Abstract
Diesel exhaust particles (DEP) are known to modulate the production of cytokines associated with acute and chronic respiratory symptoms and allergic respiratory disease. Tolerance is an important mechanism through which the immune system can maintain nonresponsiveness to common environmental antigens. We examined the effect of DEP on IL-10 and TGF-β, cytokines produced by macrophages and repressor (Tr-like) lymphocytes which influence tolerance. Human PBMCs (n = 22) were incubated with 1–100 ng/ml of DEP, and suboptimally primed with LPS. IL-10 gene expression was assessed by the S1 nuclease protection assay, and production of IL-10, TGF-β, TNF-α, IL-1β and IL-4 stimulated CD23 was evaluated by ELISA after 24 and 48 h. The effect of the order of exposure to DEP and LPS was evaluated on IL-10 protein and mRNA in cells (1) preincubated with LPS followed by DEP, or (2) exposed first to DEP followed by LPS. IL-10 was further evaluated using benzo[a]pyrene and [α]naphthoflavone as a surrogate for the polyaromatic hydrocarbons (PAHs) adsorbed to DEP. Control cells were incubated with carbon black, without PAHs. In PBMCs exposed to DEP with LPS, or preincubated with LPS before DEP, IL-10 production and mRNA fall significantly. TGF-β is similarly suppressed, IL-1β secretion is significantly stimulated, and IL-4 stimulated CD23 release rises in the atopic subjects. In contrast, when DEP is added prior to LPS, IL-10 production rises, and IL-1β falls to zero. These effects on IL-10 are reproduced with benzo[a]pyrene and reversed by the coaddition of [α]naphthoflavone, its known antagonist. The carbon black fraction has no effect on IL-10 production. The effect of DEP on IL-10 can be inhibitory or stimulatory, depending on the order of exposure to DEP and LPS. Pro-inflammatory cytokines and factors rise when IL-10 is inhibited, and are suppressed when IL-10 is stimulated. These results are duplicated with benzo[a]pyrene, suggesting that the PAH portion of the DEP is the active agent.
Keywords: pollution, asthma, allergic disease, IL-10, DEPs, PAHs, benzo[a]pyrene
INTRODUCTION
The health effects of particulate air pollution include the increased prevalence of lower respiratory tract symptoms, decrements in PEF, and increased bronchodilator use and ER visits for respiratory disease [1,2]. The increased prevalence of allergic respiratory disease has been partly attributed to respirable particulates [3–5]. DEP-induced respiratory effects have been associated with changes in inflammatory cytokines, which suggest possible mechanisms of action. However, results from in vivo and in vitro studies are conflicting, and demonstrate both pro- and anti-inflammatory activities. Mice sensitized to allergen with DEP demonstrate increased eosinophilic infiltrates, goblet cell proliferation, IL-4, IL-5 and GM-CSF, specific IgE and nonspecific bronchial hyperresponsiveness (BHR) consistent with asthma [6–8]. Nasal challenge with DEP and ragweed in humans enhances the production of inflammatory cytokines and IL-10 as well as ragweed specific IgE [9]. DEP is taken up by human bronchial epithelial cells, and is associated with increased release of IL-6, IL-8 [10], GM-CSF, sICAM-1 [11] and IL-1β [12]. DEP affects gene transcription, and binds to an NFκB site present in the IL-6 [13] and IL-8 [14] promoter sequence. However, some of DEP effects are inhibitory. MCP-1 transcripts and protein in PBMCs diminish after 48 h of DEP incubation [15], and rat alveolar macrophages exposed in vivo to DEP plus LPS decrease IL-1 and TNF-α secretion [16]. Human alveolar macrophages exposed to moderate dose DEP with LPS also suppressed IL-6 and TNF-α [17]. Some of the observed effects of DEP are dose specific. Whereas low dose DEP triggered IL-8, GM-CSF and sICAM-1 release in asthmatic bronchial epithelial cells, higher doses inhibited IL-8 and RANTES release [11].
Diesel exhaust particulates (DEP) contain several components that may affect cytokine production and allergic sensitization. DEPs consist of a carbon black core, to which are adsorbed polyaromatic hydrocarbons (PAH), SO2, heavy metals and allergens. Grass pollen allergens [18], cat and dog allergens [19], and LPS [20] have been found on respirable particulates <2·5 μm, which may be a major vehicle of delivery to the lower airway. The in vivo effects in mice of increased specific IgE production with DEP and allergen are duplicated using phenanthrene [21], benzo[a]pyrene, anthracene or fluoranthene [22] as a PAH substitute, and benzo[a]pyrene reproduces the rise in IL-1 and GM-CSF production with DEP [11]. These results suggest that the PAHs are the component responsible for these cytokine effects of DEP.
We hypothesized that crude DEP is a complex mixture of agonist and antagonist PAHs that exerts both stimulatory and inhibitory effects on cytokine expression and production. We further hypothesized that the order and timing of exposure to DEP with other inflammatory or priming agents determines the nature of the cytokine response. We were specifically interested in the effect of DEP on IL-10 and TGF-β, macrophage and repressor (TR-like) lymphocyte-derived cytokines that help determine tolerance, and are important in shaping the early immune response. Since macrophages are the initial scavenger cell for diesel particulates in the lung [23], diesel effects on macrophage function are critical to the subsequent immune response to the particles.
MATERIALS AND METHODS
Subjects
We enrolled 22 consecutive healthy adult volunteers, ages 24–48, without consideration of their atopic status. Subjects with a recent upper respiratory infection were excluded. All subjects were lifetime nonsmokers. Ten were nonatopic and had no history of asthma, allergic rhinitis or a chronic respiratory condition. Twelve were atopic with a history of mild asthma controlled with topical agents only, and/or seasonal allergies associated with positive skin tests. No subject was treated with systemic corticosteroids. All subjects gave informed consent approved by the Institutional Review Board.
Reagents
Standardized diesel exhaust particles (DEP) (National Institute of Standards, Gaithersburg, MD) and carbon black (the kind gift of Dr Joe Mauderly of the Inhalation Toxicology Research Institute) were suspended at 1 mg/ml and further diluted in sterile RPMI (Gibco/BRL). The dose of DEP (1–100 ng/ml) was based on previous reports [2–9]. LPS (Sigma) was serially diluted in RPMI from 1 mg/ml. Benzo[a]pyrene (BaP) and [α]naphthoflavone (Sigma St. Louis, MO), were solubilized as a 1 Molar solution in sterile media or DMSO and diluted further in sterile RPMI. The PAH fraction of the DEP constitutes 20–30% of the particle mass [10], which determined the concentrations used of BaP and [α]naphthoflavone from 1 nm to 100 µm, or 252·3 pg/ml to 25·2 mg/ml of BaP and 272·3 pg/ml to 27·2 mg/ml of [α]naphthoflavone.
