone (1 µg/ml), gentamicin (50 µg/ml), amphotericin B (0·05 µg/ml) and bovine brain extract (0·25% v/v), according to the manufacturer's instructions. Cells were briefly exposed to 0·25% trypsin (Sanko Pharmaceuticals Co., Tokyo, Japan) and 0·04% EDTA.
Cell enzyme-linked immunosorbent assay (ELISA) for anti-endothelial cells
A cell ELISA for the detection of anti-endothelial cells was performed according to methods reported previously with modifications [8]. Cultured HPAECs (Clonetics) between passages four and six (104 cells/well in EBM-2 medium) were seeded onto microculture plates (96 wells). Confluent cell monolayers were fixed with 0·2% v/v glutaraldehyde (200 µl) in PBS for 30 min at room temperature, and then washed three times with PBS containing 0·05% v/v Tween 20 (washing buffer). The wells were blocked with Block Ace (40 mg/ml; Dainippon Pharmaceuticals, Osaka, Japan) diluted 1:2 with PBS containing 10% goat serum (200 µl/well) at 37°C for 60 min. The wells were then washed three times with washing buffer, and 100 µl diluted IgG or purified anti-U1-RNP antibodies were added to the wells. The wells were incubated at 4°C overnight. After washing again, 0·1 ml peroxidase-conjugated goat anti-human IgG or rabbit anti-human IgG-F(ab′)2 was added to each well at the appropriate dilution for whole molecule or F(ab′)2 molecules of antibodies, respectively. Then the wells were incubated at 37°C for 60 min. Substrate solution (100 µl; 0·4 mg/ml ortho-phenylene-diamine and 0·4 µl/ml 30% H2O2 in 10 mm citrate and 20 mm phosphate buffer, pH 4·0) was added, then the wells were incubated for 5–20 min at room temperature. The colour reaction was stopped by adding 100 µl 2·5 m H2SO4. The absorbance at 490 nm of the solution in each well was read using an automatic ELISA reader (Immuno Mini NJ-2300; Nalge Nunc Int. Co. Ltd, Tokyo, Japan). The binding activity of pulmonary artery endothelial cells is expressed as optical density at 490 nm (OD490).
ELISA for anti-U1-RNP
The titre of the antibodies against U1-RNP was assayed by ELISA, as described previously [33]. Micro-plates were coated with 100 µl U1-RNP at 10 µg/ml in 0·15 m TB (pH 7·6) and incubated at 4°C overnight. Wells were washed three times with Tris-buffered saline (0·015 m TB, 0·135 m NaCl) containing 0·5% v/v Tween 20 (TBS–Tween, washing buffer) and blocked with 1% bovine serum albumin in TB (BSA–TB) for 30 min at room temperature (RT). Then, 100 µl of samples in BSA–TB were added to each well and incubated for 1 h at RT. After washing, the colour reaction and colorimetric measurement were carried out as described above for the cell ELISA for anti-endothelial cells. The binding activity of anti-U1-RNP is expressed as OD490.
ELISA for anti-double-stranded DNA (dsDNA), cardiolipin (CL) and dextran sulphate (DXS)
The titres of the antibodies against dsDNA, CL and DXS were assayed by ELISA, as described previously [34,35]. Briefly, polystyrene microtitre plates were pre-coated with 40 µg/ml poly l-lysine (Sigma Chem. Co. St. Louis, MO, USA) in TB, followed by coating with purified dsDNA (10 µg/ml in TB). Cardiolipin (Sigma) in ethanol (50 µg/ml) was absorbed onto the surface of ELISA plates after evaporation of ethanol. PLL (0·4 µg/ml in TB) pre-coated plates were coated with DXS (250 µg/ml in TB, Sigma). After blocking these plates with BSA-TB, IgG fractions or purified IgG anti-U1-RNP antibodies at 25 µg/ml in TB were added. After washing, peroxidase-labelled rabbit anti-human IgG (diluted to 1:2500) was added. The colour reaction and colorimetric measurement were carried out as described above for the cell ELISA for anti-endothelial cells. The binding activities of these antigens are expressed as OD490.
Membrane preparation of HPAECs
A membrane fraction was prepared from HPAECs according to methods reported previously, with modifications [36]. Briefly, 5 × 107 HPAECs were washed three times with PBS. The pellet of HPAECs was mixed with 1 ml isolation buffer (0·1% IGEPAL CA-630; NP40, [Sigma] in TBS containing 0·02% NaN3 and 1 mm PMSF, pH 8·3) in a vortex mixer for 30 min at 4°C. The mixture was centrifuged for 20 min at 27 000 g. The supernatant fluid was obtained as NP40-treated membrane fractions of HPAECs. The membrane fractions were stored in aliquots at −80°C until use.
Immunoblotting for the detection of anti-endothelial cells
Equal volumes of membrane fraction and SDS gel sample buffer (Tris–SDS–BME sample buffer, Owl Scientific, Inc., Woburn, MA, USA) were mixed and incubated for 5 min in boiling water. The mixture was then subjected to 10–20% SDS gradient gel electrophoresis (Dai-ichi Pure Chemical Co., Tokyo, Japan) for 20 min at 60 mA. Molecular weight standards of 6·5, 16·5, 25, 32·5, 47·5, 62, 83 and 175 kD were included in each run (BioRad Laboratories, Richmond, CA, USA). After termination of the run, the membrane antigens were transferred to a nitrocellulose sheet by electroblotting for 2 h at 180 mA. The nitrocellulose sheet was incubated for 30 min in TB containing BSA–TB. The nitrocellulose paper was inserted in a screener blotter (Sanplatec Co., Osaka, Japan) and incubated with IgG fractions or purified anti-U1-RNP antibodies in BSA–TB at 4°C overnight. The fractions were washed with washing buffer, then reactions with membrane antigens were detected by affinity-purified goat anti-human IgG alkaline phosphatase conjugate (EY Laboratories Inc., San Mateo, CA, USA) at 4°C overnight. The sheet was washed with washing buffer, incubated with 50 ml substrate solution (0·1 m Tris-HCl, 0·5 mm MgCl2, pH 9·5) containing 30 µl nitro-blue tetrazolium (NBT, 50 mg/ml) in 70% dimethylformamide and 5-bromo-4-chloro-3-indolyl phosphate (BCIP, 5 mg/ml) in 100% dimethylformamide for 10–20 min, and then washed thoroughly with deionized water.
