Abstract
Activation and proliferation of lymphocytes requires the active signal transducer Ras. Activation of lymphocytes, associated with autoimmunity, may therefore be modified by S-farnesylthiosalicylic acid (FTS), a synthetic substance that detaches Ras from the inner cell membrane and induces its rapid degradation. The MRL/lpr mouse is a genetic model of a generalized autoimmune disease sharing many features and organ pathology with systemic lupus erythematosus (SLE) and the primary antiphospholipid syndrome (APS). The objective of the present study was to examine the effect of FTS on laboratory and clinical pathology in the MRL/lpr mouse. Female MRL/lpr (n = 50) and MRL/++ control (n = 35) mice were treated intraperitoneally with either FTS (5 mg/kg/day) or saline between 6 and 18 weeks of age. The mice were weighed, tested for proteinuria and lymphadenopathy, lymphocyte proliferation, antibodies, grip strength and behaviour in an open field. FTS treatment resulted in a 50% decrease in splenocyte proliferation to ConA, LPS and a disease specific antigen, β2-glycoprotein-I, and in a significant decrease in serum antibody levels against cardiolipin and dsDNA. Proteinuria and grip strength were normalized and lymphadenopathy and postmortem lymph node and spleen weights were significantly reduced in FTS treated MRL/lpr mice. These findings indicate that modulation of Ras activation has a significant impact on the MRL/lpr model and may represent a new therapeutic approach for the treatment of systemic autoimmune diseases such as SLE and APS.
Keywords: antiphospholipid syndrome, Ras, lymphocyte activation, MRL/lpr
Introduction
Autoimmune diseases are a group of disorders involving dysfunction of the immune system that results in tissue damage. Such processes may affect any organ through antibody binding, cellular immunity or factors such as cytokines. Epidemiologically, the autoimmune diseases are significant both in the numbers of patients involved and by the serious morbidity and mortality which they cause.
Autoimmune diseases are probably initiated by genetic and environmental factors and are mediated and propagated through controlling factors in the immune system, especially lymphocytes. The activation of lymphocytes, both T and B subtypes, involves a complex interaction of cell surface receptors resulting in equally complex signal transduction pathways that eventually affect gene regulation [1,2]. Full activation of lymphocytes requires parallel stimulation of several signal transduction pathways [3,4]. One of these pathways involves the GTP-binding protein Ras, and therefore inhibition of Ras activation may result in suppression of T lymphocyte activation [5,6].
Ras-dependent signalling requires not only that Ras be GTP bound, but also that it be associated with the inner leaflet of the cell membrane [7]. Specific anchorage of Ras proteins in the cell membrane is promoted inter alia by their carboxy terminal S-farnesyl cysteine [8–10]. A recently developed farnesyl analogue, S-trans-trans-farnesylthiosalicylic acid (FTS), destabilizes the attachment of Ras to the cell membrane [11,12] and is a potent inhibitor of Ras-mediated signal transduction pathways [13–15]. Indeed, FTS appears to act on specific membrane domains that associate with Ras, since the compound and its analogues present distinctive structure–function relationships with respect to dislodgement of Ras from cell membranes [11,12,14] and inhibition of Ras-dependent signalling [11,15]. Importantly, FTS exhibits significant selectivity towards Ras in its active, GTP-bound form. This was evident in experiments demonstrating FTS inhibition of growth of Ras-transformed cells and inhibition of ligand-stimulated fibroblast proliferation [12,14,15]. The unique properties of FTS among other compounds that mimic Ras anchorage moieties [14,16] confers on it the ability to disrupt the interactions of Ras with the cell membrane in live cells without cytotoxicity. The selectivity of FTS to active Ras may explain the relative nontoxic nature of this compound, which makes it a good candidate for clinical use in autoimmune disease.
Systemic lupus erythematosus (SLE) and the primary antiphospholipid (Hughes) syndrome (APS) are relatively common chronic autoimmune diseases affecting multiple organs. The treatments of SLE and APS include corticosteroids and antineoplastic/chemotherapeutic agents [17], which are only partially effective. Experimental models of SLE and APS serve as useful tools for the investigation of experimental therapies. The genetically determined MRL/lpr and NZB/W mice serve as models for both systemic and neurological manifestations of SLE and APS which include circulating autoantibodies, thrombocytopenia, renal dysfunction, spontaneous abortions, motor deficits, neuromuscular disorders, cognitive deficits and behavioural changes [18–20] and high levels of Ras [21]. In the present study we evaluated the chronic use of FTS on disease manifestations in the MRL/lpr model.
Materials and methods
Mice
Female MRL/MpJ/lpr/lpr (MRL/lpr, n = 50) mice and age-matched MRL/MpJ/+/+(MRL/++, n = 35) mice were purchased from Jackson Laboratories (Bar Harbor, Maine, USA) at 4 weeks of age and ICR mice, aged 3 months. The mice were housed in the Laboratory Animal Housing Facility at the Tel Aviv University Medical School. This facility is maintained under standard conditions, 23 ± 1°C, 12-h light cycle (7 a.m.−7 p.m.) with ad libitum access to food and drink. The mice were weighed prior to the start of the experiment and weekly thereafter. The Animal Welfare Committee approved all procedures.
Drug
FTS was synthesized as previously described [16]. FTS was stored in chloroform, which was evaporated under a stream of nitrogen immediately before use. The powder was dissolved in absolute ethanol and diluted to the desired concentration in sterile saline made basic with NaOH. Carrier solution (200 µl) containing 100 µg of FTS (5 mg/kg) were injected intraperitoneally (i.p.) into each mouse. Control solution was prepared at the same time starting with a chloroform solution.
We performed three experiments with three protocols of treatment: (1) mice were treated once a day, three times a week starting from 6 weeks of age until 18 weeks of age; (2) mice were treated once a day, five times a week starting from 10 weeks of age until 18 weeks of age; and (3) mice were treated once a day, five times a week starting from 6 weeks of age until 18 weeks of age. In the first experiment there were groups of five mice and in the next two experiments there were groups of 5–10 mice.