Cells and culture
Human PBMCs were prepared by Ficoll-Hypaque (Pharmacia, Uppsala, Sweden) density centrifugation, and cultured at 1–2 × 106 cells/ml in RPMI-1640 medium (Gibco/BRL) supplemented with 10% heat inactivated fetal bovine serum (Gemini Bio Products Inc., Calabasas, CA) or 10% Nuserum (Collaborative Biomedical Products, Becton Dickinson, Bedford, MA), 10 mm HEPES (Gibco/BRL), 200 U/ml Pen/Strep (Gibco/BRL), 10 mg/ml Gentamycin (Gemini Bio Products), 4 mm l-glutamine, 0·1 mm nonessential amino acids (NEAA), 1 mm sodium pyruvate, and 0.05 mm 2-β-mercaptoethanol (all Gibco/BRL). DEP was added at 0, 1, 3, 10, 30 or 100 ng/ml, with a median dose of 10 ng/ml LPS. In those samples evaluating the effect of the order of addition, either 10 ng/ml LPS was added first for 24 h, and DEP at 0, 1 and 10 ng/ml added for a subsequent 48 h, or DEP at 0, 1 and 10 ng/ml was added first for 24 h, and 10 ng/ml LPS added for a further 48 h of culture. In those samples assayed for CD23 release, 4 units of human recombinant IL-4 (Genzyme, Cambridge, MA) per ml were added without LPS. A neutralizing anti-IL-10 monoclonal antibody (Pharmingen, San Diego, CA) was added to samples containing 10 ng/ml LPS and 0, 1 and 10 ng/ml DEP. Benzo[a]pyrene was added to cultures at 0, 1, 10 and 100 nm, and 1, 10 or 100 µm, with and without [α]naphthoflavone in similar concentrations. All cultures were kept at 37°C in an atmosphere of 5% CO2 and an aliquot of cells was removed at 16 h for RT-PCR, and at 6, 16 or 48 h for the S1 nuclease protection assay. Cell culture supernatants were assayed by ELISA for the cytokine of interest at 24 and 48 h.
Endotoxin and cytotoxicity controls
The media, DEP dilutions and cell preparations were tested for the presence of endotoxin using the Limulus Amoebocyte Lysate gel clot method (Associates at Cape Cod, Falmouth, MA) according to kit directions, and were found to be free of endotoxin. The assay was sensitive to 0·03 EU (endotoxin units)/ml. Aliquots from each sample condition were resuspended in trypan blue and the percentage of cells unable to exclude the dye was counted in triplicate, and compared to cells incubated in media alone for 48–72 h. The average percentage of viable cells was 96·1 ± 0·3% and did not differ significantly under any of the culture conditions. LDH release was evaluated in aliquots of cells from each sample condition. Briefly, 100 μl of the cell culture supernatant was added to phosphate buffer (50 mm, pH 7·4) and NADH (0·18 mm) supplemented with pyruvate (0·6 mm). The mean extinction diminution per minute was detected at 350 nm after 30 s, 1, 2 and 3 min at 25°C, and multiplied by a temperature coefficient to determine the concentration of LDH in each sample. Results were compared to a sample containing cells and media only, and did not significantly differ.
Reverse Transcriptase Polymerase Chain Reaction (RT-PCR)
Total cellular RNA was extracted from 106 cells per sample condition using a commercial extraction kit (Clontech, Palo Alto, CA). First strand cDNA was synthesized from 500 ng of total RNA using oligo dT and MMLV reverse transcriptase (Clontech). Based on similar amounts of the housekeeping gene Glyceraldehyde-3-phosphate dehydrogenase (G3PDH), equivalent amounts of cDNA were used in PCR for IL-10. Primers crossed one intron of genomic DNA and produced an amplified cDNA product of 328 bp for IL-10 and 452 bp for G3PDH. The following sequences of primers were used: IL-10 5′ primer: AAGCTGAGAACCAAG ACCCAGACATCAAGGCG; 3′ primer: AGCTATCCCAGAGC CCCAGATCCGATTTTGG; G3PDH 5′ primer: ACCACAGTCC ATGCCATCAC; 3′ primer: TCCACCACCCTGTTGCTGTA. cDNA was amplified 30 cycles for G3PDH, and 35 cycles for IL-10 in a reaction buffer containing 1·5 ml MgCl2 (Hot Wax MgCl2, InVitroGen, San Diego, CA), 2·5 units Taq Polymerase (Fisher Scientific, Pittsburgh, PA), 0·4 mm of each of the primers, and 0·2 mm dNTPs (Clontech). PCR cycling conditions were: 94°C for 1 min, 1 min at 60°C for G3PDH or 58°C for IL-10, 72°C for 2 min, and ended with 7 min of extension at 72°C. Products were run on 1% agarose gels (Gibco/BRL) and stained with ethidium bromide.
RNA quantification by S1 nuclease protection assay
PBMCs collected at 6, 16 or 48 h were snap frozen, and stored at −80 °C. Total RNA was extracted in RNAzol (TelTest, Inc., Friendswood, TX) according to the manufacturer's directions, and quantified using a commercial ribonuclease protection assay (Pharmingen; San Diego, CO). Briefly, sample mRNA was hybridized overnight to a panel of specific 32P-labelled single-stranded antisense RNA probes present in excess. Probes were of unique size and specific for IL-2, IL-4, IL-5, IL-9, IL-10, IL-13, IL-14, IL-15 and IFN-γ (human cytokine multiprobe template set hCK-1). Remaining nonhybridized (single-stranded) probe was lysed following a 1 h exposure to the S1 nuclease. Samples were washed, denatured, and electrophoresed on a 6% acrylamide urea denaturing gel. Protected probes of an appropriate size were identified by comparison to a ladder of undigested probe, and quantified utilizing a PhosphorImager SI (Molecular Dynamics, Sunnyvale, CA). Data were normalized to the expression of the housekeeping probes, L32 and G3PDH, and analysed as comparisons of each of the cell culture conditions.
Elisa
Cytokine production was measured by commercially available ELISA kits for IL-1β (Predicta, Genzyme, Cambridge, MA), CD23 (The Binding Site, San Diego, CA), TGF-β1 (Quantikine, R & D Systems, Minneapolis, MN), and TNF-α (EASIA, Biosource, Europe SA) according to kit directions. The effect of the anti-IL-10 neutralizing antibody on IL-1β was assessed by IL-1β EASIA (Biosource, Europe SA). Our IL-10 ELISA uses a sandwich technique with two rat monoclonal antibodies specific for different epitopes (Pharmingen, San Diego, CA). Sensitivity for the ELISAs were 0·1 ng/ml for IL-10, 0·7 ng/ml for CD23, and <10 pg/ml for IL-1β and TGF-β. The EASIAs for both IL-1β and TGF-β were sensitive to 2 pg/ml, and to 3 pg/ml for TNF-α.
Statistical analyses
Each set of data was analysed using a repeated measures analysis of variance which modelled a random subject effect. Data are expressed as the concentration of cytokine/ml and analysed between the paired control and the DEP-containing sample. Data for CD23 and IL-1β were square root transformed for these analyses, which normally distributed the data and had no bearing on the outcome of these analyses. A compound symmetric covariance structure was assumed. In all, statistical significance was analysed at the level of P-value = 0·05. Statistical analyses were performed using JMP 3.25 software (systat) on a Macintosh 6500.