Immunoabsorption
U1-RNP-Sepharose was prepared as described above. U1-RNP-Sepharose containing 5 mg RNP/ml of swollen beads was prepared. BSA-Sepharose containing 10 mg BSA/ml of swollen beads was also prepared by conjugating BSA with BrCN-activated Sepharose. Both gels were mixed independently with an equal volume of 1% BSA–TB. IgG fractions positive for anti-U1-RNP were mixed with these gels at 25 µg/ml of final concentration, and rotated end-over-end at 4°C overnight. After centrifugation, supernatant fluids of the mixture were tested for activity of anti-endothelial cells and anti-U1-RNP antibody.
F(ab′)2 preparation
IgG F(ab′)2 fragments were prepared by pepsin digestion of affinity-purified anti-U1-RNP antibody or control IgG (IgG:pepsin = 100:3 w/w) in 0·2 m acetate buffer (pH 4·5) at 37°C overnight. The digestion was terminated by adding 0·1 m Tris, and dialysed against TB. The digests were passed through a Sephadex G-100 column (1·2 × 100 cm) that had been pre-equilibrated with TB. Only fractions that contained Fd fragments and light chains on SDS–polyacrylamide gel electrophoresis (Laemmli's method) were collected.
Statistical analysis
Data are expressed as the median and interquartile levels (Q1–Q3), and analysed statistically by Wilcoxon's t-test and the Mann–Whitney U-test. Correlations were determined using Spearman's rank correlation test. Differences with P-values of < 0·05 are considered significant.
Results
Binding activity to HPAECs of patients' serum, the IgG fraction obtained from anti-U1-RNP-positive serum, and purified anti-U1-RNP antibodies
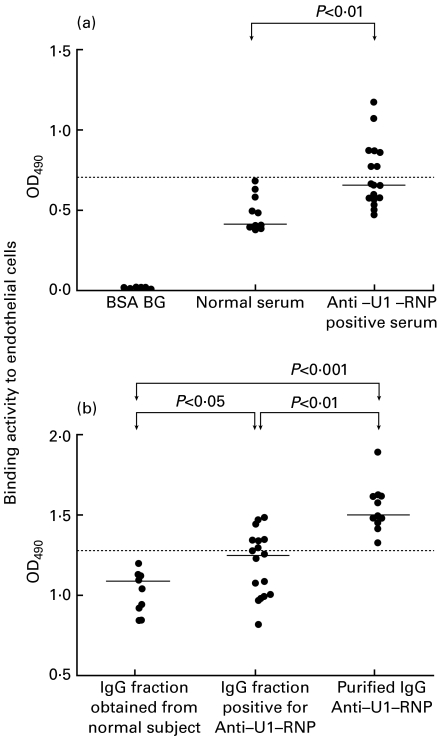
The binding activity to endothelial cells of patients' sera positive for anti-U1-RNP antibody diluted to at 1:600 (median 0·657 (OD490); interquartile levels (Q1–Q3), 0·570–0·882; n = 17) was significantly higher than that of normal sera (median 0·409; Q1–Q3 0·396–0·582; n = 10; P < 0·01 by the Mann–Whitney U-test, Fig. 1a). The binding activity to endothelial cells of IgG fractions positive for anti-U1-RNP antibody at 25 µg/ml (median 1·337; Q1–Q3 1·058–1·439; n = 17) and those of purified anti-U1-RNP antibodies at 25 µg/ml (median 1·610, 1·553–1·743; n = 13) was significantly higher than that of IgG fractions obtained from normal subjects at the same concentration (1·154; 0·968–1·189; n = 10; P < 0·05, P < 0·001 by the Mann–Whitney U-test; Fig. 1b). The binding activity of purified anti-U1-RNP antibody was also significantly higher than that of IgG fraction positive for anti-U1-RNP (P < 0·01). The percentage of sera positive for anti-endothelial cell activity was 41% (7/17, exceeding 0·707; the mean + 2 s.d. in normal serum, Fig. 1a). The percentage of anti-U1-RNP-positive-IgG fractions positive for anti-endothelial cell activity was also 41% (7/17, exceeding 1·368; the mean + 2 s.d. in IgG fractions obtained from normal subjects, Fig. 1b).
Fig. 1.
Binding activity to human pulmonary artery endothelial cells of patients' serum, the IgG fraction obtained from anti-U1-RNP-positive serum and purified anti-U1-RNP antibodies. (a) The binding activity of anti-U1-RNP-positive sera diluted 1:600 (n = 17) was significantly higher than that of normal sera (n = 10). (b) The binding activity to endothelial cells of the IgG fractions positive for anti-U1-RNP antibody (n = 17) and of purified anti-U1-RNP antibodies (n = 13) was significantly higher than that of IgG fractions obtained from normal subjects (n = 10). Binding activity to endothelial cells is expressed as OD490. Horizontal bars indicate the median values. Dotted lines indicate the mean + 2 s.d. of (a) normal serum and (b) IgG fraction obtained from normal subject.
