Abstract
IL-15 is produced by a wide variety of tissues in response to inflammatory stimuli. We examined the effect of IL-15 in supporting the maturation of monocytes to dendritic cells in ex vivo culture. IL-15 transformed CD14+ monocytes to mature dendritic cells. These dendritic cells were similar to those obtained from monocyte cultures treated with a combination of the cytokines GM-CSF, IL-4 and TNF-α. The effects of IL-15 did not depend on endogenously produced GM-CSF. The IL-15-induced dendritic cells also expressed chemokines and stimulated strong allo-responses that were characteristic of mature dendritic cells. These data indicate that CD14+ monocytes respond to IL-15 by undergoing morphological transformation and acquiring characteristic dendritic cell features that facilitate antigen-specific responses of T cells. Thus, the release of IL-15 by inflammatory stimuli may induce the conversion of monocytes to immuno-stimulatory dendritic cells to support primary immune responses against pathogens.
Keywords: monocytes, cellular differentiation, dendritic cells, MHC, cytokines
Introduction
Dendritic cells (DC) are potent APCs that activate naïve T cells and maintain adaptive immune responses [1,2]. Experimental methods for producing DC from progenitor cells were previously described [3–8]. The differentiation of DC is driven by inflammatory stimuli or microenvironmental factors such as bacterial products, lipopolysaccharides, and locally produced cytokines such as granulocyte-macrophage colony stimulating factor (GM-CSF), tumour necrosis factor α (TNF-α), and interleukin-1β (IL-1β). Monocytes and CD34+ precursors of DC require GM-CSF for differentiation and a secondary signal such as TNF-α or lipopolysaccharides (LPS) for maturation [9], whereas lymphoid DC require IL-3 [10]. T-cells, monocytes and other cells collaborate in the inflammatory response to produce these cytokines. For example, most tissues express IL-15 and IL-15Rα in response to inflammatory stimuli, whereas IL-2 and IL-2Rα is expressed primarily by antigen-activated T cells [11,12]. While these two cytokines are only distantly related by amino acid sequence homology, some degree of structural homology is suggested by the fact that both IL-2R and IL-15R use the same β- and γ-chains [13]. Ligand-specificity is determined by the α-chain [14,15]. Both cytokines are potent growth factors for activated T and NK cells [16,17].
Activated T-cells and T cell-derived factors play an important role in the functional maturation and increased survival of DC [18–20]. Because IL-15 and IL-15Rα are expressed by most cells in response to inflammatory stimuli [21,22], it is likely that this cytokine has an integral role in primary immune responses. Agostini et al. [23] reported that macrophages activated with IL-15 up-regulated the expression of costimulatory molecules, such as CD80 and CD86, which enhance signal transduction between T cells and APCs. Furthermore, the long-term effects of IL-15 may be necessary for maintaining antigen-specific memory T cells [24,25], and for the recruitment of DC and NK cells from CD34+ stem cells [7]. Monocytes represent an abundant source of DC precursors. However, it is not known if inflammatory cytokines can induce the transformation of monocytes into DC. To study this issue more closely, we examined the effect of IL-15 in supporting the maturation of CD14+ monocytes to DC.
Materials and methods
Reagents and antibodies
Recombinant cytokines GM-CSF and IL-4 were obtained from Immunex (Seattle, WA), TNF-α from Genzyme Corporation (Cambridge, MA). Pooled human AB sera were obtained from Pel-Freez (Brown Deer, WI). Mouse anti‐human CD14 and CD3 MoAbs conjugated with magnetic beads were purchased from Milteneyi Biotech Inc., Auburn, CA. The FITC-conjugated MoAbs Leu M3 (anti-CD14), Leu HLA DR (anti-DR), IL-2R (anti-CD25), anti-CD4, Leu 11a (anti-CD16) and Ig isotype control antibodies were purchased from Becton Dickinson (San Jose, CA); anti-CD86, anti-CD11c, anti-HLA-ABC and anti‐human GM-SCF MoAbs were purchased from Pharmingen (San Diego, CA); anti-CD83, Anti-CD45, anti-CD40 and anti-CD80 from Immunotech (Marseille, France); anti-CD1a antibody (cortical thymocytes) were obtained from Dako (Carpinteria, CA). IL-15 was purchased from PeproTech, Inc (Rocky Hill, NJ). Ficoll-Hypaque was purchased from Pharmacia (Uppsala, Sweden). The [methyl-3H]thymidine was purchased from Amersham Life Sciences (Arlington Heights, IL).
