Abstract
It has been postulated that T lymphocytes orchestrate the chronic inflammation in bronchial asthma. In animal models, infiltration of CD8+ T lymphocytes into the bronchial mucosa prevented bronchial hyperresponsiveness and decreased early and late phase reaction. IFN-γ antagonizes IL-4-dependent IgE production as well as IL-5-induced proliferation and activation of eosinophils. We therefore investigated the secretion of IFN-γ of isolated CD8+ T lymphocytes from peripheral blood of patients with allergic asthma (n = 6) and from healthy controls (n = 7) in vitro. In this setting we compared the effect of stimulation with anti-CD3 antibodies with that of phorbol myristate acetate (PMA) and calcium-ionophore. As expected, CD8+ T lymphocytes from peripheral blood of healthy volunteers produced significantly more IFN-γ in the presence of PMA and calcium-ionophore than after stimulation with anti-CD3 antibodies. However, in subjects with allergic asthma, IFN-γ secretion of CD8+ T cells was significantly higher when incubated with anti-CD3 antibodies than after activation with PMA and calcium-ionophore. While IFN-γ secretion of CD8+ T lymphocytes of patients with allergic asthma was lower than that of healthy controls in the presence of PMA/calcium-ionophore, it was significantly elevated when compared with normal controls after stimulation with anti-CD3 antibodies. Thus, potent activators of cytokine secretion, such as PMA and calcium-ionophore, induce a cytokine profile different from that induced by weaker stimulants, such as anti-CD3 antibodies. These findings have implications for further studies investigating cytokine production of inflammatory cells in vitro.
Keywords: Interferon-γ, bronchial asthma, CD8+ T lymphocytes, in vitro stimulation
Introduction
Allergic asthma is an inflammatory disease of the airways triggered by T lymphocytes and dominated by eosinophils and mast cells. Contact with the specific allergen leads to IgE-mediated release of mast cell mediators in the bronchi followed by inflammation. Atopic inflammation has been linked to excess production of the T helper 2 (Th2) cytokines IL-4 and IL-5 relative to the Th1 cytokine IFN-γ [1]. This is based on the division of CD4+ T lymphocytes into two subsets first established in 1986 by Mosmann et al. [2]. Th1 cells produce IL-2, IFN-γ and TNF-α, and mediate immunity to viral and bacterial pathogens, whereas Th2 cells produce IL-4, IL-5, IL-6, IL-10 and IL-13, and are involved in allergic diseases as well as in defence against parasitic infections. A similar dichotomy has been described for CD8+ T lymphocytes [3–6]. In recent years, the strict categorization into Th1- and Th2-type responses was replaced by the concept that it is the balance between these antagonistic immune responses that leads to disease resolution [7].
The presence of CD8+ T lymphocytes in bronchoalveolar lavage after experimental allergen challenge in patients with allergic asthma has been associated with the suppression of the late phase response [8]. In an animal model of asthma, depletion of CD8+ T lymphocytes increased both the early phase and the late phase immune response [9,10]. CD8+ T lymphocytes have been shown to secrete IFN-γ [11], which negatively regulates IL-4 and IL-5 production [12–14]. IFN-γ is therefore an antagonistic cytokine for the IL-4-dependent IgE switch as well as for the IL-5-induced proliferation, activation and transmigration of eosinophils [15,16]. In a rat model of bronchial hyperresponsiveness, application of IFN-γ attenuated airway hyperresponsiveness, and reduced infiltration of eosinophil and neutrophil granulocytes and CD8+ T lymphocytes [17]. These data suggest that CD8+ T lymphocytes might play a protective role in the pathogenesis of allergic inflammation via their ability to produce IFN-γ. We therefore investigated the secretion of IFN-γ by isolated CD8+ T lymphocytes from peripheral blood of patients with allergic asthma and healthy controls.
Our knowledge of regulatory phenomena in the immune system is still widely based on in vitro experiments performed with various in vitro stimuli. Nevertheless, very little is known about the differential effects of these stimuli on cytokine secretion. In this study, we compared the effect of stimulation with PMA and calcium-ionophore to stimulation with antibodies to the T-cell epitope CD3. On the cell surface of CD8+ T lymphocytes, CD3 is associated with the T-cell receptor (TCR), the CD8 molecule and CD45. Binding of the specific antigen initiates a signalling cascade, which results in the activation of protein kinase C (PKC) and an increase in the intracellular calcium concentration [18,19]. Antibodies to CD3 can efficiently imitate the effect of antigen binding [20,21], whereas PMA directly activates PKC and calcium-ionophore promotes an increase in cytoplasmatic calcium levels [22,23].