Binding activity to HPAECs of purified anti-U1-RNP antibodies and anti-U1-RNP-depleted IgG
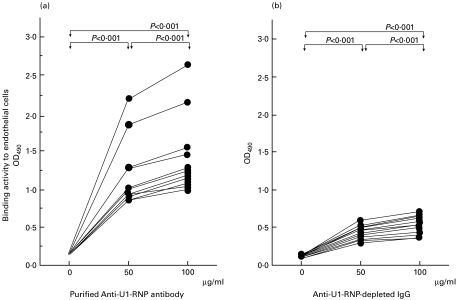
The binding activity of purified anti-U1-RNP antibody to endothelial cells was significantly higher than that of the 0 µg/ml buffer control (0 µg/ml: OD490 = 0·158, 0·151–0·161; 50 µg/ml: 1·006, 0·924–1·280, P < 0·001; 100 µg/ml: 1·233, 1·126–1·464, P < 0·001; n = 13; Wilcoxon's t-test), and the binding at 100 µg/ml was also significantly higher than that at 50 µg/ml (P < 0·001). Therefore, there was a dose-dependent increase in the binding of purified anti-U1-RNP antibodies to endothelial cells (Fig. 2a). On the other hand, the binding activity of anti-U1-RNP-depleted IgG which had been passed through an RNP-Sepharose column was also significant among the different concentrations of anti-U1-RNP-depleted IgG (0 µg/ml: OD490 = 0·139, 0·125–0·144; 50 µg/ml: 0·426, 0·369–0·515, P < 0·001 compared with 0 µg/ml; 100 µg/ml: 0·531, 0·521–0·766, P < 0·001 compared with 0 and 50 µg/ml; n = 13), although the levels of binding activity were lower than those of purified antibodies (Fig. 2b).
Fig. 2.
Binding activity to human pulmonary artery endothelial cells of purified anti-U1-RNP antibodies and anti-U1-RNP-depleted IgG. Binding activity (as OD490) of purified IgG anti-U1-RNP antibody to endothelial cells tended to increase dose-dependently. The activity was significantly higher than that of the control (0 µg/ml) (50 µg/ml: P < 0·001; 100 µg/ml: P < 0·001 by Wilcoxon's t-test; n = 13) and the binding at 100 µg/ml was also significantly higher than that at 50 µg/ml (P < 0·001) (Fig. 2a). On the other hand, the binding activity of anti-U1-RNP-depleted IgG which had been passed through an RNP-Sepharose column was also significantly different among the different concentrations of anti-U1-RNP-depleted IgG (P < 0·001 compared between 0 and 50 µg/ml, 0 and 100 µg/ml, 50 and 100 µg/ml, n = 13). However, the levels of binding activity were lower than those of purified antibodies (Fig. 2b).
Correlation between anti-U1-RNP antibodies and binding activities to endothelial cells in IgG preparations
There was a significant correlation between anti-U1-RNP antibodies and binding activities to endothelial cells in IgG preparations obtained from patients positive for anti-U1-RNP antibody (n = 17) (rs = 0·632; P < 0·01 by Spearman's rank correlation test; figure not shown).
Reduction by U1-RNP–Sepharose gel of binding activity to HPAECs, and anti-U1-RNP antibody activity in IgG fractions positive for anti-U1-RNP antibody
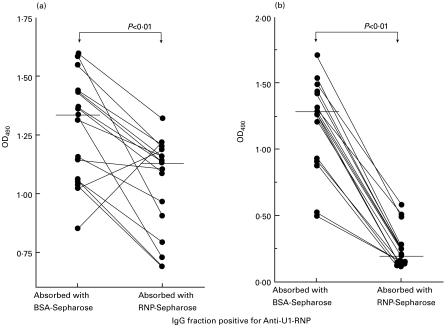
The binding activity to endothelial cells of IgG positive for anti-U1-RNP antibody was significantly reduced by U1-RNP–Sepharose gel (1·131, 0·878–1·194) compared with the control BSA–Sepharose gel (1·337, 1·058–1·439; P < 0·01 by Wilcoxon's t-test, Fig. 3a). A considerable amount of endothelial cell-binding activity was absorbed by U1-RNP. The anti-U1-RNP-antibody activity of IgG positive for anti-U1-RNP antibody was almost completely absorbed by U1-RNP-Sepharose gel (0·193, 0·142–0·284) compared with BSA-Sepharose gel (1·283, 0·925–1·447; P < 0·001, Fig. 3b).
Fig. 3.
Reduction by U1-RNP-Sepharose gel of binding activity to human pulmonary artery endothelial cells, and anti-U1-RNP antibody activity in IgG fractions positive for anti-U1-RNP antibody. The binding activity of IgG positive for anti-U1-RNP antibody to endothelial cells was significantly absorbed by U1-RNP-Sepharose gel (P < 0·01 by Wilcoxon's t-test) compared with control BSA-Sepharose gel (Fig. 3a). The anti-U1-RNP antibody activity of IgG positive for anti-U1-RNP antibody was almost completely absorbed by U1-RNP-Sepharose gel (P < 0·001) compared with BSA-Sepharose gel (Fig. 3b). Horizontal bars indicate medians.
Binding activities to HPAECs of F(ab′)2 preparations and whole molecules of purified IgG anti-U1-RNP antibody
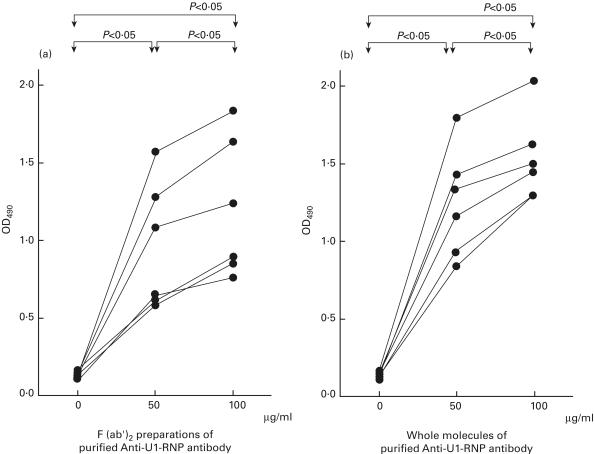
The endothelial cell-binding activities of F(ab′)2 preparations of purified IgG anti-U1-RNP antibody (Fig. 4a) increased dose-dependently (0 µg/ml: OD490 = 0·132, 0·108–0·156; 50 µg/ml: 0·663, 0·618–1·278; 100 µg/ml: 0·915, 0·852–1·632). The activities of whole molecules of purified IgG anti-U1-RNP antibody obtained from the same patients as controls (Fig. 4b) were also increased dose-dependently (0 µg/ml: OD490 = 0·136, 0·124–0·148; 50 µg/ml: 1·268, 0·920–1·416; 100 µg/ml: 1·521, 1·296–1·616). The significance of differences between each of the concentrations of antibodies is P < 0·05 by Wilcoxon's t-test; n = 6; Fig. 4a, b). The Fab portions of purified anti-U1-RNP antibodies may have specifically recognized epitopes on the surface of endothelial cells.