Cell separation and dendritic cell cultures
PBMC were obtained from normal healthy volunteers. Mononuclear cells were separated from blood by standard gradient centrifugation with Ficoll-Hypaque (Pharmacia). Mononuclear cells were harvested from the interface, washed twice and CD14+ monocytes were isolated by positive selection using immuno-magnetic beads. Briefly, purified mononuclear cells were suspended (107 cells/80 µl) in cold PBS supplemented with 2 mm EDTA and 0·5% bovine serum albumin (Fraction V, Sigma Chemical Co., St. Louis, MO). Paramagnetic beads coated with anti CD14 MoAb (Miltenyi Biotech Inc., Auburn, CA) were mixed with the mononuclear cells (20 µl per 107 cells). The CD14-labelled cells were incubated for 15 min (4°C), washed and passed through a type RS or VS iron-fibre column placed within a strong magnetic field (Miltenyi Biotech, Inc.). CD14+ cells bound to the column were eluted. For purified T cells, mononuclear cells were centrifuged through discontinuous Percoll (Pharmacia) gradients (25–60%) and T cells (purity 95–98%) were obtained from the high density (45–60%) Percoll fraction as previously described [26]. The isolated CD14+ monocytes did not express either IL-2Rα (CD25) or CD1a (data not shown). CD14+ monocytes were cultured (37°C, 5% CO2) in RPMI-1640, supplemented with 5% human AB serum. To generate DC, cultures were also supplemented with 800 U/ml GM-CSF and 500 U/ml IL-4. For experiments involving addition of cytokines, optimal doses were determined (data not shown), and based on these results, 100 ng/ml of TNF-α and 100 ng/ml of IL-15 were used for all studies.
FACS analysis
CD14+ monocytes were incubated with an anti-Fc receptor MoAb (Miltenyi) to block Fc-receptor binding sites, then incubated (45 min, 4°C) with different FITC-labelled or PE-labelled MoAbs, or control isotype-matched MoAbs. Unbound antibody was removed by washing the cells with medium (4°C). After two washes, cells were fixed with 1% paraformaldehyde and the cell-associated immuno-fluorescence was measured by flow cytometry (FACSort, Becton Dickinson).
T cell proliferation assay
CD14+ cells were cultured with either GM-CSF/IL-4 for 6 d followed by TNF-α treatment for 24 h or only IL-15 for 7 d, harvested, irradiated (2000 Rad), and used as stimulator cells. T cells (1–3 × 105/well) were cultured (6 d, 37°C, 5% CO2, humidified air) with irradiated DC as stimulators (1–10 × 104/well) in 96-well, round-bottom cell culture plates (Costar, Cambridge, MA) in RPMI medium containing 5% human AB serum. T-cell proliferation was measured in triplicate after 6 d of culture by incubating (12 h) cultures with 1 µCi [3H]thymidine/well, harvesting the cells onto microplate unifilters and measuring radioactivity in a liquid scintillation counter (Packard, Meriden, CT).