RESULTS
PBMCs exposed to DEP before or with LPS down-regulate IL-10 and TGF-β production and IL-10 mRNA, without effect on TNF-α
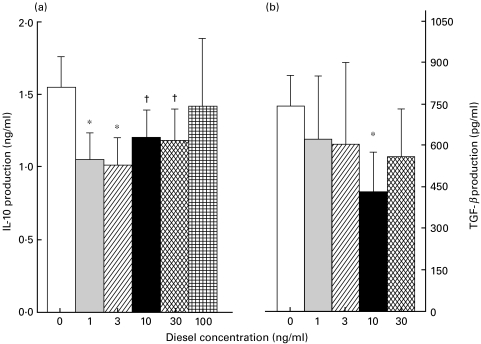
Circulating PBMCs constitutively produce IL-10, but at low levels [24]. We screened 22 consecutive donors for unstimulated IL-10 production, which in some atopics was below the limits of detection. A median dose of 10 ng/ml of LPS primed all cells to produce detectable levels of IL-10. At LPS concentrations higher than 100 ng/ml we failed to find a suppressive effect of DEP. In response to DEP alone, subjects with spontaneous detectable levels down-regulated IL-10 (data not shown), and with the coaddition of LPS all subjects down-regulated IL-10 in a dose-dependent manner. IL-10 production was 1·55 ± 0·21 ng/ml with LPS, and fell to 1·05 ± 0·18 ng/ml with 1 ng/ml of DEP and to 1·01 ± 0·19 ng/ml with 3 ng/ml of DEP (P < 0·05). IL-10 remained low with 10 and 30 ng/ml of DEP (P = 0·06), but was no longer suppressed with 100 ng/ml of DEP (Fig. 1a). We did not a priori hypothesize a difference in response to DEP by atopic status, and there was no difference in IL-10 response between atopic and nonatopic subjects when analysed retrospectively.
Fig. 1.
DEPs down-regulate IL-10 and TGF-β protein in a dose dependent manner. (a) PBMCs from 22 donors were cultured at 2 × 106 cells/ml, primed with a median dose of 10 ng/ml LPS, and stimulated with 0, 1, 3, 10, 30 or 100 ng/ml of DEP. IL-10 production was assessed by ELISA after 48 h. IL-10 production is significantly (P < 0·05) suppressed by 1 and 3 ng/ml DEP, and is also decreased (P < 0·06) by 10 and 30 ng/ml DEP. (b) Cultures from 16 subjects were also examined by ELISA for TGF-β production after 48 h of incubation. TGF-β decreased with 1 and 3 ng/ml of DEP, and fell significantly with 10 ng/ml DEP. *P < 0·05 compared to 0; †P = 0·06 compared to 0.
Supernatants were also evaluated by ELISA for TGF-β (n = 16) and TNF−α (n = 5). TGF-β production was similarly suppressed with increasing doses of DEP, from 741 ± 302 pg/ml without DEP, to 430 ± 144 pg/ml with 10 ng/ml DEP, P < 0·05 (Fig. 1b). Under similar conditions, TNF-α production was unaffected (data not shown).
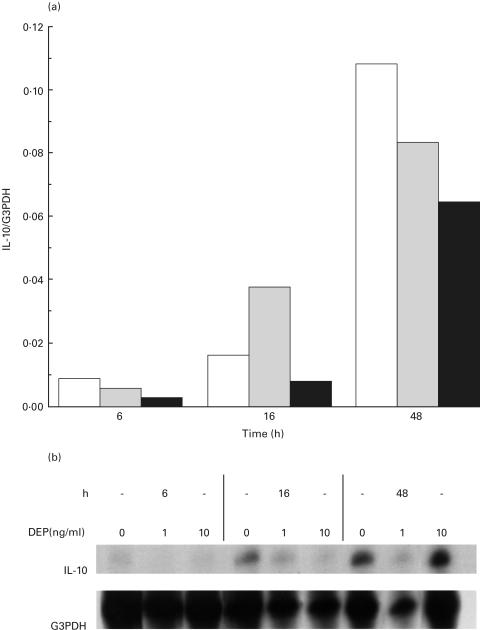
We used an S1 nuclease protection assay to evaluate the effect of DEP on IL-10 mRNA. Cells from four subjects were stimulated with 10 ng/ml of LPS, together with 0, 1 or 10 ng/ml of DEP. Levels of IL-10 mRNA were normalized to G3PDH, and compared after 6, 16 and 48 h of incubation. Sixteen hours was chosen because this has previously been shown to be the time point at which maximal modulation of IL-10 mRNA transcription occurs [25]. IL-10 mRNA decreased with 10 ng/ml DEP at all time points (Fig. 2).
Fig. 2.
DEPs down-regulate IL-10 mRNA in PBMCs in a dose dependent manner. PBMCs (n = 4) were incubated with 10 ng/ml of LPS and 0, 1 or 10 ng/ml of DEP. RNA was sampled at 6, 16 and 48 h of incubation, hybridized overnight to a panel of 32P-labelled cytokine probes which included IL-10, after which nonhybridized probes were lysed. Hybridized IL-10 probes were quantified using a PhosphorImager SI, and normalized to the housekeeping probe G3PDH. (a) Graph of IL-10 levels with 1 and 10 ng/ml DEP normalized to G3PDH from a representative subject, compared to those without added DEP at each time point. IL-10 mRNA is decreased by 10 ng/ml of DEP at all time points. □ 0 ng/ml DEP;  1 ng/ml DEP; ▪ 10 ng/ml DEP. (b) Representative gel of S1 nuclease protection assay demonstrating inhibition of IL-10 mRNA with DEP.
1 ng/ml DEP; ▪ 10 ng/ml DEP. (b) Representative gel of S1 nuclease protection assay demonstrating inhibition of IL-10 mRNA with DEP.
PBMCs exposed to DEP with LPS increase both IL-1β production and IL-4 stimulated CD23 release
As IL-10 is known to suppress IL-1β expression, we hypothesized that DEP inhibition of IL-10 would permit increased IL-1β production. Supernatants from the same DEP-stimulated cultures with or without LPS (n = 12) were sampled for IL-1β. IL-1β production doubled with 10 ng/ml of DEP in cultures without LPS, from 135 ± 58 pg/ml to 293 ± 114 pg/ml. It also increased significantly in LPS-primed cultures, from 2120 ± 543 pg/ml without DEP to 3793 ± 899 pg/ml with 30 ng/ml DEP, P = 0·011 (data not shown). To assess if the effect on IL-1β was due to changes in IL-10 levels or to the specific actions of DEP, we compared IL-1β production in cells (n = 6) suboptimally primed with a lower dose of LPS (1 ng/ml) to cells under similar conditions treated with a neutralizing anti-IL-10 monoclonal antibody (MoAb). When biologically active IL-10 was neutralized, IL-1β production nearly doubled from 556 ± 388 ng/ml with LPS alone to 998 ± 332 ng/ml with anti-IL-10 MoAb, but did not increase further with the concurrent addition of 1 or 10 ng/ml DEP.