Spleen lymphocyte proliferation
The following method was used for the ex-vivo spleen lymphocyte proliferation assay. Mice were killed by cervical dislocation and spleens removed with sterile precautions, and placed in disposable plastic Petri dishes containing Dulbecco's phosphate-buffered saline (DPBS). Single cell suspensions were obtained by passing DPBS through the spleen using a syringe and 19-gauge needle. The cells were suspended in DPBS and centrifuged at 1100 r.p.m. for 7 min. Erythrocytes were lysed by a 7-min incubation in 0·83% (weight/volume) ammonium chloride, and cells were immediately washed thrice with DPBS. Spleen lymphocytes were suspended to a concentration of 3 × 106 cells/ml in RPMI-1640 medium containing 5% fetal calf serum (FCS), 100 units/ml penicillin, 100 µg/ml streptomycin, 2 mm l-glutamine, 0·1 mm non-essential amino acids, 1 mm sodium pyruvate and 50 µm 2-mercaptoethanol. Cells were cultured at a concentration of 6 × 105 cells/200 µl culture medium/well in 96-well, flat-bottomed, microculture plates, and were incubated for 72 h in a humidified atmosphere of 95% air and 5% CO2 at 37°C. At the end of this time, 1 µCi tritiated thymidine ([3H]TdR) was added to each well in a 10-µl volume and the cultures were incubated for a further 18 h. Cells from each microculture were harvested on fibroglass filters with multiharvester and counted in a liquid scintillation β counter.
Mitogens and antigens were diluted to appropriate concentrations in the incubation medium and added to the wells at the beginning of incubation period to give a final concentration of 1·0 µg/ml lipopolysaccharide (LPS), 1·0 µg/ml concanavalin A (ConA) or 10 µg/ml beta2-glycoprotein I (β2-GPI). Spontaneous proliferation (without mitogen or antigen) was also assessed.
For determining the effect of FTS on the spleen lymphocyte proliferation in vitro, cell suspensions were prepared from two naive ICR mice. The spleen lymphocyte proliferation was evaluated in the presence of LPS or ConA, and in the presence of 0, 10 and 50 µm FTS.
To determine the effect of FTS treatment from 6 to 18 weeks of age, five times a week, on the MRL/lpr and MRL/++ mouse spleen lymphocyte proliferation ex vivo, spleen cells suspensions were prepared from three individual spleens per group, and were analysed separately in culture (in triplicate). Spleen lymphocyte proliferation was evaluated in the presence of LPS, ConA or β2-GPI. The ex vivo proliferative assays performed 24 h after the last dose of FTS. Since the preliminary estimate of FTS half-life is about 30 min, it is unlikely that there were significant levels of FTS in the assay as performed.
The results are expressed as the mean value of stimulation index per group. The stimulation index was calculated as follows: the mean decomposition per minutes (dpm) of cells cultured in the presence of mitogen/antigen divided by the mean dpm of cells cultured in the absence of mitogen/antigen. Both the in vitro and the ex vivo experiments represent at least three replications which gave similar results.
Determination of Ras in spleen lymphocytes
Spleen lymphocytes, obtained from six mice as described above, were pooled and plated in 10-cm dishes containing RPMI/5% FCS at a density of 2·3 × 107 cells per plate. Cultures were maintained at 37°C in a humidified incubator (5% CO2/95% air) and FTS (12·5 and 25 µm) or the vehicle (0·1% DMSO) were added 1 h after plating. Twenty-four hours later the cells were collected (pools of two plates for each treatment) and washed in PBS. The cells were then homogenized in homogenization buffer containing protease inhibitors. Ras was determined in total cell membranes (P100) and cytosol (S100) obtained by centrifugation (100 000 g, 30min, 4°C) as detailed elsewhere. Briefly, 25 µg of total cellular proteins were separated by 12·5% sodium dodecylsulphate polyacrylamide gel electrophoresis (SDS-PAGE) and blotted onto nitrocellulose membranes. Immunoblotting with pan-Ras, enhanced chemiluminescense assays (ECL) and densitometric analysis were then performed as detailed previously [11].
Serological evaluations
The mice were bled at 16 weeks of age by retro-orbital sinus puncture and the sera were separated by centrifugation and stored at −70°C until assayed. The sera were tested by ELISA for the presence of different autoantibodies as described previously [22,23]. This included serum-dependent (β2-GPI dependent) and -independent antibodies to cardiolipin (aCL) and antibodies to single and double-stranded DNA (antissDNA, antidsDNA).
Lymphadenopathy and splenomegaly
The development of generalized lymphadenopathy in the MRL/lpr mice (saline and FTS-treated) was evaluated by palpation of axillary and inguinal lymph nodes. The maximal lymph nodes score in each mouse was 4, when both axillary and inguinal lymph nodes (on both sides) were palpable. For mice that died the last observation was carrying forward for analysis.
At 18 weeks of age the mice were sacrificed and the spleens and lymph nodes (axillary, inguinal and cervical) were removed and weighed.
Renal function
The presence of proteinuria was measured on freshly expressed urine samples at weekly intervals. The protein content was evaluated semiquantitatively using a commercial dipstick method (Macherey-Nagel, Germany). This colorimetric assay is specific for albumin, with approximate protein concentration as follows: 0, 30, 100 and 500 mg/dL (mg%). For mice that died the last observation was carried forward for analysis.
Rodent neurological examination
The four groups of mice were examined weekly using the grip strength test. Muscle strength was measured by the number of seconds the mouse was able to hang suspended on a stationary bar. A neurologically normal mouse is able to remain suspended (hang time) for 30 s or longer [24]. For mice that died the last observation was carrying forward for analysis.
Open field
Saline-treated and FTS-treated MRL/lpr and saline-treated MRL/++ mice (n = 5) were tested for spatial behaviour in a novel environment in an open field. The apparatus comprised a circular pool (120 cm diameter by 50 cm high) placed in a lighted room. The test was conducted during the light phase of day–night cycle. The mice were brought to the experimental room 1 h before testing. Each mouse was removed gently from its cage, placed individually at the centre of the field, and videotaped with a camcorder for 20 min. At the end of each session the pool was cleaned with paper towels moistened with ammonium glass cleaner to remove urinary trails. The frequency and duration of progression or stopping in the open field was analysed during playback of the video records by software custom-written by Dr David Eilam (Life Sciences Department, Tel Aviv University). Total distance travelled, locomotion time, speed of moving, spatial distribution of explorations and latency for establishing a home base, and duration of remaining there, were extracted.