Fig. 4.
Binding activities to human pulmonary artery endothelial cells of F(ab′)2 preparations and whole molecules of purified anti-RNP antibody. Both the endothelial-cell-binding activity (as OD490) of F(ab′) 2 preparations (a) and the whole molecules (b) of IgG anti-U1-RNP antibody increased dose-dependently (differences between each of the concentrations of antibodies were significant at P < 0·05 by Wilcoxon's t-test, n = 6).
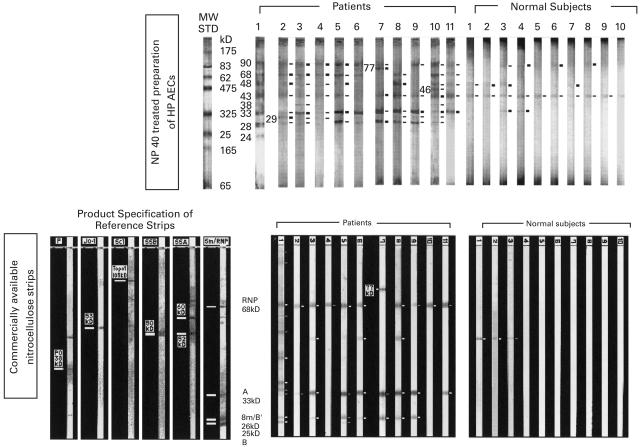
Immunoblotting assay of purified IgG anti-U1-RNP antibody to membrane preparations of HPAECs and to nitrocellulose strips impregnated with known antigens
The binding of purified IgG anti-U1-RNP antibodies (n = 11) to endothelial cells was assayed by immunoblotting of NP40-treated cell membrane preparations of HPAECs (Fig. 5, upper panel). Purified IgG anti-U1-RNP antibodies bound to membrane proteins of 90 kD (10/11), 77 kD (1/11), 68 kD (9/11), 48 kD (8/11), 46 kD (1/11), 43 kD (11/11), 38 kD (3/11), 33 kD (11/11), 29 kD (6/11), 28 kD (9/11) and 24 kD (1/11). IgG preparations from normal subjects bound to some membrane proteins of 90 kDa (4/11), 48 kD (4/10), 43 kD (10/10) and 33 kD (2/10). Membrane proteins of 68, 33, 28 and 24 kD seemed to correspond to the components of U1-RNP—68 kD, A, B′ and C peptides, respectively.
Fig. 5.
Immunoblotting assay of purified IgG anti-U1-RNP antibody to membrane preparations of human pulmonary artery endothelial cells (HPAECs) and to nitrocellulose strips impregnated with known antigens. The binding activities of the purified IgG anti-U1-RNP antibodies (n = 11) to endothelial cells were assayed by immunoblotting of NP40-treated cell membrane preparations of HPAECs (upper panel). Purified IgG anti-U1-RNP antibodies bound to membrane proteins of 90 kD (10/11), 77 kD (1/11), 68 kD (9/11), 48 kD (8/11), 46 kD (1/11), 43 kD (11/11), 38 kD (3/11), 33 kD (11/11), 29 kD (6/11), 28 kD (9/11) and 24 kD (1/11). Membrane proteins of 68, 33, 28 and 24 kD seemed to correspond to the components of U1-RNP 68 kD, A, B′ and C peptides, respectively. The binding activities of the same antibodies (n = 11) to endothelial cells were also tested using nitrocellulose strips which had been impregnated with the following antigenic components from cellular extract: P, Ribosomal P with a band at 38 kD; Jo-1, Jo-1 with a band at 53 kD; Scl, Scl-70 (Topoisomerase) with a band at 105 kD; SSB, SS-B with a band at 50 kD; SSA, SS-A (Ro) with a band at 60 kD and 52 kD; Sm/RNP, Sm with bands at 26 kD (Sm/B′) and 25 kD (B); and RNP, with bands at 68 kD and 33 kD (A) (Affini Tech NC strips, commercially available NC strips, lower panel). Purified IgG anti-U1-RNP antibodies bound to Affini Tech NC strips at 68 kD (10/11), 33 kD (9/11), 26 kD (7/11), and 25 kD (2/11). The same antibody was arranged in the same order on the two nitrocellulose strips. B′ (26 kD), which was the product specification of the reference strips, seemed to correspond to 28 kD of NP40-treated cell membrane preparations of HPAECs.
The binding activities of the same anti-U1-RNP antibodies (n = 11) to endothelial cells were also tested by another immunological test (commercially available Western blot nitrocellulose (NC) strips; Affini Tech NC strips, Fig. 5, lower panel) which had been impregnated with RNP or Sm antigens such as 68 kD, A (33 kD), B′ (26 kD) and B (25 kD) (Western Blot Cellular Extract Coated Nitrocellulose, Affini Tech, Ltd. Bentonville, AR, USA). Purified IgG anti-U1-RNP antibodies bound to Affini Tech NC strips of 77 kD (1/11), 68 kD(10/11), 33 kD (9/11), 26 kD (7/11) and 25 kD (2/11). There were some bands other than U1-RNP-related peptides detected on the Affini Tech NC strips, and it was unknown which antigen could bind to the purified IgG anti-U1-antibodies.