Measurement of transcriptional activation of chemokine genes by RT-PCR
Total cellular RNA was extracted from 1 to 2 × 106 cells using TRI REAGENT (Molecular Research Center Inc., Cincinnati, OH), according to the manufacturer's instructions. The concentration of purified RNA was estimated spectrophometrically by measuring the absorbance at 260 nm. Single-strand cDNA was synthesized from total RNA by AMV reverse transcriptase (Perkin Elmer, CA). Briefly, a 20-µl reaction mixture contained 250 ng of RNA template, 500 ng oligo-dT primer, 5 mm MgCl2, Tris buffer, 1 mm dNTPs, 2·5 Units rRNAsin, and 15 Units AMV reverse transcriptase. The reaction mixture was incubated for 30 min (42°C). Relative levels of chemokine mRNA were measured by PCR. The assay consisted of 2 µl of cDNA in a final volume of 50 µl that contained 800 mm Tris-HCl pH 8·9, 200 mm (NH4)2SO4, 50 mm MgCl2, 0·2 mm dNTPs (Promega, Madison, WI), 0·2 µm of each primer and 1·5 Units AmpliTaq DNA Polymerase (Perkin-Elmer, Norwalk, CT). Primers used for PCR were: hMIP-1α: 5′-CGAGCCCACATTCCGTCACC-3′ and 5′-CGCATGTTCCCAAGGCTCAGG-3′, amplifying a 309-bp product; hRANTES: 5′-CCCCGTGCCCACATCAAGGAGT-3′ and 5′-TCAAGGAGCGGGTGGGGTAGGA-3′, amplifying a 257-bp product; hPARC: 5′-AGTTTCCAAGCCCCAGCTCACTCT-3′ and 5′-TGGGGGCGGTTTCAGAATAGTCA-3′, amplifying a 208-bp product; hTARC: 5′-CCTCCTCCTGGGGGCTTCTCTG-3′ and 5′-GACTTAATCTGGGCCCTTTGTGC-3′ amplifying a 445-bp product; hELC: 5′-CACCCTCCATGGCCCTGCTACT-3′ and 5′-TAACTGCTGCGGCGCTTCATCT-3′ amplifying a 304-bp product; β-actin: 5′-ACACTGTGCCCATCTACGAGGGG-3′ and 5′-ATGATGGAGTTGAAGGTAGTTTCGTGGAT-3′, amplifying a 340-bp product. cDNA were amplified by PCR under the following conditions: 60 s at 95°C, 3 min at 55°C and 2 min at 72°C for 30 cycles. PCR products were resolved on a 1·5% agarose gel containing ethidium bromide.
Chemokine assays
Chemokines MIP-1α, MCP-1, RANTES and IL-8 were measured by enzyme immunoassay (R & D SYSTEMS, Minneapolis, MN), according to the manufacturer's instructions. Briefly, 100 µl of culture supernatant or control (standard) were added to 96-well microtiter plates precoated with MoAb to MIP-1α, MIP-1β, MCP-1, RANTES and IL-8 and incubated for 30 min at room temperature (RT). The wells were washed and an enzyme-linked polyclonal antibody specific for MIP-1α, MCP-1, RANTES or IL-8 was added (100 µl) to detect bound cytokine. After a brief incubation (30 min, RT), the wells were washed to remove any unbound antibody reagent, a substrate solution was added to the wells and incubated (20 min, RT). The colour development was stopped and the intensity of the colour was measured at the absorbance 450 nm. A standard curve was used to estimate the experimental concentration of chemokines.
Results
IL-15-treated CD14+ monocytes acquire characteristic DC morphology
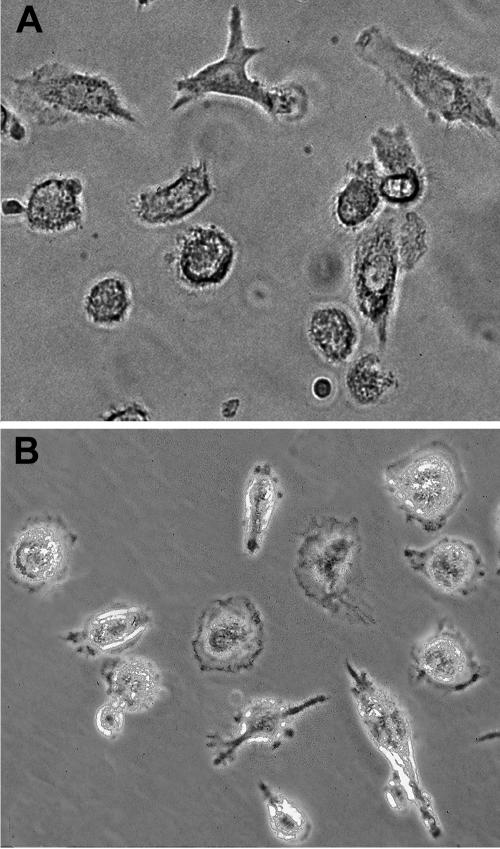
We first examined the cellular morphology of monocytes that were incubated with IL-15. We observed that IL-15 treatment resulted in CD14+ monocytes that had pronounced dendritic morphology, such as cytoplasmic protrusions, processes and veils (Fig. 1b). This change in morphology indicated that monocytes responded to exogenous IL-15 and acquired cellular processes that were quite distinct from monocytes before culture but similar to CD14+ monocytes cultured in the presence of GM-CSF plus IL-4, followed by stimulation with TNF-α (Fig. 1a).