Results
Ras inhibition in lymphocytes in vitro
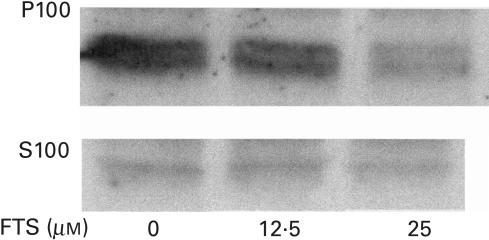
The effect of FTS on the levels of activated Ras in normal naive lymphocytes cultured in vitro is presented in Fig. 1. The levels of soluble (S100) and insoluble (P100) Ras were measured by Western immunobloting with pan Ras antibodies, as described in the Methods. As can be seen, culturing the lymphocytes in the presence of 12·5 and 25 µm of FTS resulted in a significant, dose-dependent reduction in the amount of insoluble (activated) Ras (FTS 0 µm – 100 ± 8%, FTS 12·5 µm – 84 ± 9·8%, FTS 50 µm – 52 ± 16%) with no concomitant observable change in the levels of soluble Ras.
Fig. 1.
FTS reduces the amount of membrane Ras in lymphocytes. Cells were treated with the indicated concentrations of FTS for 24 h and Ras was then determined in the particulate (P100) and in the cytosolic (S100) fractions with pan Ras antibody, as detailed in the Materials and methods. Results of a typical experiment (of three performed) are shown.
FTS inhibition of lymphocyte proliferation in vitro
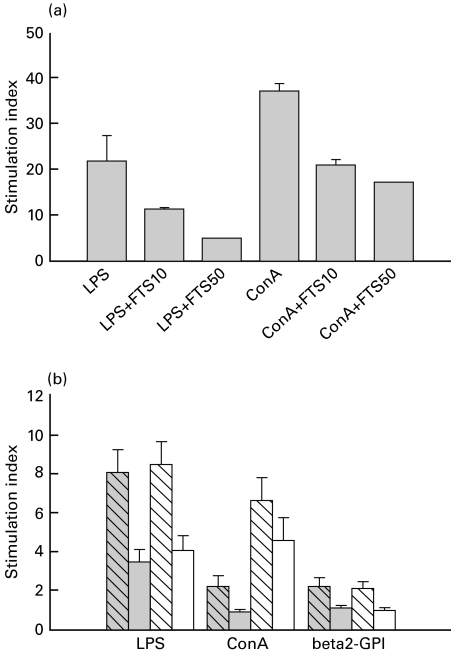
We next examined whether the effect of FTS on activated Ras levels was of functional importance in spleen lymphocytes isolated from naive ICR mice. Stimulation of [3H]TdR incorporation into DNA was used as measure of lymphocyte proliferation. Stimulation indices were measured for LPS and ConA in the presence of 10 and 50 µm FTS. As can be seen in Fig. 2a, FTS produced a significant dose-dependent reduction of LPS and ConA induced proliferation. FTS did not induce lymphocyte cell death under these conditions, evident by trypan blue exclusion staining. Also, FTS had no effect on basal [3H]TdR incorporation into DNA. The concentrations of FTS at which significant inhibition of lymphocyte proliferation were observed reflect expected levels of drug when given to animals at doses of 5–10 mg/kg. Such doses were therefore used in subsequent experiments in the MRL/lpr mouse model.
Fig. 2.
Inhibition by FTS of normal mouse splenocyte proliferation in vitro (a) and of MRL/lpr and MRL/++ mouse spleen lymphocyte proliferation ex vivo (b). A: Stimulation index to LPS and ConA in the presence of 0, 10 (FTS10) and 50 (FTS50) µm FTS was determined in triplicate by uptake of [3H]TdR as described in the Materials and methods and presented as the mean ±SE (SE for FTS50 was too small to be visible). The mean value of dpm in cells cultured in the absence of mitogen was 1192 ± 239. B: Spleen lymphocytes were prepared from mice treated from age 6–18 weeks with FTS, five times a week and assayed for proliferation in response to LPS, ConA or beta2-GPI as described in the Methods and Results sections are presented as mean ±SE stimulation index. The mean values of dpm in cells cultured in the absence of mitogen/antigen were 3146 ± 891 for the saline-treated MRL/lpr group ( ), 5321 ± 1106 for the FTS-treated MRL/lpr group (
), 5321 ± 1106 for the FTS-treated MRL/lpr group ( ), 5863 ± 1382 for the saline-treated MRL/++ group (
), 5863 ± 1382 for the saline-treated MRL/++ group ( ) and 9092 ± 1678 for the FTS-treated MRL/++ group (□).
) and 9092 ± 1678 for the FTS-treated MRL/++ group (□).
Effect of FTS on lymphocyte proliferation in vivo in MRL/lpr and control MRL/++ mice as determined by ex-vivo experiments
The mice received 5 mg/kg FTS (5 days a week) for 3 months and spleen lymphocyte proliferation assays were then performed in vitro with no added FTS (Fig. 2b). Baseline [3H]TdR incorporation was similar in all four groups examined, except for a trend towards higher values in the MRL/++ mice treated with FTS. Stimulation indices revealed a two-fold reduction of the response to LPS in FTS-treated mice (P < 0·01, t-test). As is known to occur in MRL/lpr mice [25,26], the response of the MRL/lpr mice to ConA was markedly lower than in the MRL/++ control mice. This response was further reduced in lymphocytes of the FTS-treated MRL/lpr mice (P < 0·01, t-test), but not in lymphocytes of the FTS-treated MRL/++ mice (P < 0·06, t-test). A significant proliferative response to β2-GPI was measured which was reduced by 50% in lymphocytes of FTS-treated mice (P < 0·02, t-test).