In Fig. 5, as the same antibody was arranged in the same order on the two nitrocellulose strips (upper and lower panels), almost the same bands for nRNP antigens were obtained by the two strips. B′ (26 kD), the product specification of the reference strips for the Affini Tech NC strips, seemed to correspond to 28 kD of NP40 of the treated cell membrane preparations of HPAECs. Although some bands were found on both NC strips which reacted with IgG from normal subjects, there was no band related to nRNP antigens.
Examination for cross-reactive properties of anti-U1-RNP positive for IgG, purified anti-U1-RNP antibody and IgG fractions obtained from normal subjects
The binding properties of IgG fractions at 25 µg/ml positive for anti-U1-RNP (n = 17), purified anti-U1-RNP antibodies (n = 13) and IgG from normal subjects (n = 10) were assayed for other autoantibodies showing specific binding to dsDNA, negatively-charged phospholipids, cardiolipin (CL) and dextran sulphate (DXS) (Table 1). While some of the purified anti-U1-RNP antibodies had slight binding activity to dsDNA and DXS, the correlations between the levels of these antibody activities were not significant (rs = 0·121, rs = 0·016, respectively). IgGs or anti-U1-RNP did not have binding activity to a BSA-TB-coated plate without cells. The BSA background without added IgG or anti-U1-RNP antibodies in the ELISA for HPAEC, anti-U1-RNP, anti-DNA, anti-cardiolipin or anti-dextran sulphate is shown as low level (Table 1).
Table 1.
Examination for cross-reactive property of IgG fraction positive for anti-U1-RNP and purified IgG anti-U1-RNP antibodies
| OD490 | IgG fraction obtained from normal subject median (Q1-Q3) | IgG fraction positive for Anti-U1-RNP median (Q1-Q3) | Purified IgG anti-U1-RNP median (Q1-Q3) | BSA median (Q1-Q3) |
|---|---|---|---|---|
| AECA | 1·154 (0·968–1·189) | 1·337 (1·058–1·439)* | 1·610 (1·553–1·743)*** | 0·024 (0·021–0·027) |
| Cell (+) | n = 10 | n = 17 | n = 13 | n = 10 |
| AECA | 0·065 (0·056–0·078) | 0·056 (0·051–0·083) | 0·061 (0·046–0·078) | 0·044 (0·043–0·045) |
| Cell (–) BG | n = 10 | n = 17 | n = 13 | n = 10 |
| ARNP | 0·140 (0·132–0·155) | 1·283 (0·925–1·447)*** | 1·659 (1·283–1·740)*** | 0·043 (0·039–0·049) |
| n = 10 | n = 17 | n = 13 | n = 10 | |
| ADNA | 0·065 (0·059–0·068) | 0·047 (0·034–0·071) | 0·209 (0·048–0·273)* | 0·011 (0·011–0·014) |
| n = 10 | n = 17 | n = 13 | n = 10 | |
| ACL | 0·035 (0·034–0·037) | 0·034 (0·031–0·039) | 0·045 (0·036–0·054)* | 0·026 (0·023–0·027) |
| n = 10 | n = 17 | n = 13 | n = 10 | |
| ADXS | 0·035 (0·025–0·051) | 0·014 (0·009–0·027) | 0·141 (0·080–0·188)** | 0·016 (0·011–0·016) |
| n = 10 | n = 17 | n = 13 | n = 10 |
AECA: anti-endothelial cell antibody; ARNP: anti-ribonucleoprotein antibody; ADNA: anti-dsDNA antibody; ACL: anti-cardiolipin antibody; ADXS: anti-dextransulphate antibody.
P < 0·05
P < 0·01
P < 0·001 compared with IgG fractions obtained from normal subjects.
Data are expressed as the median and interquartile levels (Q1-Q3) at OD490 nm and analysed statistically by Mann–Whitney U-test.
Discussion
Our results show that IgG positive for anti-U1-RNP from patients with CTD binds to human pulmonary artery endothelial cells (HPAECs). Furthermore, whole molecules and F(ab′)2 IgG anti-U1-RNP purified by affinity chromatography also bind to endothelial cells. The binding activity to endothelial cells of IgG positive for anti-U1-RNP could be significantly absorbed by U1-RNP-Sepharose. These results suggest that almost all anti-U1-RNP antibodies in patients with CTD play a role like anti-endothelial cell antibodies.
However, both the percentage of sera positive for anti-endothelial cell activity and the percentage of anti-U1-RNP-positive-IgG fractions positive for anti-endothelial cell activity were 41% (exceeding the means + 2 s.d. in normal subjects). This means that not all of the sera or IgG containing anti-U1-RNP reacted with endothelial cells; some anti-U1-RNP-positive-IgG fractions or anti-U1-RNP-positive-sera were negative for anti-endothelial-cell activity. This suggests that varieties of anti-U1-RNP antibodies are responsible for the binding activity to endothelial cells.
While the anti-U1-RNP-antibody activity of the IgG that was positive for anti-U1-RNP antibody was almost completely absorbed by U1-RNP-Sepharose gel, part of the binding activity of the IgG to endothelial cells of IgG was reduced by the same gel. This suggests that part of the IgG fraction positive for anti-U1-RNP antibody is anti-U1-RNP antibody, which has binding activity for endothelial cells. The rest of the IgG fraction may have binding activity to endothelial cells, though the antibody that possesses binding activity to endothelial cells in the remaining IgG has not been identified.
The serum that had the highest titre of anti-endothelial cell reactivity was obtained from the patient with MCTD complicated by pulmonary hypertension (PH; nearly half the deaths of patients with MCTD [6]). However, the activity of anti-endothelial cell antibody of another serum from a patient with MCTD complicated by pulmonary hypertension was found to be within normal limits. It will be necessary to study the relationship between the activity of anti-endothelial cell antibody and diagnostic group, disease activity or clinical symptom such as PH.