As DEP-stimulated B cells release more CD23 [26], we evaluated soluble CD23 (sCD23) release in DEP-exposed PBMCs. Without exogenous IL-4, levels of sCD23 did not differ between atopics and nonatopics (6·3 ± 0·6 ng/ml versus 5·0 ± 0·8 ng/ml, P = NS), and the addition of DEP did not affect CD23. With exogenous IL-4, atopics released more sCD23 than did nonatopics, 33·5 ± 7·1 ng/ml compared to 12·3 ± 4·55 ng/ml, P = 0·02. With 30 ng/ml DEP, sCD23 rose further in the atopic subjects to 44·4 ± 9·3 ng/ml, P = 0·06.
The order of exposure to DEP and LPS is critical to the effect on IL-10 and IL-1β
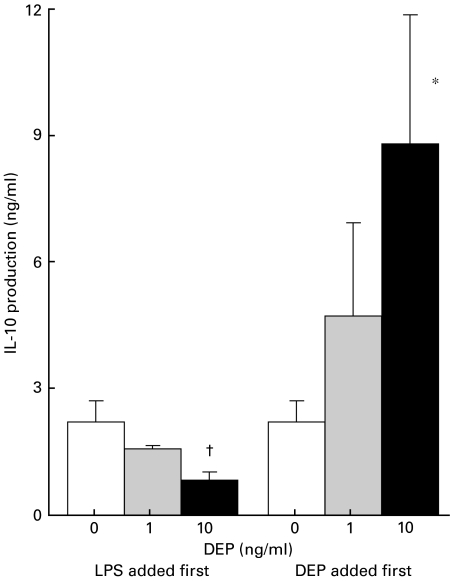
It was not clear from the initial experiments whether DEP needed to be present from the beginning of the LPS stimulus to exert its inhibitory effects, or whether DEP would inhibit IL-10 if added after LPS priming. In parallel cultures (n = 5), half were exposed to LPS for 24 h, followed by DEP, and the other half received DEP for 24 h, followed by LPS. When LPS was added first, subsequent DEP had a dose-dependent inhibitory effect by 24 h on IL-10 production, which was 2·21 ± 0·62 ng/ml with LPS alone, and fell to 1·58 ± 0·18 ng/ml with 1 ng/ml DEP, and to 0·83 ± 0·32 ng/ml with 10 ng/ml DEP (Fig. 3). The inhibitory effect was also seen at 48 h (data not shown). In contrast, when DEP was added 24 h before LPS, IL-10 increased in a dose dependent manner (Fig. 3), from 2·21 ± 0·62 ng/ml without DEP, to 4·71 ± 2·34 ng/ml with 1 ng/ml DEP, and 8·79 ± 3·29 ng/ml with 10 ng/ml DEP at 24 h (P < 0·05). Similar findings were seen at 48 h (data not shown). Concurrent with the rise in IL-10 when DEP was added first, IL-1β production fell to zero with 10 ng/ml DEP. These findings show that the rise in IL-10 had biologically demonstrable effects, and suggest that the effect of DEP on IL-1β was mediated through altered IL-10 levels.
Fig. 3.
The order of exposure to DEP and LPS is critical to the effect on IL-10 protein. Parallel cultures from 5 subjects were either exposed first to DEP at 0, 1 or 10 ng/ml for 24 h, followed by 10 ng/ml of LPS, or exposed first to 10 ng/ml LPS for 24 h followed by 0, 1 or 10 ng/ml of DEP. Supernatants were sampled at 24 and 48 h after the second addition; data is shown for 24-h samples. When LPS is added first to PBMCs, IL-10 protein is decreased in a dose-dependent manner. When DEP is added first, IL-10 protein rises with 1 ng/ml of DEP, and increases significantly with 10 ng/ml of DEP. *P = 0·09; †P < 0·05.
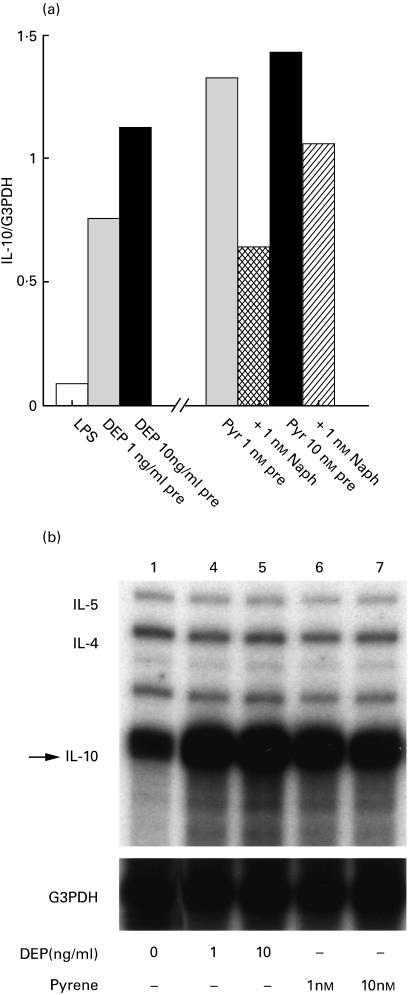
IL-10 mRNA transcripts (n = 3) were also evaluated by the S1 nuclease protection assay after 24 h of preincubation with 0, 1 and 10 ng/ml of DEP and 16 h of LPS exposure. IL-10 transcripts normalized to G3PDH rose eight-fold with 1 ng/ml DEP and over 12-fold with 10 ng/ml DEP preincubation (Fig. 4a, left). A representative gel (Fig. 4b, left) demonstrates increased intensity of the IL-10 bands when DEP was added first.
Fig. 4.
IL-10 mRNA is increased in cells exposed first to DEP or benzo[a]pyrene and then to LPS. (a, left) PBMCs (n = 3) were incubated with 0, 1 and 10 ng/ml of DEP, and 10 ng/ml LPS was added after 24 h. RNA was extracted and hybridized to a panel of 32P-labelled probes, and the hybridized IL-10 probe was quantified using a PhosphorImager SI, and normalized to the housekeeping probe G3PDH. Results are shown for a representative subject, and show a dose-dependent increase in IL-10 mRNA with increasing doses of DEP. (a, right) PBMCs were also incubated for 24 h with 1 or 10 nm benzo[a]pyrene as a surrogate for the PAH component of the DEP, then 10 ng/ml of LPS was added and RNA extracted after a further 16 h. Parallel cultures also received 1 nm of the competitive antagonist [α]naphthoflavone with each dose of benzo[a]pyrene. IL-10 mRNA was quantified and normalized to G3PDH. Results from a representative subject show increased IL-10 mRNA with benzo[a]pyrene preincubation, which is diminished with the coaddition of [α]naphthoflavone. Pyr = benzo[a]pyrene; Naph = [α]naphthoflavone. (b) Gel of a representative S1 nuclease protection assay showing increased IL-10 mRNA in cells exposed first to DEP (left) or benzo[a]pyrene (right), followed by LPS.