Methods
Subjects
Six patients suffering from allergic asthma [24], who were out-patients at our pulmonary clinic, were recruited to participate in this study. Three male and three female patients were selected with a mean serum IgE of 210 ± 63 IU/ml (range 10·5–407 IU/ml), a mean FEV1 of 85·8 ± 8·3% of predicted (range 47–111%), and a positive skin prick test to common aeroallergens. All subjects had a history of intermittent wheeze, chest tightness, cough, sputum production and bronchial hyperreactivity. Patients used topical steroids and/or long- or short-acting beta-2-agonists and/or theophylline for treatment of asthma (see Table 1). Subjects taking systemic steroids were excluded from the study. Seven healthy volunteers with no history of asthma served as controls. There was no evidence to suggest a recent infection in any of the subjects participating in the study. All patients gave their informed consent and the study protocol was approved by the Ethics Committee of the University of Freiburg.
Table 1.
Patient characteristics
| Age (years) | Sex | Duration of asthma (years) | Eosinophils (%) | Prick test | IgG (U/ml) | RAST | FEV-1 (%) | Medication |
|---|---|---|---|---|---|---|---|---|
| 36 | m | 22 | 6·5 | pos. | 407 | 2 | 47 | TS, B |
| 22 | m | 5 | 2·8 | pos. | 93·2 | 4 | 111 | TS, B |
| 42 | m | 2 | 2·5 | pos. | 277 | 4 | 85 | TS, B |
| 46 | f | 1 | 3·7 | pos. | 10·5 | 2 | 77 | TS, (B) |
| 33 | f | 15 | 3·3 | pos. | n.d. | 4 | 97 | B, T |
| 33 | f | 2 | 3·5 | pos. | 263 | 4 | 98 | (B) |
TS, topical steroids; B, inhaled β2-agonists; T, theophylline; (), medication as needed.
Isolation of CD8+ T lymphocytes by magnetic cell sorting
Blood was drawn from the cubital vein into EDTA tubes. The separation of peripheral blood mononuclear cells (PBMCs) was performed on a gradient of Ficoll with a density of 1·077 g/ml (Seromed, Berlin, Germany) as previously reported [25]. Isolated PBMCs were washed twice and resuspended in phosphate-buffered saline (PBS) supplemented with 2% heat-inactivated fetal calf serum (FCS, Gibco, NY, USA). CD8+ T lymphocytes were negatively selected by magnetic cell sorting with a CD8+ T-cell isolation kit (Miltenyi Biotec, Bergisch-Gladbach, Germany). PBMCs were incubated with a mixture of hapten-conjugated antibodies to CD4, CD11b, CD16, CD19, CD36 and CD56. After two washing steps, paramagnetic microbeads conjugated to a monoclonal anti-hapten antibody were added. Cell separation was then performed with a depletion column in a magnetic field, as previously described [26].
Flow cytometry
Specific staining of the respective cell-surface molecules was performed by anti-human CD3-Cy5 (clone UCHT1; Dako, Hamburg, Germany), anti-human CD4-Cy5 (Dako), anti-human CD8-FITC (Guildhay Ltd, Guildford, UK), anti-human CD8-PE (clone DK25, Dako), anti-human CD11b-FITC (Immuno Quality Products, UK), anti-human CD14-FITC (Dako), anti-human CD16-PE (clone 3G8; Immunotech, Hamburg, Germany), anti-human CD56-PE (Immuno Quality Products) and anti-human CD19-PE (Immunotech). A 100 µl volume of the cell suspension (1 × 105 cells) was incubated with 10 µl fluorescence-conjugated antibody for 30 min at 4°C. The cells were then washed once and resuspended in 100 µl PBS. For determination of the percentage of dead cells, staining with propidium iodide (Sigma, Deisenhofen, Germany) was employed. Flow cytometry was performed on at least 104 cells per sample with a FACScan (Becton Dickinson, Heidelberg, Germany).
Cell culture and stimulation
Culture medium consisted of RPMI 1640 supplemented with 10% heat-inactivated FCS, 2 mm l-glutamine, 100 IU/ml penicillin and 100 µg/ml streptomycin. CD8+ T lymphocytes (3 × 106 cells/ml) or unseparated PBMCs (3 × 106 cells/ml) were cultured for 24 h at 37°C in a humidified atmosphere with 5% CO2. For stimulation with anti-CD3, culture plates were coated with monoclonal anti-CD3 antibody (20 µg/ml, clone OKT3, kindly provided by K. Blaser, Davos, Switzerland) at 4°C overnight according to the study protocols of Jung et al. and Walker et al. [27,28]. Phorbol myristate acetate (PMA) and calcium-ionophore A23187 (Sigma) were used in concentrations of 10−8 and 10−6 m, as previously described [28]. Culture supernatant fluids were stored in small aliquots at − 70°C until assayed for IFN-γ.