Fig. 1.
CD14+ monocytes cultured with IL-15 acquired the characteristic morphology of mature dendritic cells. (a) CD14+ monocytes cultured for 6 d with GM-CSF and IL-4, followed by TNF-α stimulation for 1 d; (b) CD14+ monocytes cultured for 7 d with IL-15 alone. Magnification 40 ×.
Cell surface expression of MHC class II and costimulatory molecules on monocytes are induced by IL-15
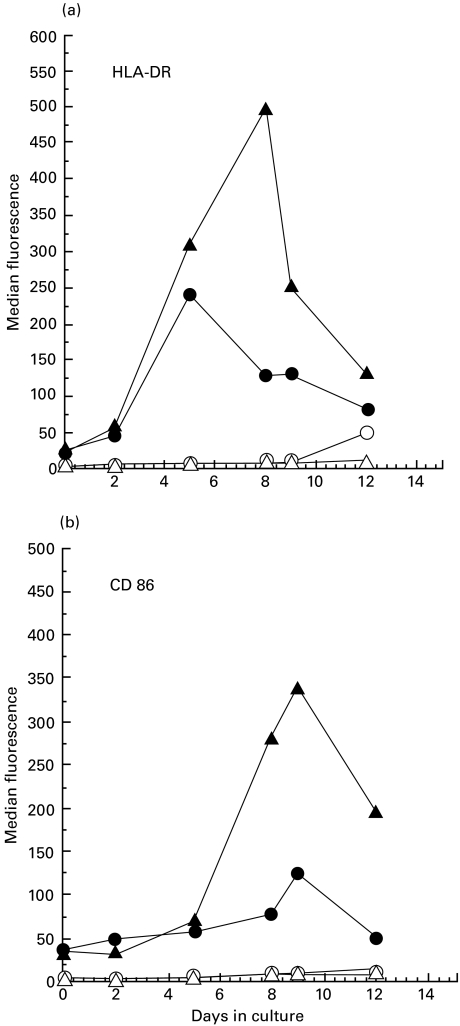
Activation of naïve T cells by DC requires neo-expression of HLA-DR and costimulatory molecules. Surface HLA-DR and CD86 expression on IL-15 treated CD14+ monocytes were examined by flow cytometry. Results shown in Fig. 2a,b indicate that IL-15-treated monocytes expressed significantly higher levels of HLA-DR and CD86 when compared to untreated monocytes. Peak expression occurred by 8 d for HLA-DR and 9 d for CD86. These results indicated that transformation of CD14+ monocytes by IL-15 was accompanied by strong induction of HLA-DR and CD86 expression. Monocytes treated with IL-15 expressed cell-surface levels of the costimulatory molecules CD86, CD80 and CD40 that were equivalent to DC obtained from cultures of CD14+ monocytes treated with GM-CSF plus IL-4 and TNF-α (Fig. 3a, b). CD14 expression was down-regulated in both IL-15-induced DC as well as DC derived from culture treated with combination of GM-CSF, IL-4 and TNF-α (Fig. 3a, b) and these cells did not express CD1a (data not shown). The number of DC recovered by either IL-15 or combination treatment with GM-CSF, IL-4 and TNF-α was about the same. Since expression of CD83 is a late event in DC maturation we examined expression of this marker at 24 h (day 7) and 72 h (day 9) after TNF-α addition in GM-CSF/IL-4 culture and for IL-15 treated culture of monocytes at day 7 or 9. CD83 expression was found to be increased in both cultures treated with GM-CSF/IL-4/TNF-α or IL-15 and suggest maturation of DC (Fig. 3c). These results suggest that the IL-15-induced transformation of CD14+ monocytes to DC occurs without maturation stimuli like TNF-α or LPS.