Benzo[a]pyrene mimics the effect of DEP on IL-10, which is reversed by the coaddition of [α]naphthoflavone
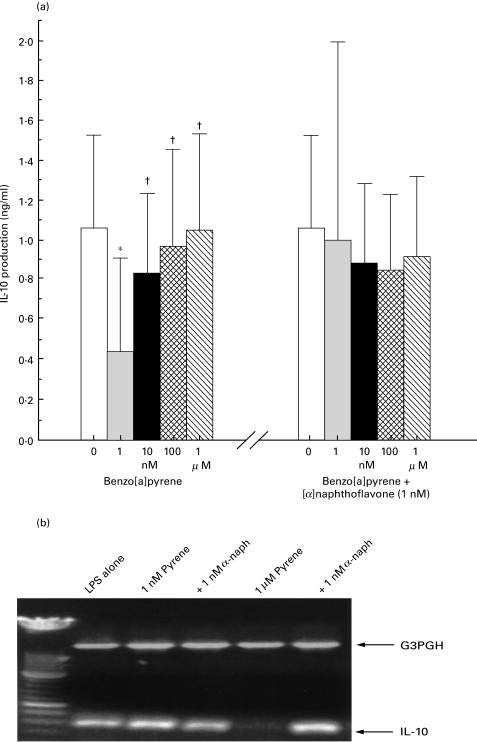
We next examined whether benzo[a]pyrene could duplicate the DEP effect on IL-10. Cell cultures (n = 6) were stimulated for 48 h with LPS together with BaP from 253 pg/ml to 25·3 mg/ml (1 nm to 100 µm). IL-10 was 1·17 ± 055 ng/ml with LPS alone, and fell to 0·44 ± 0·36 ng/ml with 1 nm BaP, P = 0·0003 (Fig. 5a, left). IL-10 production was also suppressed at 10 nm, 100 nm and 1 μm BaP, but to a lesser extent, and not suppressed with higher doses of BaP (data not shown). With the coaddition of 1 nm [α]naphthoflavone, a competitive antagonist of pyrene at low doses [27], to samples containing 1 nm BaP, IL-10 production was no longer suppressed (Fig. 5a, right). Higher concentrations of [α]naphthoflavone had no further effect on IL-10 production (data not shown).
Fig. 5.
IL-10 protein and mRNA are suppressed by low dose benzo[a]pyrene; the effect is reversed by the addition of [α]naphthoflavone. (a, left) PBMCs (n = 6) were primed with LPS at 10 ng/ml and exposed to benzo[a]pyrene at 0, 1, 10 and 100 nm, and 1 μm for 48 h. IL-10 protein is significantly suppressed by 1 nm benzo[a]pyrene, but returns to normal levels with higher doses. *P < 0·0005 compared to 0; †P < 0·005 compared to 1 nm. (a, right) 1 nm [α]naphthoflavone was added to parallel cultures, and IL-10 protein assayed by ELISA after 48 h. IL-10 protein is no longer suppressed when both benzo[a]pyrene and [a]napthoflavone are present in culture. P = NS compared to 0. (b) RT-PCR of IL-10 mRNA from PBMCs incubated for 18 h with 10 ng/ml of LPS alone (lane 1), with 1 nm (lanes 2 and 3) or 1 µm (lanes 4 and 5) of benzo[a]pyrene, and with 1 nm [α]naphthoflavone (lane 3 and 5). IL-10 message is expressed with LPS and with 1 nm pyrene, but is suppressed by 1 µm pyrene (lane 4). The coaddition of 1 nm [α]naphthoflavone to 1 μm pyrene (lane 5) restores IL-10 message. Gels were loaded with cDNA normalized to the equivalent expression of the housekeeping gene G3PDH.
The effect of BaP and [α]naphthoflavone on IL-10 mRNA was similar to that found for IL-10 production, although at a higher dose of BaP. IL-10 mRNA was inhibited by 1 µm pyrene, and reversed with the coaddition of 1 nm [α]naphthoflavone (Fig. 5b). These results demonstrate inhibition of IL-10 by BaP similar to crude DEP, occurring at the level of mRNA regulation. They suggest that IL-10 suppression was not due to cell toxicity, as the coaddition of [α]naphthoflavone restored IL-10 message.
We also evaluated the effect of preincubation with BaP on IL-10 mRNA by the S1 nuclease protection assay. PBMCs (n = 3) were exposed first to 0, 1 or 10 nm BaP for 24 h, and then to 10 ng/ml LPS (Fig. 4a, right). IL-10 mRNA rose 15-fold with 1 nm and 10 nm BaP when normalized to G3PDH. The coaddition of 1 nm [α]naphthoflavone blunted the rise in IL-10 transcripts by 50% with 1 nm pyrene and 25% with 10 nm pyrene. A representative gel (Fig. 4b, right) demonstrates increased intensity of IL-10 bands when BaP was added first.
Carbon black is not active
IL-10 production was not inhibited by the carbon black fraction of DEP, without the PAHs. With LPS, IL-10 levels were 0·80 ± 0·16 ng/ml, 0·60 ± 0·17 ng/ml with 10 ng/ml and 0·67 ± 0·22 ng/ml with 30 ng/ml DEP, P = NS. These results suggest that the carbon black particle core is not responsible for the effects of DEP on IL-10 production.
DISCUSSION
In this study we report that DEPs inhibit IL-10, a cytokine critical for the maintenance of tolerance, under specific conditions of exposure. In PBMCs primed with LPS and exposed concurrently or even after 24 h to DEP, IL-10 production was inhibited by ∼50%. Although circulating mononuclear phagocytic cells may not perfectly reflect the behaviour of airway antigen presenting cells, such as alveolar macrophages or the different families of dendritic cells, we chose to study the mononuclear phagocyte as a paradigm of an antigen-presenting cell to determine influences of diesel exhaust particulates on this class of immune cell. We reasoned that the behaviour of monocytes in response to these particulates would largely extend to their more differentiated tissue-based counterparts. IL-10 is known to inhibit production of IL-1, IL-6, TNF-α, IFN-γ, IL-4, IL-5 [28] and IL-8 [29], NFκB activation in human monocytes [30], and T-cell and monocyte accessory and antigen-presenting cell function [31–4]. Thus a fall in IL-10 levels during exposure to allergen or other inflammatory stimuli would permit an inflammatory response that otherwise would be inhibited. Interestingly, we found that maximal inhibition of IL-10 in LPS-primed human PBMCs occurred only at low doses of DEP and LPS. IL-10 production was not inhibited at doses of DEP greater than 30 ng/ml, or LPS greater than 100 ng/ml. Other studies have similarly demonstrated opposite effects of low compared to high dose DEP. For example, the initiation of a TH2 response to antigen plus DEP is more pronounced at lower doses of DEP [35]. DEP augments IL-6 and IL-8 production by TNF-α primed human bronchial epithelial cells only at low (50–200 pg/ml) concentrations of both DEP and TNF-α [10]. In our current studies, DEP also diminished IL-10 mRNA. However, these experiments cannot distinguish whether this effect is mitigated by decreased specific gene transcription, increased mRNA degradation, or other post-transcriptional effects leading to decreased expression of IL-10 mRNA transcripts. These data are consistent with observations that DEP binds to NFκB sites in cytokine promoter regions, and may exert inhibitory as well as stimulatory effects directly on gene transcription.