Determination of IFN-γ in culture supernatant fluids of CD8+ T lymphocytes
ELISA plates (Maxisorp, Nunc, Denmark) were coated with 1 µg/ml of a monoclonal anti-human IFN-γ antibody (clone 69, Intex, Muttenz, Switzerland) in coating buffer (0·1 m NaHCO3, pH 8·3) over 6 h at room temperature, washed with PBS/0·05% Tween, and blocked with 3% bovine serum albumin dissolved in PBS for 2 h. Culture supernatant fluids of stimulated CD8+ T lymphocytes and controls, as well as serial dilutions of recombinant IFN-γ (Hoffmann-La Roche, Basel, Switzerland), were then applied to each well and incubated at 4°C overnight. For detection, a 1 µg/ml dilution of biotinylated anti-human IFN-γ antibody (clone 69) was added for 45 min. After extensive washing with PBS/0·05% Tween, bound antibodies were detected by application of a conjugated avidin peroxidase (2·5 µg/ml) (Sigma) at room temperature for 30 min. ABTS substrate (Sigma) dissolved in 0·1 m citronic acid and 0·03% H2O2 was then added. The reaction was read at 450 nm (reference filter: 620 nm), and results were derived from a standard curve established with recombinant IFN-γ.
Detection of intracellular cytokines
Flow-cytometric detection of intracellular cytokines was performed as previously described [29]. In brief, isolated CD8+ T lymphocytes were stimulated with PMA and calcium-ionophore in the presence of brefeldin A (10 µg/ml). After incubation for 6 h at 37°C in a humidified atmosphere of 5% CO2, cells were washed in a washing buffer (PBS, 1% FCS, 0·1% NaN3) and fixed in 1 ml 4% ice-cold paraformaldehyde (Merck, Darmstadt, Germany) for 30 min. After a further wash in washing buffer, cells were resuspended in 20 µl saponin buffer (PBS, 1% FCS, 0·1% saponin). Saponin was purchased from Fluka, Buchs, Switzerland. Anti-IFN-γ-FITC (clone B27, Pharmingen, Hamburg, Germany) was added to the cell suspension and incubated for 15 min. As controls, FITC-labelled non-specific mouse IgG1 antibodies were used (Pharmingen). After two further washes in saponin buffer, cells were resuspended in 100 µl PBS for flow-cytometric evaluation.
Statistical analysis
Results are expressed as arithmetic means ± s.e.m. Differences between groups were analysed using the Mann–Whitney U-test. Differences with P-values < 0·05 were considered significant.
Results
PBMC subpopulations in allergic asthma and healthy controls
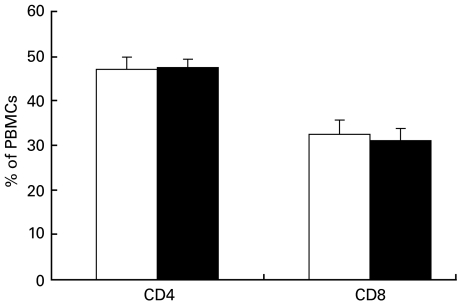
After Ficoll separation, PBMCs were incubated with fluorescence-labelled antibodies to the CD4 and CD8 surface markers. The percentage of CD4 and CD8 positive cells in PBMCs was analysed by flow cytometry. As shown in Fig. 1, there was no difference between PBMCs from patients with allergic asthma and healthy controls with regard to the percentage of CD4 and CD8 positive cells. In healthy controls, PBMCs consisted of 47 ± 2·9% CD4 and 32 ± 3·4% CD8 positive cells. In patients with allergic asthma, there were 48 ± 1·9% CD4 and 31 ± 2·7% CD8 positive events (Fig. 1).
Fig. 1.
Percentage of CD4+ and CD8+ in PBMCs after Ficoll separation. In patients with allergic asthma (▪), PBMCs consisted of 48 ± 1·9% CD4+ and 31 ± 2·7% CD8+ cells. In healthy controls (□), 47 ± 2·9% of PBMCs were CD4+ and 32 ± 3·4% were CD8+. Values are mean ± s.e.m., n = 7 in healthy controls, n = 6 in patients with allergic asthma.
Isolation of CD8+ T lymphocytes by magnetic cell sorting
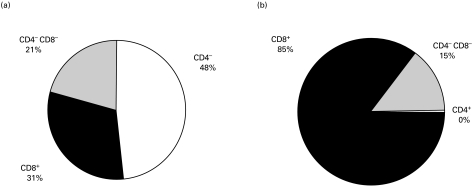
PBMCs and isolated CD8+ T lymphocytes were analysed by flow cytometry after incubation with fluorescence-labelled antibodies to CD4 and CD8 (Fig. 2), as well as antibodies to CD3, CD14, CD16, CD56, CD19 and CD11b (data not shown). As depicted in Fig. 2, the suspension of isolated CD8+ T lymphocytes consisted of 85 ± 7% CD8+ events (compared with 31 ± 7% CD8+ events in the PBMCs), 14 ± 7% CD4−CD8− cells and practically no contaminating CD4+ T lymphocytes. The CD4−CD8− cells did not stain with antibodies to CD3, CD14, CD16, CD56, CD19 and CD11b (data not shown).
Fig. 2.
Isolation of CD8+ T lymphocytes by magnetic cell sorting. Percentages of CD4+, CD8+ and CD4−CD8− cells are shown before (a) and after (b) magnetic cell sorting. The isolated CD8+ fraction consisted of 85 ± 7% CD8+ T lymphocytes, practically no contaminating CD4+ T lymphocytes and 14 ± 7% CD8−CD4− cells, which could not further be characterized by CD3, CD14, CD16, CD56, CD19 and CD11b surface markers (data not shown). Values are mean ± s.e.m., n = 22.