Effects of FTS on autoantibody levels
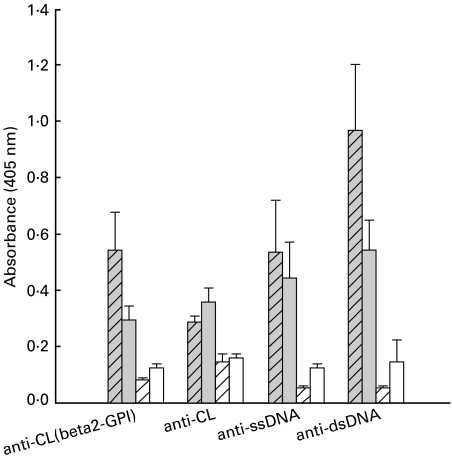
In view of the effects of FTS on lymphocyte proliferation, we also measured mouse antibodies to relevant autoantigens including β2-GPI-dependent aCL, serum-independent aCL, antissDNA and antidsDNA. As can be seen in Fig. 3, there were significantly higher levels of all these antibodies in the MRL/lpr groups compared to the MRl/++ controls (P < 0·014 by one-way anova). FTS treatment did lower the levels of β2-GPI-dependent aCL (β2-GPI) antibodies and antidsDNA antibodies significantly in the MRL/lpr mice (P < 0·049 one-way anova), but did not affect the levels of non-serum-dependent aCL and of antissDNA antibodies in this group (Fig. 3).
Fig. 3.
Autoantibody levels in sera of FTS-treated and saline-treated MRL/lpr and MRL/++ mice at 16 weeks of age. The levels of autoantibodies are represented as mean absorbance values with SE. High levels of serum (β2-GPI) dependent aCL antibodies (anti-CL(beta2-GPI)), serum-independent aCL antibodies (anti-CL), antissDNA and antidsDNA antibodies were measured in the MRL/lpr mice (filled-hatched bars, n = 10) compared to the saline-treated (open-hatched bars, n = 10) and FTS-treated (open bars, n = 10) MRL/++ mice. There were significantly less antibeta2-GPI and antidsDNA antibodies in the FTS-treated MRL/lpr mice (filled bars, n = 10) compared with the saline-treated MRL/lpr mice (P < 0·05, one-way anova).
Effect of FTS on lymphadenopathy
An important clinical measure of disease progression in MRL/lpr mice is lymphadenopathy. This was found in the course of the experiments only in the MRL/lpr group and was significantly delayed in the FTS treated mice (3·625 ± 0·166 lymph node score compared to 3·026 ± 0·237 at 15 weeks of age, P = 0·034 by repeated-measures anova), although all mice developed measurable lymphadenopathy at 17 weeks of age. At this stage the animals were sacrificed and lymph nodes could be easily identified, excised and weighed in MRL/lpr mice, revealing a significant beneficial effect of FTS (1·79 ± 0·15 g compared to 1·24 ± 0·17 g, P < 0·017 one-way anova). Similar measurements of the spleens at 17 weeks in all groups of mice revealed reduced spleen weight in FTS-treated MRL/lpr mice (0·44 ± 0·04 g, mean ±SE) compared to untreated mice (0·53 ± 0·03 g, P < 0·038 by t-test). MRl/++ mice had smaller spleens (0·13 ± 0·01 g) and spleen weight was significantly increased by FTS (0·19 ± 0·03 g P < 0·042 by t-test).
Effects of FTS on parameters of autoimmune-induced damage in MRL/lpr mice
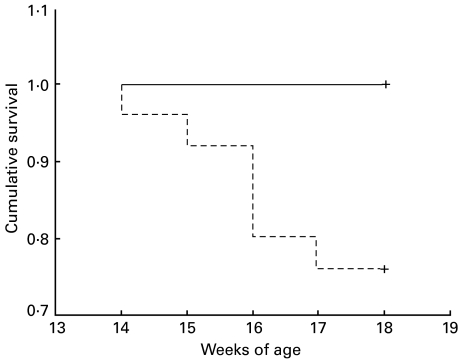
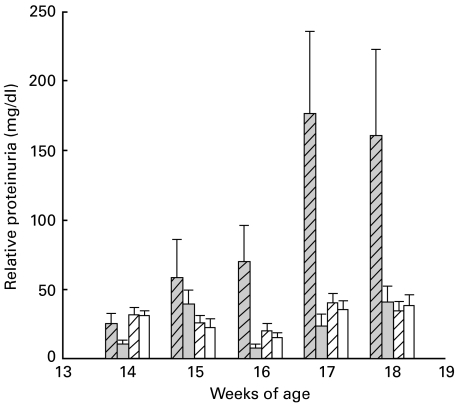
In view of the effects of FTS on the immune system we examined whether this was reflected in measures of autoimmune disease. Saline-treated MRL/lpr mice displayed an overall mortality of 24% up to 18 weeks of age and, in contrast, there was no mortality in the FTS-treated MRL/lpr mice or in the MRl/++ groups (P < 0·011, Fisher's exact test). A detailed Kaplan–Meier analysis of mortality is presented in Fig. 4, demonstrating significant difference between the MRL/lpr treated with FTS compared to saline (P = 0·005, log rank test). Proteinuria is a consistent feature of this model [27] and indeed, as presented in Fig. 5, beginning at 15 weeks of age the MRL/lpr mice developed significant proteinuria. In contrast, MRL/lpr mice treated with FTS had very low levels of proteinuria, similar to the MRl/++ group (P = 0·007 for all four groups by repeated-measures anova). The results presented are from mice (10 in each group) treated 5 days a week from 6 weeks of age. The results from mice treated from 10 weeks of age or only three times a week displayed similar but less pronounced effects (not shown).
Fig. 4.
Effect of FTS on survival of female MRL/lpr mice. Saline-treated MRL/lpr mice (n = 25, dashed line) displayed an overall mortality of 24% up to 18 weeks of age; in contrast, there was no mortality in the FTS-treated MRL/lpr mice (n = 25, solid line).
Fig. 5.
Proteinuria (mean ±SE) measured in the urine of FTS-treated and saline-treated MRL/lpr and MRL/++ mice at the indicated weeks. There were 10 mice in each group. FTS treatment was given five times a week from age 6 to 18 weeks. Beginning at 16 weeks age, saline-treated MRL/lpr mice ( ) exhibited significant proteinuria compared with trace amounts of protein detected in the FTS-treated MRL/lpr (
) exhibited significant proteinuria compared with trace amounts of protein detected in the FTS-treated MRL/lpr ( ) and MRL/++ (
) and MRL/++ ( ) and saline-treated MRL/++ mice (□) (P < 0·001, repeated-measures anova).