Interestingly, the effect of DEP on IL-10 was dependent on the order of its addition relative to LPS. DEP may have opposite effects through an ability to mediate inhibitory effects on IL-10 transcription while stimulating receptor expression, coupling, or other facets of the LPS-signalling cascade. If PBMCs were first exposed to DEP and then to LPS, IL-10 production and mRNA increased in a dose-dependent fashion. These results may explain the finding of increased IL-10 transcripts together with other pro-inflammatory cytokines following high dose DEP challenge with allergen [9]. Recently, IL-10 has been shown to be necessary for airway hyperresponsiveness following allergic sensitization [36,37]. It is plausible therefore that when the airway is primed with DEP and then exposed to an IL-10-inducing stimulus such as endotoxin these conditions may promote the development of airway hyperresponsiveness. Other studies demonstrate that the timing of IL-10 release is critical to its effect. If exogenous IL-10 is added within 3 days to cultures costimulated with IL-4, IgE transcripts and production fall. If, however, IL-10 is added after 3 days of culture, IgE and IgG4 production will be enhanced [38]. Thus a late rise in IL-10 following an inflammatory stimulus may have a pro-inflammatory effect. Together, these results demonstrate mixed agonist–antagonist effects of DEP on IL-10 production and mRNA.
We found that DEP also inhibited TGF-β production at doses similar to those that inhibited IL-10. Similar to IL-10, TGF-β is an inhibitory cytokine that blocks T cell activation by modulation of antigen presenting cell function [39], inhibits antigen-induced eosinophilic inflammation [40] and down-regulates the switch to IgE production [41]. Although the observed decreases were small, changes in complementary cytokines may have a profound and amplified effect in permitting an inflammatory response to bystander allergens that normally would be blocked. In a model of rheumatoid arthritis, TGF-β expression was increased together with IL-10 [42], and mRNA of both IL-10 and TGF-β increased with glucocorticoid treatment [43,44]. Thus under certain conditions the two cytokines are regulated in concert, possibly due to putative Th3(Tr1)-like cells, and concurrent suppression of IL-10 and TGF-β may be due to an effect of DEP on a transcription factor or cellular target common to both.
The IL-1β response of DEP and LPS exposed PBMCs is directly affected by IL-10 levels, and experiments with a neutralizing anti-IL-10 antibody confirm that the increase in IL-1β was a direct effect of IL-10 inhibition, rather than an effect specific to DEP itself. Under these conditions of LPS and DEP exposure, TNF-α production is unaffected, suggesting that the influence of DEP on cytokine production is cytokine specific and not due to a general toxic effect. When PBMCs were stimulated with IL-4, the atopic subjects released significantly more sCD23 than the nonatopic subjects, and also released more sCD23 with DEP. This suggests that atopic subjects are more sensitive to some of the effects of DEPs [11], and that DEP may synergize with IL-4, or possibly IL-13 or GM-CSF in regulating CD23 production.
Studies using purified PAHs have determined that these agents are responsible for some effects of DEP on cytokine secretion [45] and IgE expression and production [21–3]. We used benzo[a]pyrene (BaP) as a surrogate for the PAH fraction and found that at a dose similar to DEP, BaP suppressed IL-10 production and mRNA. Because we suspected that the action of DEP on IL-10 involved the aryl hydrocarbon receptor (AhR), we repeated the dose–response curve adding [α]naphthoflavone. Alpha-naphthoflavone is a competitive antagonist of BaP, especially at lower doses [27], and acts through the AhR. With both BaP and [α]naphthoflavone present at molar concentrations, IL-10 production and mRNA were no longer suppressed. Different concentrations of BaP produced optimal suppression of IL-10 when measured by secreted protein or by levels of specific mRNA. However, these assays represent very different measures of IL-10. The ELISA reflects the total concentration of secreted IL-10 over the course of the entire culture period, corrected for that which is degraded by proteases or bound to receptors or other IL-10 binding proteins that may thereby not be available for the ELISA. In contrast RT-PCR reflects the concentration of mRNA transcripts only at the exact moment the RNA was extracted, and is at best only an indirect reflection of protein. Similar to DEP, BaP stimulated IL-10 mRNA when added 24 h before LPS and [α]naphthoflavone blunted the rise when added concurrently.
DEP, similar to purified PAHs, can bind to the cytosolic aryl hydrocarbon receptor (AhR) and induce transcription of reporter cytokine genes [46]. These actions are significantly decreased by the AhR antagonist [α]naphthoflavone [47]. Our findings of IL-10 modulation with BaP, with reversal with [α]naphthoflavone, suggest that the effect of DEP on IL-10 is mediated by PAHs through the AhR system. These results lend further credence to the hypothesis that adsorbed PAHs are responsible for many of the immunological effects seen with DEP. Although under some experimental conditions, PAHs have been shown to suppress immunity by inducing apoptosis [48], several results in this study suggest that IL-10 and TGF-β suppression is not due to cell death. IL-10 was inhibited only at low doses of DEP, whereas IL-1β was enhanced in all subjects and sCD23 was increased in atopic subjects. Cell counts and LDH exclusion did not fall with increasing concentrations of DEP. When the same dose of DEP or BaP was added before LPS, IL-10 production and mRNA rose, also suggesting that cell toxicity is not important at these doses.
These results demonstrate that DEP can stimulate or inhibit cytokine expression and production, depending on the dose and timing of coexposure to other inflammatory stimuli. Inhibition of IL-10 expression and secretion by low dose DEP during the critical early phase of allergen presentation may permit the establishment and progression of an allergic response that normally would be suppressed, aided by diminished TGF-β and increased IL-1β and sCD23. Enhanced IL-10 expression and production following late exposure to inflammatory agents may lead to increased bronchial hyperreactivity. This pattern of cytokine responses may explain some of the respiratory and allergic effects seen with exposure to particulates.
Acknowledgments
The authors gratefully acknowledge the contribution of David R. McCormick, MS, who provided the statistical analysis of the data, and Mary Peterson for her excellent secretarial support. Funding was provided by National Jewish Medical and Research Center, Clinical Investigation Research funds, by NIH-AI 35156, and by EPAGrant R825702-01, Immunologic Basis of Environmental Lung Disease. K.P. was the recipient of the Robert S. Olnick fellowship in Occupational and Environmental Medicine. M.T. was the recipient of a Fullbright fellowship as a postdoctoral fellow from the Nofer Institute of Occupational Medicine, Lodz, Poland.