IFN-γ secretion of CD8+ T lymphocytes from peripheral blood of patients with allergic asthma and healthy controls
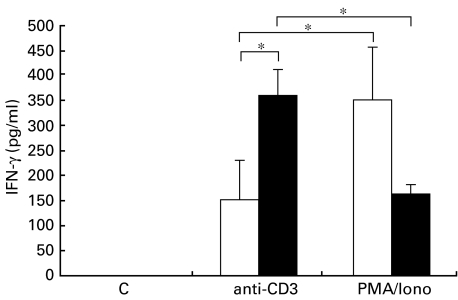
IFN-γ secretion of CD8+ T lymphocytes from peripheral blood of patients with allergic asthma was compared with the secretion of CD8+ T lymphocytes from peripheral blood of healthy control subjects. Neither group demonstrated detectable spontaneous secretion of IFN-γ. After stimulation with anti-CD3 antibodies, IFN-γ levels in supernatant fluids of CD8+ T lymphocytes were significantly higher in patients with allergic asthma than in healthy control subjects (359 ± 51 pg/ml versus 151 ± 79 pg/ml) (Fig. 3).
Fig. 3.
IFN-γ secretion of peripheral blood CD8+ T lymphocytes. Isolated CD8+ T lymphocytes from peripheral blood of (□) healthy volunteers (n = 7) and (▪) patients with allergic asthma (n = 6) were stimulated for 24 h with anti-CD3 antibodies (20 µg/ml) or PMA (10−8 m) and calcium-ionophore (10−6 m). Release of IFN-γ in culture supernatant fluids was detected by ELISA (pg/ml). Values are mean ± s.e.m., *P < 0·05.
In the presence of PMA/calcium-ionophore, the mean concentration of IFN-γ in culture supernatant fluids of CD8+ T lymphocytes of healthy volunteers was twice as high as in patients with allergic asthma (352 ± 104 pg/ml versus 163 ± 19 pg/ml). However, this difference failed to reach statistical significance (Fig. 3).
IFN-γ secretion was significantly higher in healthy volunteers after stimulation with PMA/calcium-ionophore than in the presence of anti-CD3 antibodies (352 ± 104 pg/ml compared with 151 ± 79 pg/ml, P < 0·05). However, in patients with allergic asthma, IFN-γ secretion of CD8+ T lymphocytes was significantly higher after stimulation with anti-CD3 antibodies than after stimulation with PMA/calcium-ionophore (359 ± 51 pg/ml compared with 163 ± 19 pg/ml, P < 0·05) (Fig. 3).
After either stimulation, the percentage of dead cells was less than 1%, as determined by PI staining (data not shown).
IFN-γ secretion of unseparated mononuclear cells from peripheral blood of patients with allergic asthma and healthy controls
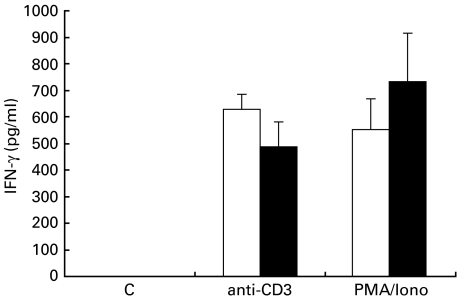
IFN-γ secretion of unseparated mononuclear cells from peripheral blood (PBMCs) of patients with allergic asthma was compared with the secretion of PBMCs of healthy control subjects. There was no detectable spontaneous secretion of IFN-γ. After stimulation with anti-CD3 antibodies, IFN-γ levels in supernatant fluids of PBMCs were slightly higher in healthy control subjects than in patients with allergic asthma (631 ± 55 pg/ml versus 486 ± 96 pg/ml). In the presence of PMA/calcium-ionophore, the mean concentration of IFN-γ in culture supernatant fluids of PBMCs of healthy volunteers was lower than in patients with allergic asthma (550 ± 114 pg/ml versus 733 ± 183 pg/ml). These differences, however, failed to reach statistical significance (Fig. 4).
Fig. 4.
IFN-γ secretion of unseparated peripheral blood mononuclear cells. PBMCs from (□) healthy volunteers (n = 7) and (▪) patients with allergic asthma (n = 6) were stimulated for 24 h with anti-CD3 antibodies (20 µg/ml) or PMA (10−8 m) and calcium-ionophore (10−6 m). Release of IFN-γ in culture supernatant fluids was detected by ELISA (pg/ml). Values are mean ± s.e.m.