In the ELISA for the binding activity to HPAECs of whole molecules and F(ab′)2 preparations of purified anti-U1-RNP antibodies, peroxidase-conjugated antibodies, goat anti-human IgG (γ chain specific) and rabbit anti-human IgG [F(ab′)2 specific] were used at different concentrations. These differences in dilution may be due to the binding affinity of the antibodies. Both the binding activity to endothelial cells of the whole molecules and F(ab′)2 preparations of purified IgG anti-U1-RNP antibody increased dose-dependently, suggesting that the Fab portions of purified anti-U1-RNP antibodies may have specifically recognized epitopes on the surface of the endothelial cells.
Immunoblotting assay of purified IgG anti-U1-RNP antibodies showed that these antibodies bound to various membrane proteins of NP40-treated HPAECs of 68, 48, 43, 38, 33, 29, 28 and 24 kDa. Some bands — at 68, 33, 28 and 24 kDa — seemed to correspond to U1-RNP components 68 kDa, A, B′ and C peptides, respectively. Almost the same results for binding activities of anti-U1-RNP antibodies to nRNP antigens were obtained in another immunological test, using Western Blot Autoantibody Strips impregnated with the RNP or Sm antigens. Bands related to U1-RNP on the Affini Tech NC strips were clearly revealed, easily corresponding to the bands on membrane preparations of HPAECs bound to the anti-U1-RNP antibodies.
Endogenous autoantigens are likely to be presented on MHC class II molecules and to be recognized by corresponding T cells, which are tolerant under ordinary circumstances [9]. One hypothesis — that anti-U1-RNP antibody binds to fragments of U1-RNP presented by MHC class II molecules — remains to be confirmed. Another hypothesis is that anti-U1-RNP antibody, which may act as anti-endothelial cell antibody, induces apoptosis on the endothelial cells and binds to fragments of nucleosome RNP blebs on the EC surface [37,38].
Binding activities to endothelial cells in purified anti-U1-RNP-depleted IgG decreased to less than half of those before absorption. Enough binding activity to react with epitopes other than U1-RNP on the surface of endothelial cells remained in the anti-U1-RNP-depleted IgG fractions. Endothelial cell-binding activities of normal IgG detected by cell ELISA might also be due to binding to membrane proteins other than RNP components.
Although anti-endothelial cell antibodies develop in many diseases [10–31], there has been no report of anti-endothelial cell activity shown by anti-U1-RNP antibody. With regard to human anti-DNA antibodies, Chan et al. reported autoantibodies that bound to endothelial cells from human umbilical vein, both indirectly via immunoglobulin-bound DNA and directly through cross-reactivity [11]. They showed that polyclonal anti-DNA antibodies bound to membrane proteins of 30–180 kDa, and suggested that these mechanisms of cellular binding by anti-DNA antibodies may correspond to pathogenic steps in human systemic lupus erythematosus.
IgG positive for anti-U1-RNP, purified anti-U1-RNP antibodies and IgG from normal subjects were assayed for other autoantibodies binding specifically to dsDNA, and to other negatively-charged molecules, such as cardiolipin and dextran sulphate (DXS), which might contribute to cell binding. IgG positive for anti-U1-RNP and IgG from normal subjects did not have binding activity to dsDNA, CL or DXS. While some purified anti-U1-RNP antibodies have slight binding activity to dsDNA and DXS, the correlations between the levels of these antibody activities were not significant.
Koenig et al. showed that antibodies from thrombotic thrombocytopenic purpura recognized 43 kDa proteins obtained from microvascular endothelial cells from human kidney [31]. They suggested that the band may be related to injury of endothelial cells. Although we also showed reactivity of the same 43 kDa protein by immunoblotting with purified anti-U1-RNP antibodies, normal IgG could also bind the same protein.
Several mechanisms have been reported by which anti-endothelial cell antibodies may play a role in the pathophysiology of inflammatory disease. Papa et al. reported up-regulated expression of the endothelial adhesion molecules E-selectin, ICAM-1 and VCAM-1 after incubation of endothelial cells with IgG anti-endothelial cell antibodies [23]. We have previously reported up-regulation of ICAM-1 and ELAM-1 on HPAEC after incubation of endothelial cells with anti-U1-RNP antibody [8]. All these results suggest that anti-U1-RNP antibodies may act as anti-endothelial cell antibodies, and may induce the activation and up-regulation of adherent molecules of HPAECs.
In contrast, anti-endothelial cell antibodies may trigger the pathogenesis of some diseases by complement-dependent cytotoxicity or antibody-dependent cellular cytotoxicity [39]. The mechanism of up-regulation of adherent molecules by anti-endothelial cell antibodies remains unclear.
In our previous studies using human pulmonary artery endothelial cells (HPAECs), we demonstrated the roles of anti-U1-RNP antibody in inducing some soluble factors [7] and up-regulating adhesion molecules [8]. In the present study, we confirmed that the anti-U1-RNP antibody from patients with CTD can directly recognize a variety of antigens on the endothelial surface of the pulmonary artery, including the components of U1-RNP or other unknown polypeptides. These results suggest that binding to HPAECs of this autoantibody may be one of the triggers of endothelial cell inflammation in CTDs.
Acknowledgments
The authors would like to thank Ms Michiyo Shimizu for her technical assistance, and Ms Midori Horibe and Ms Hiromi Hata for their secretarial help. This work was supported by a research grant for mixed connective tissue disease from the Ministry of Labour, Health and Welfare of Japan.