REFERENCES
- 1.van der Zee S, Hoek G, Boezen HM, et al. Acute effects of urban air pollution on respiratory health of children with and without chronic respiratory symptoms. Occup Environ Med. 1999;56:802–12. doi: 10.1136/oem.56.12.802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Atkinson RW, Bremner SA, Anderson HR, et al. Short-term associations between emergency hospital admissions for respiratory and cardiovascular disease and outdoor air pollution in London. Arch Environ Health. 1999;54:398–411. doi: 10.1080/00039899909603371. [DOI] [PubMed] [Google Scholar]
- 3.Ishizaki T, Kotzumi K, Ikemori R, Ishiyama Y, Kushibiki E. Studies of the prevalence of Japanese cedar pollinosis among residents in a densely cultivated area. Ann Allergy. 1987;56:270–9. [PubMed] [Google Scholar]
- 4.Omran M, Russell G. Continuing increase in respiratory symptoms and atopy in Aberdeen schoolchildren. BMJ. 1996;312:34. doi: 10.1136/bmj.312.7022.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nakagawa T, Nakagomi T, Hisamatsu S, et al. Increased prevalence of elevated serum IgE and IgG4 antibodies in students over a decade. J Allergy Clin Immunol. 1996;97:1165–6. doi: 10.1016/s0091-6749(96)70272-5. [DOI] [PubMed] [Google Scholar]
- 6.Fujimake H, Nohara O, Ichinose T, Watanabe N, Saito S. IL-4 production in mediastinal lymph node cells in mice intratracheally instilled with diesel exhaust particulates and antigen. Toxicology. 1994;92:261–8. doi: 10.1016/0300-483x(94)90182-1. [DOI] [PubMed] [Google Scholar]
- 7.Takano H, Yoshikawa T, Ichinose T, et al. Diesel exhaust particles enhance antigen-induced airway inflammation and local cytokine expression in mice. Am J Respir Crit Care Med. 1997;156:36–42. doi: 10.1164/ajrccm.156.1.9610054. [DOI] [PubMed] [Google Scholar]
- 8.Takano H, Ichinose T, Miyabara Y, et al. Inhalation of diesel exhaust enhances allergen-related eosinophil recruitment and airway hyperresponsiveness in mice. Toxicol Appl Pharmacol. 1998;150:328–37. doi: 10.1006/taap.1998.8437. 10.1006/taap.1998.8437. [DOI] [PubMed] [Google Scholar]
- 9.Diaz-Sanchez D, Dotson AR, Takenaka H, Saxon A. Combined diesel exhaust particulate and ragweed allergen challenge markedly enhances human in vivo nasal ragweed-specific IgE and skews cytokine production to a T helper cell 2-type pattern. J Immunol. 1997;158:2406–13. [PubMed] [Google Scholar]
- 10.Steerenberg PA, Zonnenberg JA, Dormans JA, et al. Diesel exhaust particles induced release of interleukin 6 and 8 by (primed) human bronchial epithelial cells (BEAS 2B) in vitro. Exp Lung Res. 1998;24:85–100. doi: 10.3109/01902149809046056. [DOI] [PubMed] [Google Scholar]
- 11.Bayram H, Devalia JL, Sapsford RJ, et al. The effect of diesel exhaust particles on cell function and release of inflammatory mediators from human bronchial epithelial cells in vitro. Am J Respir Cell Mol Biol. 1998;18:441–8. doi: 10.1165/ajrcmb.18.3.2882. [DOI] [PubMed] [Google Scholar]
- 12.Boland S, Baeza-Squiban A, Fournier T, et al. Diesel exhaust particles are taken up by human airway epithelial cells in vitro and alter cytokine production. Am J Physiol. 1999;276:L604–13. doi: 10.1152/ajplung.1999.276.4.L604. [DOI] [PubMed] [Google Scholar]
- 13.Quay J, Reed W, Samet J, Devlin RB. Air pollution particles induce IL-6 gene expression in human airway epithelial cells via NF-κB activation. Am J Respir Cell Mol Biol. 1998;19:98–106. doi: 10.1165/ajrcmb.19.1.3132. [DOI] [PubMed] [Google Scholar]
- 14.Takizawa H, Ohtoshi T, Kawasaki S, et al. Diesel exhaust particles induce NF-kappa B activation in human bronchial epithelial cells in vitro: importance in cytokine transcription. J Immunol. 1999;162:4705–11. [PubMed] [Google Scholar]
- 15.Fahy O, Tsicopoulos A, Hammad H, et al. Effects of diesel organic extracts on chemokine production by peripheral blood mononuclear cells. J Allergy Clin Immunol. 1999;103:1115–24. doi: 10.1016/s0091-6749(99)70187-9. [DOI] [PubMed] [Google Scholar]
- 16.Yang HM, Barger MW, Castranova V, et al. Effects of diesel exhaust particles (DEP), carbon black, and silica on macrophage responses to lipopolysaccharide: evidence of DEP suppression of macrophage activity. J Toxicol Environ Health. 1999;58:261–78. doi: 10.1080/009841099157232. [DOI] [PubMed] [Google Scholar]
- 17.Amakawa K, Terashima T, Matsumaru A, et al. Suppressive effects of exhaust particles on the release of cytokines from alveolar macrophages. Am J Respir Crit Care Med. 2000;161:A913. [Google Scholar]
- 18.Knox RB, Suphioglu C, Taylor P, et al. Major grass pollen allergen Lol p 1 binds to diesel exhaust particles: implications for asthma and air pollution. Clin Exp Allergy. 1997;27:246–51. [PubMed] [Google Scholar]
- 19.Ormstad H, Johansen BV, Gaarder PI. Airborne house dust particles and diesel exhaust particles as allergen carriers. Clin Exp Allergy. 1998;28:702–8. doi: 10.1046/j.1365-2222.1998.00302.x. 10.1046/j.1365-2222.1998.00302.x. [DOI] [PubMed] [Google Scholar]
- 20.Monn C, Koren HS. Bioaerosols in ambient air particulates: a review and research needs. Rev Environ Health. 1999;14:79–89. doi: 10.1515/reveh.1999.14.2.79. [DOI] [PubMed] [Google Scholar]
- 21.Tsien A, Diaz-Sanchez D, Ma J, Saxon A. The organic component of diesel exhaust particles and phenanthrene, a major polyaromatic hydrocarbon constituent, enhances IgE production by IgE-secreting EBV-transformed human B cells in vitro. Toxicol Appl Pharmacol. 1997;142:256–63. doi: 10.1006/taap.1996.8063. 10.1006/taap.1996.8063. [DOI] [PubMed] [Google Scholar]
- 22.Kanoh T, Suzuki T, Ishimori M, et al. Adjuvant activities of pyrene, anthracene, fluoranthene and benzo(a)pyrene in production of Anti-IgE antibody to Japanese cedar pollen allergen in mice. J Clin Lab Immunol. 1996;46:133–47. [PubMed] [Google Scholar]
- 23.Kobzik L. Lung macrophage uptake of unopsonized environmental particulates. J Immunol. 1995;155:367–76. [PubMed] [Google Scholar]
- 24.Borish L, Aarons A, Rumbyrt J, Cvietusa P, Negri J, Wenzel S. Interleukin-10 regulation in normal and asthmatic subjects. J Allergy Clin Immunol. 1996;97:1290–6. doi: 10.1016/s0091-6749(96)70197-5. [DOI] [PubMed] [Google Scholar]