Fig. 2.
Kinetics of HLA-DR and CD86 cell-surface expression. CD14+ monocytes were cultured with IL-15, harvested at different time points and labelled with FITC-conjugated MoAb. Cell surface expression of HLA-DR and CD86 was analysed by flow cytometry. (a) Kinetics of HLA-DR expression; (b) kinetics of CD86 expression. ○ and ▵ represent matched Ab isotype control, • represent expression without cytokine treatment and ▴ represent expression in the presence of IL-15. Data are presented as the median value of fluorescence intensity.
Fig. 3.
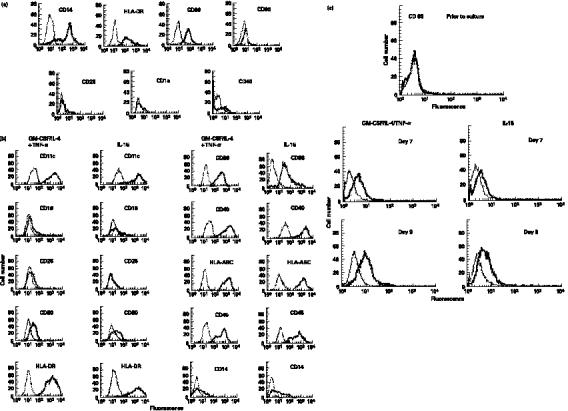
Comparison of cell surface receptors of CD14+ monocytes cultured with IL-15 or combination of GM-CSF, IL-4 and TNF-α. DC were harvested after 7 d in culture with IL-15 or GM-CSF and IL-4 for 6 d followed by TNF-α treatment for 24 h and labelled with FITC- or PE-conjugated specific MoAb or isotype matched-control MoAb. Cell surface antigen expression was measured by flow cytometry. (a) CD14+ cells prior to culture. (b) 7 d of culture. (c) CD83 expressed by CD14+ monocytes prior to culture, 7 or 9 of culture. Isotype-matched control MoAb, thin lines; surface antigen-specific MoAb, thick lines.
Conversion of CD14+ monocytes to mature DC by IL-15 does not require GM-CSF
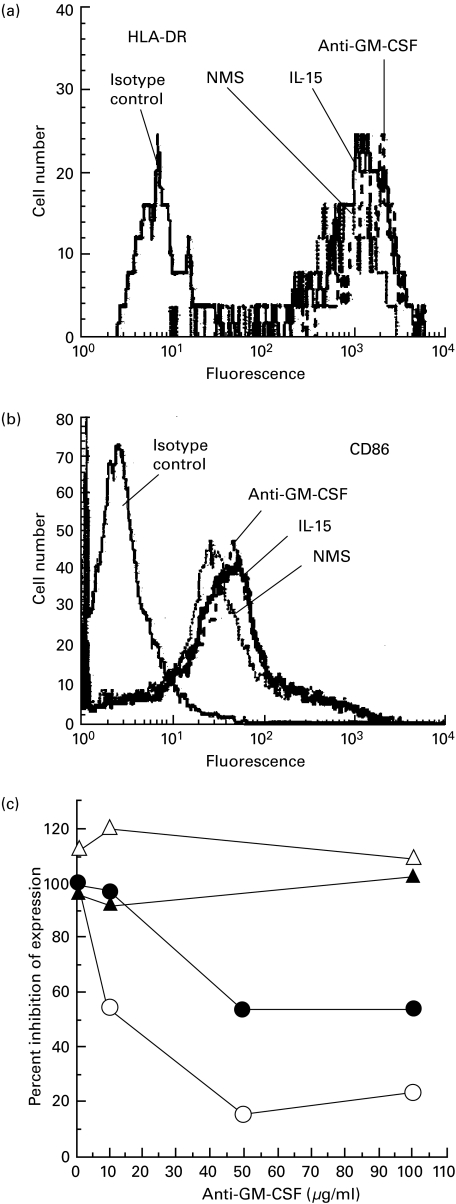
GM-CSF is a key factor for both murine and human DC development that stimulates the growth and differentiation of pluripotential progenitors into DC. We examined the monocytes' response to IL-15 to see if the transformation to DC depended on the release of GM-CSF. Varying concentrations (1–100 µg/ml) of neutralizing anti-GM-CSF MoAb [27] were added to monocytes cultured with IL-15. As determined by the increased expression of HLA-DR and CD86 molecules (Fig. 4a, b), the anti-GM-CSF antibody did not block the transformation of monocyte to DC. To confirm that the anti-GM-CSF-antibody neutralized GM-CSF activity, CD14+ monocytes were cultured with IL-15 either for 7 d or GM-CSF and IL-4 for 6 d followed by TNF-α for 24 h in the presence of blocking of anti-GM-CSF antibody. Cell surface expression of HLA-DR and CD 86 were examined by flow cytometry. Our results indicate