) and saline-treated MRL/++ mice (□) (P < 0·001, repeated-measures anova).
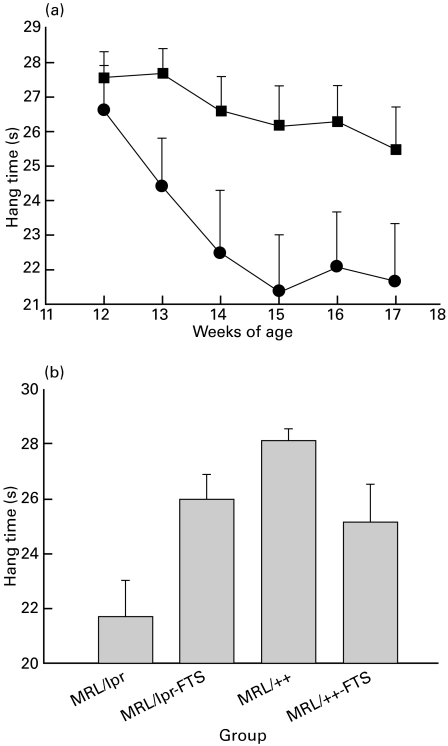
Neurological function, as measured by hanging time on a horizontal bar, is presented in Fig. 6. Figure 6a compares the time MRL/lpr mice and FTS-treated MRL/lpr mice could hang on to a horizontal bar in weekly tests from 12 to 17 weeks of age. As can be seen, the MRL/lpr mice were significantly impaired in this test from week 14 compared to the FTS-treated group, although this group also showed some decline in performance over the observation period (P < 0·001 by repeated-measures anova). Figure 6b summarizes the performance of all four groups of mice at 15–17 weeks of age, demonstrating that the MRL/lpr group was significantly impaired compared to the other groups (P < 0·001 one-way anova, P < 0·01 in post hoc tests) which did not differ significantly from each other.
Fig. 6.
Grip strength in saline-treated MRL/lpr mice, FTS-treated MRL/lpr mice, saline-treated MRL/++ mice and FTS-treated MRL/++ mice. Grip strength was measured as described in the Materials and methods. (a) Mean ±SE values for performance of saline-treated (circles) and FTS-treated (squares) MRL/lpr mice at 12–17 weeks of age; (b) mean ±SE values for performance of four groups at weeks 15–17. MRL/lpr mice were significantly impaired in this test from week 14 compared to the other groups (P < 0·001, repeated-measures anova). MRL/lpr mice (n = 25), FTS-treated MRL/lpr mice (n = 25), saline-treated MRL/++ mice (n = 20) and FTS-treated MRL/++ mice (n = 15).
Omitting the missing data from the animals that died did not change the statistical significance of results from lymphadenopathy, proteinuria and neurological function.
In a behavioural open field test, both FTS-treated and untreated MRL/lpr mice displayed significantly reduced locomotion compared to MRl/++ controls (158 ± 9, 189 ± 15 and 240 ± 10 total distance (m) in a 20-min task, respectively; P < 0·001 by one-way anova). Overall, there was no beneficial effect of FTS except in a purely behavioural aspect of movement, the time spent in the centre of the field, where there was a significant difference between the untreated MRL/lpr mice and FTS-treated MRL/lpr mice (81 ± 1 versus 70 ± 3% time in the centre; P < 0·048 by one-way anova), which were similar in their behavioural pattern to the MRl/++ controls (73 ± 4%).
Discussion
In the present study we explored the possible therapeutic potential of the Ras inhibitor FTS in systemic autoimmune disease such as APS and SLE by using the genetic model of these diseases, the MRL/lpr mouse. The results show that FTS treatment (5 mg/kg/day) for periods of 6–18 weeks had a number of significant beneficial effects on the diseased MRL/lpr mouse. There were neither increased mortality nor exacerbation of pathology associated with FTS treatment in these mice nor in the non-diseased MRl/++ control mice. The treatment resulted in a 50% decrease in splenocyte proliferation to ConA, LPS and a disease-specific antigen, β2-GPI, and in a significant decrease in serum-dependent antibodies to cardiolipin (antiβ2-GPI) and antibodies to dsDNA. These immunological effects were associated with amelioration of proteinuria and grip strength which were normalized in FTS-treated MRL/lpr mice. Lymphadenopathy and postmortem lymph node and spleen weights were also significantly less in FTS treated MRL/lpr mice.
Inhibitors that directly affect Ras function have not been tested as potential selective immune system modulators in models of SLE and APS. Other studies of treatment of MRL/lpr mice include immunosuppression with corticosteroids [28] and apoptosis inducing cytotoxic agents such as cyclophosphamide [28,29] and immunomodulatory agents such as cyclosporin A [30], FK506 [29], rapamycin [30], anti-ICAM antibodies [24] and antilymphocyte marker antibodies [31,32]. The effect of FTS on proteinuria, grip strength, antibody production and lymphocyte stimulation compare well with results obtained with immunosuppressive and immunomodulatory drugs. In contrast, the effects of FTS on parameters such as lymphadenopathy were less pronounced.
A possible explanation for differing effects of FTS on disease parameters lies in the complex nature of the immune abnormality in MRL/lpr mice. These mice lack an important cell death receptor, Fas, and accumulate large numbers of dysfunctional lymphocytes, most of them CD4−CD8− T lymphocytes [33–36]. These cells should probably have undergone apoptosis and the high levels of Ras found in lymphocytes in this disorder [21] may reflect a compensatory attempt at apoptosis. We have found in preliminary experiments that indeed lymph node cells from the MRL/lpr contain up to 10-fold more Ras than the MRl/++ controls. Prolonged FTS treatment, however, did not produce any detectable reduction in the levels of Ras in the MRL/lpr splenocytes. In addition, the impaired response of MRL/lpr lymphocytes to ConA is believed to result from large numbers of the cells being non-functional [25]. As can be seen in Fig. 2b, this abnormality was not corrected by chronic FTS treatment, which actually further lowered the T-cell response to ConA. Cyclophosphamide, an agent that promotes apoptosis in the abnormal lymphocytes, greatly reduces their number, normalizes the response to ConA and greatly reduces the lymphadenopathy [29,37]. In the present study we found delayed lymphadenopathy and splenomegally in FTS treated MRL/lpr mice but the effect was relatively minor compared with cyclophosphamide. It is interesting to note that one study utilizing anti-CD4 and anti-CD8 antibodies found that one antibody reduced the number of defective lymphocytes, while the other inhibited the autoimmune features of the disease [32]. There is much current interest in the interplay of cancer, apoptosis and autoimmunity. The autoimmune pathology in these mice may be due to either impaired apoptosis of autoreactive lymphocytes or to an autoimmune response to the lymphocytes that have inadequate apoptosis. The dose of FTS used in the present study seemed therefore to affect the immune pathology more than the lymphoproliferative aspects of the MRL/lpr model. This is supported by our findings in Balb/C mice immunized with β2-GPI in which FTS treatment three times a week significantly inhibited in vivo production of antiphospholipid antibodies (in preparation). Cytokine assays and other measures of immunological function were not performed in the MRL/lpr mice in this study but have been shown to be clearly affected by FTS in experimental autoimmune encephalomyelitis model (Karussis et al., submitted for publication).