- 25.Yssel H, de Waal Malefyt R, Roncarolo MG, Abrams JS, Lahesmaa R, Spits H, de Vries JE. IL-10 is produced by subsets of human CD4+ T cell clones and peripheral blood T cells. J Immunol. 1992;149:2378–84. [PubMed] [Google Scholar]
- 26.Takenaka H, Zhang K, Diaz-Sanchez D, Tsien A, Saxon A. Enhanced human IgE production results from exposure to the aromatic hydrocarbons from diesel exhaust: Direct effects on B-cell IgE production. J Allergy Clin Immunol. 1995;95:103–15. doi: 10.1016/s0091-6749(95)70158-3. [DOI] [PubMed] [Google Scholar]
- 27.Merchant M, Krishnan V, Safe S. Mechanism of action of alpha-naphthoflavone as an Ah receptor antagonist in MCF-7 human breast cancer cells. Toxicol Appl Pharmacol. 1993;120:179–85. doi: 10.1006/taap.1993.1101. 10.1006/taap.1993.1101. [DOI] [PubMed] [Google Scholar]
- 28.Del Prete G, De Carli M, Almerigogna F, et al. Human IL-10 is produced by both type 1 helper (Th1) and Type 2 helper (Th2) T cell clones and inhibits their antigen-specific proliferation and cytokine production. J Immunol. 1993;150:353–60. [PubMed] [Google Scholar]
- 29.Wang P, Wu P, Anthes JC, et al. Interleukin-10 inhibits interleukin-8 production in human neutrophils. Blood. 1994;83:2678–83. [PubMed] [Google Scholar]
- 30.Wang P, Wu P, Siegel MI, Egan RW, Billah MM. Interleukin(IL)-10 inhibits nuclear factor κB (NFκB) activation in human monocytes. J Biol Chem. 1995;270:9558–63. doi: 10.1074/jbc.270.16.9558. [DOI] [PubMed] [Google Scholar]
- 31.Curtis JL, Sonstein J, Milik AM, et al. IL-10 downregulates murine lung inflammation to intratracheal particulate antigen by decreasing T cell recruitment and cytokine production. Am J Resp Crit Care Med. 1997;S155:A205. [Google Scholar]
- 32.deWaal Malefyt R, Haanen J, Spits H, et al. Interleukin-10 (IL-10) and viral IL-10 strongly reduce antigen-specific T cell proliferation by diminishing the antigen-presenting capacity of monocytes via downregulation of class II Major Histocompatibility expression. J Exp Med. 1991;174:915–24. doi: 10.1084/jem.174.4.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ozawa H, Alba A, Nakagawa S, Tagami H. Interferon-γ and interleukin-10 inhibit antigen presentation by Langerhans cells for T helper type 1 cells by suppressing their CD80 (B7–1) expression. Eur J Immunol. 1996;26:648–52. doi: 10.1002/eji.1830260321. [DOI] [PubMed] [Google Scholar]
- 34.Groux H, Bigler M, deVries J, Roncarolo MG. Interleukin-10 induces a long-term antigen-specific anergic state in human CD4+ T cells. J Exp Med. 1996;184:19–29. doi: 10.1084/jem.184.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yoshino S, Sagai M. Induction of systemic Th1 and Th2 immune responses by oral administration of soluble antigen and diesel exhaust particles. Cell Immunol. 1999;192:72–8. doi: 10.1006/cimm.1998.1427. [DOI] [PubMed] [Google Scholar]
- 36.van Scott MR, Justice JP, Bradfield JF, et al. IL-10 reduces Th2 cytokine production and eosinophilia but augments airway reactivity in allergic mice. Am J Physiol Lung Cell Mol Physiol. 2000;278:L667–74. doi: 10.1152/ajplung.2000.278.4.L667. [DOI] [PubMed] [Google Scholar]
- 37.Makela MJ, Kanehiro A, Borish L, et al. IL-10 is necessary for the expression of airway hyperresponsiveness but not pulmonary inflammation after allergic sensitization. Proc Natl Acad Sci USA. 2000;97:6007–12. doi: 10.1073/pnas.100118997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jeannin P, Lecoanet S, Delnest Y, Gauchat JF, Bonnefoy JY. IgE versus IgG4 production can be differentially regulated by IL-10. J Immunol. 1998;160:3555–61. [PubMed] [Google Scholar]
- 39.Tsunawaki S, Sporn M, Ding A, Nathan C. Deactivation of macrophages by transforming growth factor-β. Nature. 1988;334:260–2. doi: 10.1038/334260a0. [DOI] [PubMed] [Google Scholar]
- 40.Haneda K, Sano K, Tamura G, et al. Transforming growth factor-β secreted from CD4+ T cells ameliorates antigen-induced eosinophilic inflammation: a novel high-dose tolerance in the trachea. Am J Respir Cell Mol Biol. 1999;21:268–74. doi: 10.1165/ajrcmb.21.2.3576. [DOI] [PubMed] [Google Scholar]
- 41.Machold KP, Carson DA, Lotz M. Transforming growth factor-beta (TGF beta) inhibition of Epstein-Barr virus (EBV)- and interleukin-4 (IL-4)-induced immunoglobulin production in human B lymphocytes. J Clin Immunol. 1993;13:219–27. doi: 10.1007/BF00919975. [DOI] [PubMed] [Google Scholar]
- 42.Bucht A, Larsson P, Weisbrot L. Expression of interferon gamma (IFN-gamma), IL-10, IL-12 and Transforming Growth Factor-beta (TGF-beta) mRNA in synovial fluid cells from patients in the early and late phases of rheumatoid arthritis. Clin Exp Immunol. 1996;103:357–67. doi: 10.1111/j.1365-2249.1996.tb08288.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Matthias J, Lim S, Seybold J, et al. Inhaled corticosteroids increase Interleukin-10 but reduce macrophage inflammatory protein-1α, granulocyte-macrophage colony-stimulating factor, and interferon-γ release from alveolar macrophages in asthma. Am J Respir Crit Care Med. 1998;157:256–62. doi: 10.1164/ajrccm.157.1.9703079. [DOI] [PubMed] [Google Scholar]
- 44.Ayanlar Batuman O, Ferrero AP, Diaz A, Jimenez SA. Regulation of transforming growth factor-β1 gene expression by glucocorticoids in normal human T lymphocytes. J Clin Invest. 1991;88:1574–80. doi: 10.1172/JCI115469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ohtoshi T, Takizawa H, Okazaki H, et al. Diesel exhaust particles stimulate human airway epithelial cells to product cytokines relevant to airway inflammation in vitro. J Allergy Clin Immunol. 1998;101:778–85. doi: 10.1016/S0091-6749(98)70307-0. [DOI] [PubMed] [Google Scholar]
- 46.Meek MD. Ah receptor and estrogen receptor-dependent modulation of gene expression by extracts of diesel exhaust particles. Environ Res. 1998;79:114–21. doi: 10.1006/enrs.1998.3870. [DOI] [PubMed] [Google Scholar]
- 47.Chang CY, Puga A. Constitutive activation of the aromatic hydrocarbon receptor. Mol Cell Biol. 1998;18:525–35. doi: 10.1128/mcb.18.1.525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Burchiel SW, Luster MI. Signaling by environmental polycyclic aromatic hydrocarbons in human lymphocytes. (Review.) Clin Immunol. 2001;98:2–10. doi: 10.1006/clim.2000.4934. 10.1006/clim.2000.4934. [DOI] [PubMed] [Google Scholar]