that neutralizing anti-GM-CSF antibody did not affect HLA-DR or CD86 surface expression in IL-15-induced DC. In contrast, anti-GM-CSF antibody inhibited cell surface expression of HLA-DR as well as CD86 in a dose dependent manner in cultures treated with combination of GM-CSF, IL-4 and TNF-α (Fig. 4c). Further, no IL-15 was detected in media of activated monocytes cultured with GM-CSF and IL-4 (data not shown). These results suggest that the effect of IL-15 on monocytes was specific and independent of the GM-CSF-driven maturation pathway.
Fig. 4.
Conversion of CD14+ monocytes to mature DC by IL-15 was independent of GM-CSF. CD14+ monocytes were cultured with IL-15 or IL-15 in the presence of anti-GM-CSF or control antibody (normal mouse serum, NMS). Cells were harvested at day 7 and cell surface HLA-DR (a) and CD86 (b) were measured by flow cytometry with FITC-labelled specific or isotype matched-control MoAbs. Cell surface HLA-DR and CD86 expression were assessed. Histogram (a and b) represents for isotype matched control antibody and HLA-DR or CD86 expression by CD14+ monocytes when cultured with IL-15 (no antibody) or expression when cultured with IL-15 in the presence of NMS (control antibody) or anti-GM-CSF antibody as indicated. Data represent optimum dose of antibody concentration. (c) Inhibition of HLA-DR and CD86 expression by anti-GM-CSF antibody in GM-CSF, IL-4 and TNF-α induced culture of CD14+ monocytes but not in IL-15-induced culture. To confirm that the anti-GM-CSF neutralized GM-CSF activity, CD14+ monocytes were cultured with IL-15 or combination of GM-CSF, IL-4 and TNF-α in the presence of varying concentrations (1–100 µg/ml) of anti-GM-CSF antibody. Cells were harvested at day 7 and cell surface HLA-DR and CD86 expression were measured by flow cytometry with FITC-labelled specific or isotype matched-control MoAbs. Cell surface HLA-DR and CD86 expression were assessed. Data presented as percent inhibition of expression. Combination treatment with GM-CSF, IL-4 and TNF-α DC (○, •); IL-15 treated DC, (▵, ▴). CD86 expression, filled symbols; HLA-DR expression, open symbols. Results are representative of three experiments.