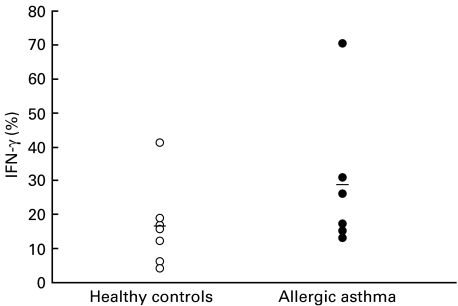
Percentage IFN-γ producing cells in isolated CD8+ T lymphocytes from patients with allergic asthma and healthy controls
The percentage of IFN-γ-producing cells in isolated CD8+ T lymphocytes from patients with allergic asthma and healthy controls was analysed by intracellular cytokine staining and flow-cytometric analysis after 6 h of stimulation with PMA and calcium-ionophore. The percentage of positively stained cells ranged from 4 to 70% with a mean IFN-γ-producing population of 29 ± 8% in patients with allergic asthma and of 16 ± 4% in healthy volunteers. Though the percentage of IFN-γ-producing CD8 lymphocytes tended to be greater in patients with allergic asthma, these differences were not statistically significant (Fig. 5). There was no correlation between the amount of IFN-γ in culture supernatant fluids and the percentage of IFN-γ-producing cells.
Fig. 5.
Intracellular cytokine detection. Isolated CD8+ T lymphocytes from peripheral blood of (□) healthy volunteers (n = 7) and (▪) patients with allergic asthma (n = 6) were stimulated for 6 h with PMA (10−8 m) and calcium-ionophore (10−6 m) in the presence of brefeldin A (10 µg/ml). Percentage of IFN-γ-positive cells was measured by flow cytometry. The arithmetic mean is indicated by a bar.
Discussion
T lymphocytes orchestrate the generation of a chronic inflammation dominated by mast cells and eosinophils in the pathogenesis of allergic asthma. In addition to their role in the killing of infected cells, CD8+ T lymphocytes are able to secrete a large variety of cytokines and strongly influence the activation and regulation of CD4 cells and other immune cells [3–6].
In vitro studies of regulatory mechanisms in the immune system are based on stimulation with different in vitro stimuli. As yet, knowledge about the effects of these stimuli on cytokine production of cell cultures is still very limited. In this study, we compared the effect of stimulation with anti-CD3 antibodies and PMA/calcium-ionophore on IFN-γ secretion of isolated CD8+ T lymphocytes of patients with allergic asthma and healthy controls. In subjects with allergic asthma, IFN-γ secretion of CD8+ T cells after stimulation with antibodies to the T-cell epitope CD3 was significantly higher than after stimulation with PMA and calcium-ionophore. In contrast, CD8+ T lymphocytes from peripheral blood of healthy volunteers produced significantly more IFN-γ in the presence of PMA and calcium-ionophore than after stimulation with anti-CD3 antibodies. While IFN-γ secretion of CD8+ T lymphocytes of patients with allergic asthma was lower than that of healthy controls in the presence of PMA/calcium-ionophore, it was significantly elevated when compared with normal controls after stimulation with anti-CD3 antibodies. This observation appears to be specific for CD8+ T lymphocytes, as analysis of IFN-γ secretion from PBMCs did not reveal differences in susceptibility to the employed stimuli in the same donors. In PBMCs, IFN-γ secretion was generally higher than in isolated CD8+ T lymphocytes.
Isolation of CD8+ T lymphocytes from peripheral blood was performed by magnetic cell sorting. The suspension of isolated CD8+ T lymphocytes consisted of 85 ± 7% CD8+ events, 14 ± 7% CD4−CD8− cells and practically no contaminating CD4+ T lymphocytes. The CD4−CD8− cells did not stain with antibodies to CD3, CD14, CD16, CD56, CD19 and CD11b. We therefore assume that those CD4−CD8− events in flow cytometry resulted from agglutinated erythrocytes and other cell detritus, rather than from cells that would have influenced cytokine production of CD8+ T lymphocytes. Nevertheless, it cannot be excluded that these contaminants might contain cells that have down-regulated the markers used for the negative selection.
To our knowledge, this is the first study investigating IFN-γ secretion of isolated CD8+ T lymphocytes from peripheral blood of humans. Earlier examinations of IFN-γ production in peripheral blood cells were performed in unseparated PBMCs [12,30,31]. In these studies, decreased IFN-γ secretion in PBMCs of allergic subjects was found after stimulation with PMA and calcium-ionophore, PHA and concanavalin A. The authors postulated an intrinsic defect of IFN-γ production in blood cells of these patients, which suited the Th1/Th2 hypothesis for the pathogenesis of allergy. This conclusion, however, is in contrast to our study. As we were able to show, CD8+ T lymphocytes from peripheral blood of patients with allergic asthma have a strong potential to secrete IFN-γ. Interestingly, our observations are confirmed by recent findings in whole blood cultures of patients with bronchial asthma, which reported an increase in IFN-γ-containing CD8+ T lymphocytes [32]. Krug et al. found an increased number of T cells producing IFN-γ in Bronchoalveolar lavage fluid from subjects with atopic asthma compared with atopic non-asthmatics and healthy controls. However, no such difference was detected in peripheral blood T cells from the same donors [33]. This observation is in agreement with our findings in which intracellular staining of IFN-γ in CD8+ T lymphocytes did not reveal any significant differences between patients with allergic asthma and healthy controls.