The dose effect of FTS was most apparent on proteinuria. In other parameters of disease there were no significant differences in the effect of treatment three times a week from 6 weeks of age, or treatment five times a week from either 6 or 10 weeks of age. From experiments carried out in the experimental autoimmune encephalomyelitis and neuritis (EAE, EAN) models, increasing the daily dosage of FTS by a factor of 3 does not produce significantly more effect [38,39]. Results from short-term models such as EAN, a brief pulse of therapy at the onset of clinical signs was superior to continuous therapy. Experiments with chronic models will reveal whether short pulses of FTS may ameliorate autoimmune disease more effectively.
Because full activation of lymphocytes requires parallel stimulation of several signal transduction pathways, including the Ras pathway [3,40], we assumed that interfering with Ras functions would inhibit lymphocyte activation. The presumed mechanism of action involves a dual effect on membrane Ras where initially (within 30 min) FTS releases Ras from constrains on its lateral mobility leading to Ras degradation while later (> 6 h) FTS induces a decrease in the lateral mobility of Ras that remains associated with the cell membrane [11,12]. The reduced amount of Ras and the altered membrane mobility of Ras in FTS-treated fibroblasts and human tumour cells are then manifested in the inhibition of Ras mediated signalling to the MAPK Erk [13,14]. In the present study we show that FTS induced in vitro a significant reduction (16–48%) in membrane associated Ras of lymphocytes (Fig. 1), and that at exactly the same concentration range (10–50 µm) it inhibited LPS and ConA stimulated lymphocyte proliferation (Fig. 2a). Consistent with early observations in other cell types [11], we found no accumulation of cytosolic Ras in lymphocytes following FTS treatment (Fig. 1). This and the previously described lack of FTS effects on Ras processing [41] suggest that FTS affected membrane Ras in lymphocytes and rendered the protein susceptible to proteolytic degradation, as in fibroblasts [11]. The observed short-term FTS-induced reduction in activated Ras seems in chronic treatment to be manifested by long standing inhibition of LPS- or ConA-stimulated lymphocyte proliferation in vitro. As mentioned, it was difficult to detect in vivo effect of FTS on the very high levels of Ras in MRL/lpr mice. We have performed such assays in normal mice and found FTS to significantly lower activated Ras (data not shown). Furthermore, the ex vivo experiments that were performed in the MRL/lpr mice in the absence of FTS also show a clear effect of FTS and argue against a non-specific low-grade toxic effect of the drug. Although there is evidence that FTS affects Ras in lymphocytes, we cannot exclude other possible effects of FTS associated with non-Ras driven lymphocyte proliferation through other small G-proteins.
The mice treated with FTS did not display serious side-effects such as increased mortality. Preliminary toxicological experiments have estimated that LD50 by a single i.p. administration of FTS was 75–100 mg/kg and no effect of FTS treatment was found on animal or tissue weight and on blood counts, creatinine, liver enzymes or creatine kinase [42]. There were no adverse toxic effects at the effective FTS dosage in nude mice [42], SCID mice [43] or Wistar rats [44]. The significance of the increased spleen weight in MRL++ mice treated with FTS is uncertain, but suggests the need for further experiments in different strains of mice and caution in the use of this treatment approach in humans.
The clearest indication that FTS affected lymphocyte proliferation in vivo comes from the ex-vivo proliferation assays data. The in vitro LPS-induced or β2-GPI-induced proliferation of splenocytes of FTS-treated mice was 50–60% lower compared to the response of splenocytes of untreated mice. This lower response reflects the in vivo effect of FTS because the in vitro assays were performed without FTS. No residual FTS is present in the washed splenocytes as evident by radioabelled FTS experiments (Karusis, Chapman and Kloog, unpublished data). It appears, therefore, that FTS inhibited lymphocyte proliferation in vivo resulting in a decrease in the population of lymphocytes that can respond to LPS or to the disease-related antigen β2-GPI. This effect of FTS was apparent in both MRL/lpr and MRl/++ mice, suggesting that it did not correlate specifically with the diseased state. The strong in vivo effect of FTS on ConA sensitive lymphocytes observed in MRL/lpr mice, as opposed to lack of an effect in MRL/++ mice, suggest a specific effect on T-cells that is associated with the autoimmune diseased state. The effect of FTS on LPS induced proliferation, however, indicates that other inflammatory cell populations such as macrophages may be significantly affected. Thus, although an effect of FTS on Ras in lymphocytes is substantiated, much work remains to characterize the specificity of this effect to cell types and protein messengers.
In summary, the present results indicate that FTS, an inhibitor of Ras, is potentially useful for treating some autoimmune features of the MRL/lpr mouse, a model of SLE and APS. This supports the strategy of selective inhibition of intracellular signals as a potential therapy for autoimmune diseases.
Acknowledgments
This study was supported by the Schreiber Foundation, the Sieratzki Chair of Neurology, Tel Aviv University, the Miriam Turjanski de Gold and Dr Roberto Gold Fund for Neurological Research, and the Streifler Fund for Neurological Research.