References
- 1.Sharp GC, Irvin WS, Tan EM, et al. Mixed connective tissue disease: an apparently distinct rheumatic disease syndrome associated with a specific antibody to an extractable nuclear antigen. Am J Med. 1972;52:148–59. doi: 10.1016/0002-9343(72)90064-2. [DOI] [PubMed] [Google Scholar]
- 2.Frandsen PB, Kriegbaum NJ, Ullman S, et al. Follow-up of 151 patients with high-titer U1 RNP antibodies. Clin Rheumatol. 1996;15:254–60. doi: 10.1007/BF02229703. [DOI] [PubMed] [Google Scholar]
- 3.Asherson RA. Pulmonary hypertension in systemic lupus erythematosus. J Rheumatol. 1990;17:414–5. [PubMed] [Google Scholar]
- 4.Luchi ME, Asherson RA, Lahita RG. Primary idiopathic pulmonary hypertension complicated by pulmonary arterial thrombosis. Association with antiphospholipid antibodies. Arthritis Rheum. 1992;35:700–5. doi: 10.1002/art.1780350616. [DOI] [PubMed] [Google Scholar]
- 5.Asherson RA, Higenbottam TW, Xuan ATD, et al. Pulmonary hypertension in a lupus clinic: experience with twenty-four patients. J Rheumatol. 1990;17:1292–8. [PubMed] [Google Scholar]
- 6.Nishimaki T, Aotsuka S, Kondo H, et al. Immunological analysis of pulmonary hypertension in connective tissue diseases. J Rheumatol. 1999;26:2357–62. [PubMed] [Google Scholar]
- 7.Okawa-Takatsuji M, Aotsuka S, Uwatoko S, et al. Increase of cytokine production by pulmonary artery endothelial cells induced by supernatants from monocytes stimulated with autoantibodies against U1-ribonucleoprotein. Clin Exp Rheumatol. 1999;17:705–12. [PubMed] [Google Scholar]
- 8.Okawa-Takatsuji M, Aotsuka S, Fujinami M, et al. Up-regulation of intercellular adhesion molecule-1 (ICAM-1), endothelial leucocyte adhesion molecule-1 (ELAM-1) and class II MHC molecules on pulmonary artery endothelial cells by antibodies against U1-ribonucleoprotein. Clin Exp Immunol. 1999;116:174–80. doi: 10.1046/j.1365-2249.1999.00864.x. 10.1046/j.1365-2249.1999.00864.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Calin-Laurens V, Forguet F, Lombard PS, et al. High efficiency of endogenous antigen presentation by MHC class II molecules. Int Immunol. 1992;4:1113–21. doi: 10.1093/intimm/4.10.1113. [DOI] [PubMed] [Google Scholar]
- 10.Yoshio T, Masuyama J, Sumiya M, et al. Antiendothelial cell antibodies and their relation to pulmonary hypertension in systemic lupus erythematosus. J Rheum. 1994;21:2058–63. [PubMed] [Google Scholar]
- 11.Chan TM, Cheng IKP. Identification of endothelial cell membrane proteins that bind anti-DNA antibodies from patients with systemic lupus erythematosus by direct or indirect mechanisms. J Autoimmunity. 1997;10:433–9. doi: 10.1006/jaut.1997.9998. [DOI] [PubMed] [Google Scholar]
- 12.Hashemi S, Smith CD, Izaguirre CA. Anti-endothelial cell antibodies: Detection and characterization using a cellular enzyme-linked immunosorbent assay. J Lab Clin Med. 1987;109:434–40. [PubMed] [Google Scholar]
- 13.Van Der Zee JM, Siegert CEH, De Vreede TA, et al. Characterization of anti-endothelial cell antibodies in systemic lupus erythematosus (SLE) Clin Exp Immunol. 1991;84:238–44. doi: 10.1111/j.1365-2249.1991.tb08155.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li JS, Liu MF, Lei HY. Characterization of anti-endothelial cell antibodies in the patients with systemic lupus erythematosus: a potential marker for disease activity. Clin Immunol Immunopathol. 1996;79:211–6. doi: 10.1006/clin.1996.0070. [DOI] [PubMed] [Google Scholar]
- 15.D'Cruz D, Khamashta M, Hughes G. Antiendothelial cell antibodies (AECA) in systemic lupus erythematosus (SLE) Clin Rev Allergy Imm. 1997;15:53–63. doi: 10.1007/BF02828277. [DOI] [PubMed] [Google Scholar]
- 16.Carvalho D, Savage COS, Isenberg D, et al. IgG anti-endothelial cell autoantibodies from patients with systemic lupus erythematosus or systemic vasculitis stimulate the release of two endothelial cell-derived mediators, which enhance adhesion molecule expression and leukocyte adhesion in an autocrine manner. Arthritis Rheum. 1999;42:631–40. doi: 10.1002/1529-0131(199904)42:4<631::AID-ANR5>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 17.Frampton G, Jayne DRW, Perry GJ, et al. Autoantibodies to endothelial cells and neutrophils cytoplasmic antigens in systemic vasculitis. Clin Exp Immunol. 1990;82:227–32. doi: 10.1111/j.1365-2249.1990.tb05431.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pignone A, Scaletti C, Matucci-Cerinic M, et al. Anti-endothelial cell antibodies in systemic sclerosis: Significant association with vascular involvement and alveolo-capillary impairment. Clin Exp Rheumatol. 1998;16:527–32. [PubMed] [Google Scholar]
- 19.Salojin KV, Tonqueze ML, Saraux A, et al. Antiendothelial cell antibodies: Useful markers of systemic sclerosis. Am J Med. 1997;102:178–85. doi: 10.1016/s0002-9343(96)00404-4. 10.1016/s0002-9343(96)00404-4. [DOI] [PubMed] [Google Scholar]
- 20.Youinou P, Revelen R, Bordron A. Is antiendothelial cell antibody the murder weapon in systemic sclerosis? Clin Exp Rheumatol. 1999;17:35–6. [PubMed] [Google Scholar]