IFN-γ is associated with a variety of functions in the pathogenesis of allergic diseases. It is able to inhibit proliferation of Th2 cells in vitro and to antagonize Th2-type responses such as IL-4-dependent IgE production and IL-5-dependent activation and infiltration of eosinophils [12–14]. CD95 expression on peripheral blood lymphocytes of normal subjects, as well as the sensitivity of human T cells to CD95-mediated apoptosis, are increased in the presence of IFN-γ [34,35]. IFN-γ also facilitates CD95-mediated apoptosis of eosinophils in vitro [36,37]. Thus, the potential of CD8+ T lymphocytes to secrete IFN-γ (as this study describes) could influence the pathogenesis of allergic asthma not only by counterbalancing the development of atopy but also by regulating apoptosis of inflammatory cells. It may be speculated that in CD8+ T lymphocytes from patients with allergic asthma, IFN-γ production is up-regulated in order to compensate for the preferential Th2-type cytokine secretion pattern of other PBMCs. However, IFN-γ secretion of CD8+ T lymphocytes might not be sufficient to prevent the development of allergic disease.
In this study we have found fundamental differences in IFN-γ secretion of CD8+ T lymphocytes in response to different in vitro stimuli. Susceptibility to stimulation with PMA/calcium-ionophore and anti-CD3 antibodies differed in patients with bronchial asthma and healthy controls. Comparisons of the effects of stimulation with anti-CD3 antibodies and PMA/calcium-ionophore have not been published as yet.
Binding of antibodies to the CD3 complex can efficiently imitate the effect of antigen presentation and leads to activation of PKC and calcium influx [20,21]. As these effects are most likely mediated by the same structures and the same intracellular signalling cascade as T-cell activation by the TCR, stimulation with anti-CD3 antibodies is assumed to be a somewhat more physiological stimulus. However, the protocol of cell separation, the concentration of the in vitro stimulus and the lack of co-stimulatory events and physiological cell–cell interaction have to be considered when interpreting the results of stimulation of cell cultures with anti-CD3 antibodies. In our study, IFN-γ production of anti-CD3-activated CD8+ T lymphocytes was significantly higher in patients with allergic asthma than in healthy controls. These differences might be due to differences in the pre-activation of CD8+ T cells, where relatively weak stimuli, such as anti-CD3 antibodies, can strongly affect cells pre-stimulated in vivo.
T-cell activation with PMA and calcium-ionophore is independent of the T-cell receptor complex and its signalling cascade [22,23]. This combination is regarded as a very strong stimulus for cell cultures. As expected, IFN-γ production by CD8+ T lymphocytes from peripheral blood of healthy donors was significantly higher after stimulation with PMA/calcium-ionophore than in the presence of anti-CD3 antibodies [38]. In contrast, CD8+ T lymphocytes from patients with allergic asthma secreted significantly more IFN-γ when activated with anti-CD3 antibodies than after stimulation with PMA/calcium-ionophore. We presume that the susceptibility of cells in patients with allergic diseases to either PMA or calcium-ionophore is altered.
In conclusion, potent activators of cytokine secretion such as PMA and calcium-ionophore induce a cytokine secretion different from that induced by weaker stimulators such as anti-CD3 antibodies. These findings have implications for further in vitro studies investigating cytokine production of inflammatory cells.
Acknowledgments
The authors wish to thank Sieglinde Bock for expert technical assistance and Karin Gierke for her help in the preparation of the manuscript. This work was supported by a grant from the Bundesministerium für Bildung, Wissenschaft, Forschung und Technologie (Klinische Forschergruppe Allergologie BMBF01GC9701/7).