REFERENCES
- 1.Berridge MJ. Lymphocyte activation in health and disease. Crit Rev Immunol. 1997;17:155–78. doi: 10.1615/critrevimmunol.v17.i2.30. [DOI] [PubMed] [Google Scholar]
- 2.Weiss A, Littman DR. Signal transduction by lymphocyte antigen receptors. Cell. 1994;76:263–74. doi: 10.1016/0092-8674(94)90334-4. [DOI] [PubMed] [Google Scholar]
- 3.June CH. Signal transduction in T cells. Curr Opin Immunol. 1991;3:287–93. doi: 10.1016/0952-7915(91)90026-w. [DOI] [PubMed] [Google Scholar]
- 4.Downward J, Graves JD, Warne PH, et al. Stimulation of p21ras upon T-cell activation. Nature. 1990;346:719–23. doi: 10.1038/346719a0. [DOI] [PubMed] [Google Scholar]
- 5.Henning SW, Cantrell DA. GTPases in antigen receptor signalling. Curr Opin Immunol. 1998;10:322–9. doi: 10.1016/s0952-7915(98)80171-4. [DOI] [PubMed] [Google Scholar]
- 6.Pastor MI, Reif K, Cantrell D. The regulation and function of p21ras during T-cell activation and growth. Immunol Today. 1995;16:159–64. doi: 10.1016/0167-5699(95)80134-0. [DOI] [PubMed] [Google Scholar]
- 7.Lowy DR. Function and regulation of Ras. Annu Rev Biochem. 1993;62:851–91. doi: 10.1146/annurev.bi.62.070193.004223. [DOI] [PubMed] [Google Scholar]
- 8.Casey PJ, Der Solski PA, CJ, et al. p21ras is modified by a farnesyl isoprenoid. Proc Natl Acad Sci USA. 1989;86:8323–7. doi: 10.1073/pnas.86.21.8323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cox AD, Hisaka MM, Buss JE, et al. Specific isoprenoid modification is required for function of normal, but not oncogenic, Ras protein. Mol Cell Biol. 1992;12:2606–15. doi: 10.1128/mcb.12.6.2606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kato K, Cox AD, Hisaka MM, et al. Isoprenoid addition to Ras protein is the critical modification for its membrane association and transforming activity. Proc Natl Acad Sci USA. 1992;89:6403–7. doi: 10.1073/pnas.89.14.6403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Haklai R, Weisz MG, Elad G, et al. Dislodgment and accelerated degradation of Ras. Biochemistry. 1998;37:1306–14. doi: 10.1021/bi972032d. [DOI] [PubMed] [Google Scholar]
- 12.Niv H, Gutman O, Henis YI, et al. Membrane interactions of a constitutively active GFP-Ki-Ras 4B and their role in signaling. Evidence from lateral mobility studies. J Biol Chem. 1999;274:1606–13. doi: 10.1074/jbc.274.3.1606. 10.1074/jbc.274.3.1606. [DOI] [PubMed] [Google Scholar]
- 13.Gana-Weisz M, Haklai R, Marciano D, et al. The Ras antagonist S-farnesylthiosalicylic acid induces inhibition of MAPK activation. Biochem Biophys Res Commun. 1997;239:900–4. doi: 10.1006/bbrc.1997.7582. [DOI] [PubMed] [Google Scholar]
- 14.Aharonson Z, Gana-Weisz M, Varsano T, et al. Stringent structural requirements for anti-Ras activity of S-prenyl analogues. Biochim Biophys Acta. 1998;1406:40–50. doi: 10.1016/s0925-4439(97)00077-x. [DOI] [PubMed] [Google Scholar]
- 15.Egozi Y, Weisz B, Gana-Weisz M, et al. Growth inhibition of ras-dependent tumors in nude mice by a potent ras-dislodging antagonist. Int J Cancer. 1999;80:911–8. doi: 10.1002/(sici)1097-0215(19990315)80:6<911::aid-ijc18>3.0.co;2-4. 10.1002/(sici)1097-0215(19990315)80:6`911::aid-ijc18b3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 16.Marciano D, Ben-Baruch G, Marom M, et al. Farnesyl derivatives of rigid carboxylic acids-inhibitors of ras-dependent cell growth. J Med Chem. 1995;38:1267–72. doi: 10.1021/jm00008a004. [DOI] [PubMed] [Google Scholar]
- 17.Ballow M, Nelson R. Immunopharmacology: immunomodulation and immunotherapy. JAMA. 1997;278:2008–17. doi: 10.1001/jama.278.22.2008. [DOI] [PubMed] [Google Scholar]
- 18.Gharavi AE, Aron AL. Experimental models for antiphospholipid studies. Haemostasis. 1994;24:204–7. doi: 10.1159/000217102. [DOI] [PubMed] [Google Scholar]
- 19.Shoenfeld Y. Experimental and induced animal models of systemic lupus erythematosus and Sjogren's syndrome. Curr Opin Rheumatol. 1989;1:360–8. doi: 10.1097/00002281-198901030-00020. [DOI] [PubMed] [Google Scholar]
- 20.Brey RL, Sakic B, Szechtman H, et al. Animal models for nervous system disease in systemic lupus erythematosus. Ann NY Acad Sci. 1997;823:97–106. doi: 10.1111/j.1749-6632.1997.tb48382.x. [DOI] [PubMed] [Google Scholar]
- 21.Rapoport M, Mor A, Bistritzer T, et al. Protooncogenes autoimmunity. Isr J Med Sci. 1997;33:262–6. [PubMed] [Google Scholar]
- 22.Blank M, Palestine A, Nussenblatt R, et al. Down-regulation of autoantibody levels of cyclosporine and bromocriptine treatment in patients with uveitis. Clin Immunol Immunopathol. 1990;54:87–97. doi: 10.1016/0090-1229(90)90008-e. [DOI] [PubMed] [Google Scholar]
- 23.George J, Blank M, Levy Y, et al. Differential effects of anti-beta2-glycoprotein I antibodies on endothelial cells and on the manifestations of experimental antiphospholipid syndrome. Circulation. 1998;97:900–6. doi: 10.1161/01.cir.97.9.900. [DOI] [PubMed] [Google Scholar]
- 24.Brey RL, Amato AA, Kagan-Hallet K, et al. Anti-intercellular adhesion molecule-1 (ICAM-1) antibody treatment prevents central and peripheral nervous system disease in autoimmune-prone mice. Lupus. 1997;6:645–51. doi: 10.1177/096120339700600805. [DOI] [PubMed] [Google Scholar]