- 21.Ihn H, Sato S, Fujimoto M, et al. Characterization of autoantibodies to endothelial cells in systemic sclerosis association with pulmonary fibrosis. Clin Exp Immunol. 2000;119:203–9. doi: 10.1046/j.1365-2249.2000.01115.x. 10.1046/j.1365-2249.2000.01115.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Johnson PA, Alexander HD, McMillan SA, et al. Up-regulation of the endothelial cell adhesion molecule intercellular adhesion molecule-1 (ICAM-1) by autoantibodies in autoimmune vasculitis. Clin Exp Immunol. 1997;108:234–42. doi: 10.1046/j.1365-2249.1997.3741271.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Papa ND, Guidali L, Sironi M, et al. Anti-endothelial cell IgG antibodies from patients with Wegener's granulomatosis bind to human endothelial cells in vitro and induce adhesion molecule expression and cytokine secretion. Arthritis Rheum. 1996;39:758–66. doi: 10.1002/art.1780390507. [DOI] [PubMed] [Google Scholar]
- 24.Triolo G, Triolo G, Accardo-Palumbo A, et al. IgG anti-endothelial cell antibodies (AECA) in type I diabetes mellitus; induction of adhesion molecule expression in cultured endothelial cells. Clin Exp Immunol. 1998;111:491–6. doi: 10.1046/j.1365-2249.1998.00524.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Negi VS, Tripathy NK, Misra R, et al. Antiendothelial cell antibodies in scleroderma correlate with severe digital ischemia and pulmonary arterial hypertension. J Rheum. 1998;25:462–6. [PubMed] [Google Scholar]
- 26.Triolo G, Accardo-Palumbo A, Triolo G, et al. Enhancement of endothelial cell E-selectin expression by sera from patients with active Behcet's disease: Moderate correlation with anti-endothelial cell antibodies and serum myeloperoxidase levels. Clin Immunol. 1999;91:330–7. doi: 10.1006/clim.1999.4687. [DOI] [PubMed] [Google Scholar]
- 27.Heurkens AHM, Hiemstra PS, Lafeber GJM, et al. Anti-endothelial cell antibodies in patients with rheumatoid arthritis complicated by vasculitis. Clin Exp Immunol. 1989;78:7–12. [PMC free article] [PubMed] [Google Scholar]
- 28.Van Der Zee JM, Heurkens AHM, Van Der Voort EAM, et al. Characterization of anti-endothelial antibodies in patients with rheumatoid arthritis complicated by vasculitis. Clin Exp Rheum. 1991;8:589–94. [PubMed] [Google Scholar]
- 29.Salih AM, Nixon NB, Dawes PT, et al. Soluble adhesion molecules and anti-endothelial cell antibodies in patients with rheumatoid arthritis complicated by peripheral neuropathy. J Rheumatol. 1999;26:551–5. [PubMed] [Google Scholar]
- 30.Lanir N, Zilberman M, Yron I, et al. Reactivity patterns of antiphospholipid antibodies and endothelial cells: Effect of antiendothelial antibodies on cell migration. J Lab Clin Med. 1998;131:548–56. doi: 10.1016/s0022-2143(98)90063-4. [DOI] [PubMed] [Google Scholar]
- 31.Koenig DW, Barley-Maloney LB, Daniel TOA. western blot assay detects autoantibodies to cryptic endothelial antigens in thrombotic microangiopathies. J Clin Immunol. 1993;13:204–11. doi: 10.1007/BF00919973. [DOI] [PubMed] [Google Scholar]
- 32.White PJ, Gardner WD, Hoch SO. Identification of the immunogenically active components of the Sm and RNP antigens. Proc Natl Acad Sci USA. 1981;78:626–30. doi: 10.1073/pnas.78.1.626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Okawa-Takatsuji M, Aotsuka S, Uwatoko S, et al. The B cell repertoire in patients with systemic autoimmune diseases: analysis of Epstein-Barr virus (EBV)-inducible circulating precursors that produce autoantibodies against nuclear ribonucleoprotein (RNP) Clin Exp Immunol. 1992;90:415–21. doi: 10.1111/j.1365-2249.1992.tb05861.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Aotsuka S, Okawa-Takatsuji M, Kinoshita M, et al. Analysis of negatively charged dye-binding antibodies reactive with double-stranded DNA and heparan sulfate in serum from patients with rheumatic diseases. Clin Exp Immunol. 1988;73:436–42. [PMC free article] [PubMed] [Google Scholar]
- 35.Aotsuka S, Okawa-Takatsuji M, Uwatoko S, et al. Antibodies against sulphatide in sera from patients with autoimmune rheumatic diseases. Clin Exp Immunol. 1992;87:438–43. doi: 10.1111/j.1365-2249.1992.tb03016.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nag B, Wada HG, Deshpande SV, et al. Stimulation of T cells by antigenic peptide complexed with isolated chains of major histocompatibility complex class II molecules. Proc Natl Acad Sci USA. 1993;90:1604–8. doi: 10.1073/pnas.90.4.1604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Casciola-Rosen LA, Miller DK, Anhalt GJ, et al. Specific cleavage of the 70-kDa protein component of the U1 small nuclear ribonucleoprotein is a characteristic biochemical feature of apoptotic cell death. J Biol Chem. 1994;269:30757–60. / [PubMed] [Google Scholar]
- 38.Biggogera M, Bottone MG, Martin TE, et al. Still immunodetectable nuclear RNPs are extruded from the cytoplasm of spontaneously apoptotic thymocytes. Exp Cell Res. 1997;234:512–20. doi: 10.1006/excr.1997.3657. 10.1006/excr.1997.3657. [DOI] [PubMed] [Google Scholar]
- 39.Belizna C, Tervaert JWC. Specificity, pathogenecity, and clinical value of antiendothelial cell antibodies. Semin Arthritis Rheum. 1997;27:98–109. doi: 10.1016/s0049-0172(97)80010-8. [DOI] [PubMed] [Google Scholar]