References
- 1.Romagnani S. Lymphokine production by human T cells in disease states. Annu Rev Immunol. 1994;227:227–57. doi: 10.1146/annurev.iy.12.040194.001303. [DOI] [PubMed] [Google Scholar]
- 2.Mosmann TR, Cherwinski H, Bond MW, Giedlin MA, Coffman RL. Two types of murine helper T cell clone. J Immunol. 1986;136:2348–57. [PubMed] [Google Scholar]
- 3.Croft M, Carter L, Swain SL, Dutton RW. Generation of polarized antigen-specific CD8 effector populations: reciprocal action of interleukin (IL)-4 and IL-12 in promoting type 2 versus type 1 cytokine profiles. J Exp Med. 1994;180:1715–28. doi: 10.1084/jem.180.5.1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Maggi E, Giudizi MG, Biagiotti R, et al. Th2-like CD8+ T cells showing B cell helper function and reduced cytolytic activity in human immunodeficiency virus type 1 infection. J Exp Med. 1994;180:489–95. doi: 10.1084/jem.180.2.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sad S, Marcotte R, Mosmann TR. Cytokine-induced differentiation of precursor mouse CD8+ T cells into cytotoxic CD8+ T cells secreting Th1 or Th2 cytokines. Immunity. 1995;2:271–9. doi: 10.1016/1074-7613(95)90051-9. [DOI] [PubMed] [Google Scholar]
- 6.Salgame P, Abrams JS, Clayberger C, et al. Differing lymphokine profiles of functional subsets of human CD4 and CD8 T cell clones. Science. 1991;254:279–82. doi: 10.1126/science.254.5029.279. [DOI] [PubMed] [Google Scholar]
- 7.Borish L, Rosenwasser L. TH1/TH2 lymphocytes: doubt some more. J Allergy Clin Immunol. 1997;99:161–4. doi: 10.1016/s0091-6749(97)70090-3. [DOI] [PubMed] [Google Scholar]
- 8.Gonzalez MC, Diaz P, Galleguillos FR, Ancic P, Cromwell O, Kay AB. Allergen-induced recruitment of bronchoalveolar helper (OKT4) and suppressor (OKT8) T-cells in asthma. Am Rev Respir Dis. 1987;136:600–4. doi: 10.1164/ajrccm/136.3.600. [DOI] [PubMed] [Google Scholar]
- 9.Huang TJ, MacAry PA, Kemeny DM, Chung KF. Effect of CD8+ T-cell depletion on bronchial hyper-responsiveness and inflammation in sensitized and allergen-exposed Brown-Norway rats. Immunology. 1999;96:416–23. doi: 10.1046/j.1365-2567.1999.00699.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Olivenstein R, Renzi PM, Yang JP, et al. Depletion of OX-8 lymphocytes from the blood and airways using monoclonal antibodies enhances the late airway response in rats. J Clin Invest. 1993;92:1477–82. doi: 10.1172/JCI116725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Renz H, Lack G, Saloga J, et al. Inhibition of IgE production and normalization of airways responsiveness by sensitized CD8 T cells in a mouse model of allergen-induced sensitization. J Immunol. 1994;152:351–60. [PubMed] [Google Scholar]
- 12.Romagnani S, Maggi E, Del Prete G, et al. Role of interleukin 4 and gamma interferon in the regulation of human IgE synthesis: possible alterations in atopic patients. Int Arch Allergy Appl Immunol. 1989;88:111–3. doi: 10.1159/000234758. [DOI] [PubMed] [Google Scholar]
- 13.Maggi E, Parronchi P, Manetti R, et al. Reciprocal regulatory effects of IFN-gamma and IL-4 on the in vitro development of human Th1 and Th2 clones. J Immunol. 1992;148:2142–7. [PubMed] [Google Scholar]
- 14.Finkelman FD, Katona IM, Mosmann TR, Coffman RL. IFN-gamma regulates the isotypes of Ig secreted during in vivo humoral immune responses. J Immunol. 1988;140:1022–7. [PubMed] [Google Scholar]
- 15.Iwamoto I, Nakajima H, Endo H, Yoshida S. Interferon gamma regulates antigen-induced eosinophil recruitment into the mouse airways by inhibiting the infiltration of CD4+ T cells. J Exp Med. 1993;177:573–6. doi: 10.1084/jem.177.2.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nakajima H, Iwamoto I, Yoshida S. Aerosolized recombinant interferon-gamma prevents antigen-induced eosinophil recruitment in mouse trachea. Am Rev Respir Dis. 1993;148:1102–4. doi: 10.1164/ajrccm/148.4_Pt_1.1102. [DOI] [PubMed] [Google Scholar]
- 17.Huang TJ, MacAry PA, Wilke T, Kemeny DM, Chung KF. Inhibitory effects of endogenous and exogenous interferon-gamma on bronchial hyperresponsiveness, allergic inflammation and T-helper 2 cytokines in Brown-Norway rats. Immunology. 1999;98:280–8. doi: 10.1046/j.1365-2567.1999.00870.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Weiss A, Littman DR. Signal transduction by lymphocyte antigen receptors. Cell. 1994;76:263–74. doi: 10.1016/0092-8674(94)90334-4. [DOI] [PubMed] [Google Scholar]
- 19.Szamel M, Resch K. T-cell antigen receptor-induced signal-transduction pathways — activation and function of protein kinases C in T lymphocytes. Eur J Biochem. 1995;228:1–15. doi: 10.1111/j.1432-1033.1995.tb20221.x. [DOI] [PubMed] [Google Scholar]