- 25.Cameron R, Waterfield JD. Delineation of two defects responsible for T-cell hyporesponsiveness to concanavalin A in MRL congenic mice. Immunology. 1986;59:187–93. [PMC free article] [PubMed] [Google Scholar]
- 26.Dziarski R. Comparison of in vitro and in vivo mitogenic and polyclonal antibody and autoantibody responses to peptidoglycan, LPS, protein A, PWN, PHA and Con A in normal and autoimmune mice. J Clin Lab Immunol. 1985;16:93–109. [PubMed] [Google Scholar]
- 27.Bernstein KA, Bolshoun D, Lefkowith JB. Serum glomerular binding activity is highly correlated with renal disease in MRL/lpr mice. Clin Exp Immunol. 1993;93:418–23. doi: 10.1111/j.1365-2249.1993.tb08194.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kiberd BA, Young ID. Modulation of glomerular structure and function in murine lupus nephritis by methylprednisolone and cyclophosphamide. J Lab Clin Med. 1994;124:496–506. [PubMed] [Google Scholar]
- 29.Woo J, Wright TM, Lemster B, et al. Combined effects of FK506 (tacrolimus) and cyclophosphamide on atypical B220+ T cells, cytokine gene expression and disease activity in MRL/MpJ-lpr/lpr mice. Clin Exp Immunol. 1995;100:118–25. doi: 10.1111/j.1365-2249.1995.tb03612.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Warner LM, Adams LM, Sehgal SN. Rapamycin prolongs survival and arrests pathophysiologic changes in murine systemic lupus erythematosus. Arthritis Rheum. 1994;37:289–97. doi: 10.1002/art.1780370219. [DOI] [PubMed] [Google Scholar]
- 31.Henrickson M, Giannini EH, Hirsch R. Reduction of mortality and lymphadenopathy in MRL-lpr/lpr mice treated with nonmitogenic anti-CD3 monoclonal antibody. Arthritis Rheum. 1994;37:587–94. doi: 10.1002/art.1780370422. [DOI] [PubMed] [Google Scholar]
- 32.Merino R, Fossati L, Iwamoto M, et al. Effect of long-term anti-CD4 or anti-CD8 treatment on the development of lpr CD4–CD8− double negative T cells and of the autoimmune syndrome in MRL-lpr/lpr mice. J Autoimmun. 1995;8:33–45. doi: 10.1006/jaut.1995.0003. [DOI] [PubMed] [Google Scholar]
- 33.Watanabe-Fukunaga R, Brannan CI, Copeland NG, et al. Lymphoproliferation disorder in mice explained by defects in Fas antigen that mediates apoptosis. Nature. 1992;356:314–7. doi: 10.1038/356314a0. [DOI] [PubMed] [Google Scholar]
- 34.Katagiri T, Cohen PL, Eisenberg RA. The lpr gene causes an intrinsic T cell abnormality that is required for hyperproliferation. J Exp Med. 1988;167:741–51. doi: 10.1084/jem.167.3.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Carlsten H, Tarkowski A. Expression of heterozygous lpr gene in MRL mice. I. Defective T-cell reactivity and polyclonal B-cell activation. Scand J Immunol. 1989;30:457–62. doi: 10.1111/j.1365-3083.1989.tb02450.x. [DOI] [PubMed] [Google Scholar]
- 36.Budd RC, Winslow G, Inokuchi S, et al. Intact antigen receptor-mediated generation of inositol phosphates and increased intracellular calcium in CD4–CD8− T lymphocytes from MRL lpr mice. J Immunol. 1990;145:2862–72. [PubMed] [Google Scholar]
- 37.Smith HR, Chused TM, Steinberg AD. Cyclophosphamide-induced changes in the MRL-lpr/lpr mouse: effects upon cellular composition, immune function, and disease. Clin Immunol Immunopathol. 1984;30:51–61. doi: 10.1016/0090-1229(84)90006-0. [DOI] [PubMed] [Google Scholar]
- 38.Karussis D, Grigoriadis N, Brenner T, et al. Inhibition of the Ras-pathway of cell activation with s-trans-trans-farnesyl-thiosalicylic acid (FTS); in vivo suppression of experimental autoimmune encephalomyelitis (EAE) and differential in vitro effects on lymphocytes and astrocytes. Neurosci Lett. 1999;54(Suppl.):S23. [Google Scholar]
- 39.Kafri M, Drory V, Wang N, et al. Treatment of experimental autoimmune neuritis by a Ras inhibitor s-farnesylthiosalicylic acid (FTS) Neurosci Lett. 1999;54(Suppl.):S22. [Google Scholar]
- 40.DeFranco AL. The complexity of signaling pathways activated by the BCR. Curr Opin Immunol. 1997;9:296–308. doi: 10.1016/s0952-7915(97)80074-x. [DOI] [PubMed] [Google Scholar]
- 41.Marom M, Haklai R, Ben-Baruch G, et al. Selective inhibition of Ras-dependent cell growth by farnesylthiosalisylic acid. J Biol Chem. 1995;270:22263–70. doi: 10.1074/jbc.270.38.22263. [DOI] [PubMed] [Google Scholar]
- 42.Weisz B, Giehl K, Gana-Weisz M, et al. A new functional Ras antagonist inhibits human pancreatic tumor growth in nude mice. Oncogene. 1999;18:2579–88. doi: 10.1038/sj.onc.1202602. [DOI] [PubMed] [Google Scholar]
- 43.Jansen B, Schlagbauer-Wadl H, Kahr H, et al. Novel Ras antagonist blocks human melanoma growth. Proc Natl Acad Sci USA. 1999;96:14019–24. doi: 10.1073/pnas.96.24.14019. 10.1073/pnas.96.24.14019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Reif S, Weis B, Aeed H, et al. The Ras antagonist, farnesylthiosalicylic acid (FTS), inhibits experimentally-induced liver cirrhosis in rats. J Hepatol. 1999;31:1053–61. doi: 10.1016/s0168-8278(99)80318-3. [DOI] [PubMed] [Google Scholar]