- 20.Van Wauwe J, De Mey J, Goossens JG. OKT3: a monoclonal anti-human T lymphocyte antibody with potent mitogenic properties. J Immunol. 1980;124:2708–13. [PubMed] [Google Scholar]
- 21.Meuer SC, Hodgdon JC, Hussey RE, Protentis JP, Schlossman SF, Reinherz EL. Antigen-like effects of monoclonal antibodies directed at receptors on human T cell clones. J Exp Med. 1983;158:988–93. doi: 10.1084/jem.158.3.988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Niedel JE, Kuhn LJ, Vandenbark GR. Phorbol diester receptor copurifies with protein kinase C. Proc Natl Acad Sci USA. 1983;80:36–40. doi: 10.1073/pnas.80.1.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tsien RY, Pozzan T, Rink TJ. T-cell mitogens cause early changes in cytoplasmic free Ca2+ and membrane potential in lymphocytes. Nature. 1982;295:68–71. doi: 10.1038/295068a0. [DOI] [PubMed] [Google Scholar]
- 24.Anonymous. International Consensus Report on Diagnosis and Treatment of Asthma. Eur Respir J. 1992;5:601–41. [PubMed] [Google Scholar]
- 25.Luttmann W, Herzog V, Virchow JC, Jr, Matthys H, Thierauch KH, Kroegel C. Prostacyclin modulates granulocyte/macrophage colony-stimulating factor release by human blood mononuclear cells. Pulm Pharmacol. 1996;9:43–8. doi: 10.1006/pulp.1996.0005. 10.1006/pulp.1996.0005. [DOI] [PubMed] [Google Scholar]
- 26.Miltenyi S, Muller W, Weichel W, Radbruch A. High gradient magnetic cell separation with MACS. Cytometry. 1990;11:231–8. doi: 10.1002/cyto.990110203. [DOI] [PubMed] [Google Scholar]
- 27.Walker C, Virchow JC, Jr, Bruijnzeel PL, Blaser K. T cell subsets and their soluble products regulate eosinophilia in allergic and nonallergic asthma. J Immunol. 1991;146:1829–35. [PubMed] [Google Scholar]
- 28.Jung T, Lack G, Schauer U, et al. Decreased frequency of interferon-gamma- and interleukin-2-producing cells in patients with atopic diseases measured at the single cell level. J Allergy Clin Immunol. 1995;96:515–27. doi: 10.1016/s0091-6749(95)70296-2. [DOI] [PubMed] [Google Scholar]
- 29.Jung T, Schauer U, Heusser C, Neumann C, Rieger C. Detection of intracellular cytokines by flow cytometry. J Immunol Methods. 1993;159:197–207. doi: 10.1016/0022-1759(93)90158-4. [DOI] [PubMed] [Google Scholar]
- 30.Tang M, Kemp A, Varigos G. IL-4 and interferon-gamma production in children with atopic disease. Clin Exp Immunol. 1993;92:120–4. doi: 10.1111/j.1365-2249.1993.tb05957.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Walker C, Bode E, Boer L, Hansel TT, Blaser K, Virchow JC., Jr Allergic and nonallergic asthmatics have distinct patterns of T-cell activation and cytokine production in peripheral blood and bronchoalveolar lavage. Am Rev Respir Dis. 1992;146:109–15. doi: 10.1164/ajrccm/146.1.109. [DOI] [PubMed] [Google Scholar]
- 32.Magnan AO, Mely LG, Camilla CA, et al. Assessment of the Th1/Th2 paradigm in whole blood in atopy and asthma. Am J Respir Crit Care Med. 2000;161:1790–6. doi: 10.1164/ajrccm.161.6.9906130. [DOI] [PubMed] [Google Scholar]
- 33.Krug N, Madden J, Redington AE, et al. T-cell cytokine profile evaluated at the single cell level in BAL and blood in allergic asthma. Am J Respir Cell Mol Biol. 1996;14:319–26. doi: 10.1165/ajrcmb.14.4.8600935. [DOI] [PubMed] [Google Scholar]
- 34.Novelli F, Bernabei P, Ozmen L, et al. Switching on of the proliferation or apoptosis of activated human T, lymphocytes by IFN, -gamma is correlated with the differential expression of the alpha- and beta-chains of its receptor. J Immunol. 1996;157:1935–43. [PubMed] [Google Scholar]
- 35.Kawakami A, Eguchi K, Matsuoka N, et al. Expression and function of Fas and Fas ligand on peripheral blood lymphocytes in normal subjects. J Laboratory Clin Med. 1998;132:404–13. doi: 10.1016/s0022-2143(98)90111-1. [DOI] [PubMed] [Google Scholar]
- 36.Luttmann W, Opfer A, Dauer E, et al. Differential regulation of CD95 (Fas/APO-1) expression in human blood eosinophils. Eur J Immunol. 1998;28:2057–65. doi: 10.1002/(SICI)1521-4141(199807)28:07<2057::AID-IMMU2057>3.0.CO;2-T. 10.1002/(sici)1521-4141(199807)28:07<2057::aid-immu2057>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 37.Luttmann W, Dauer E, Schmidt S, et al. Effects of interferon-gamma and tumour necrosis factor-alpha on CD95/Fas ligand-mediated apoptosis in human blood eosinophils. Scand J Immunol. 2000;51:54–9. doi: 10.1046/j.1365-3083.2000.00645.x. 10.1046/j.1365-3083.2000.00645.x. [DOI] [PubMed] [Google Scholar]
- 38.Rostaing L, Tkaczuk J, Durand M, et al. Kinetics of intracytoplasmic Th1 and Th2 cytokine production assessed by flow cytometry following in vitro activation of peripheral blood mononuclear cells. Cytometry. 1999;35:318–28. doi: 10.1002/(sici)1097-0320(19990401)35:4<318::aid-cyto4>3.0.co;2-4. 10.1002/(sici)1097-0320(19990401)35:4<318::aid-cyto4>3.3.co;2-w. [DOI] [PubMed] [Google Scholar]