IL-15-induced DC stimulate potent T-cell responses
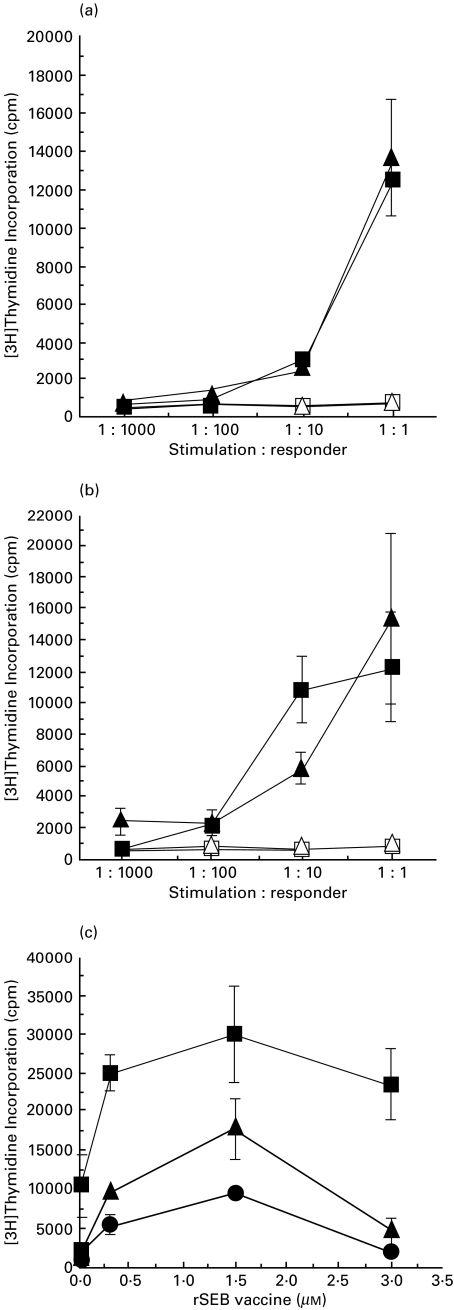
The best definition of DC relies on a confirmation of functional properties. Potent induction of a primary, T-cell dependent, allo-response can be used as one measure of DC function. Therefore, we examined the T-cell stimulatory capacity of DC generated by culturing CD14+ monocytes with IL-15. Results, shown in Fig. 5a,b, indicate that the IL-15-induced DC stimulated proliferative responses in a dose dependent manner from allogeneic T cells comparable to GM-CSF, IL-4 and TNF-α induced DC. Thus, IL-15 appeared to induce DC that were functionally similar to DC resulting from treatment with a combination of GM-CSF, IL-4 and TNF-α. We next evaluated antigen processing and presentation of IL-15-induced DC and induction of antigen-specific autologous T-cell responses to recombinant staphylococcal enterotoxin B vaccine (rSEB) [28]. These results shown in Fig. 5c indicate that in a dose dependent manner DC derived by treatment either with IL-15 or combination of GM-CSF, IL-4 and TNF-α stimulated equivalent levels of T-cell (naïve and memory) responses to a recombinant staphylococcal enterotoxin B vaccine (rSEB). In addition, we observed that both IL-15 and GM-CSF, IL-4, and TNF-α-derived DC stimulated equivalent levels of T-cell responses against tetanus toxoid (data not shown). These data suggest that IL-15-induced DC expressed appropriate phenotypic characteristics of mature DC such as CD83 expression and stimulated antigen-specific immune responses from autologous T cells. In addition to activating antigen-specific T-cell responses, IL-15-induced mature DC produced significant amount of cytokines IFN-γ and IL-12 (manuscript in preparation).
Fig. 5.
IL-15-induced DC stimulate a strong T-cell response. Allogeneic T cells (2 × 105) from two donors (a) and (b) were cultured separately for 6 d in a 96-well microtiter plate with mature DC (2 × 102−2 × 105 γ-irradiated cells/well) that were generated either by treating with GM-CSF, IL-4 and TNF-α or IL-15. T-cell proliferation was measured by incorporation of [3H]thymidine. DC without T cells, open symbols (□ and ▵); T cells and DC induced with GM-CSF, IL-4 and TNF-α, ▴; T cells and DC induced with IL-15, ▪. Data were expressed as means of triplicate determinations ± s.e.m. Results are representative of six experiments). Statistical analysis in (a) and (b) shows strong dose effect in each group (P < 0·0001 and P < 0·0004), respectively, but pair wise comparison of two sets at each dose shows no significance differences between IL-15 and GM-CSF, IL-4 and TNF-α induced DC. (c). IL-15-induced DC processed and presented recombinant staphylococcal enterotoxin vaccine (rSEB vaccine) and stimulated vaccine-specific T-cell response. CD14+ monocytes (4 × 105) were cultured with IL-15 for 7 d or with a combination of GM-CSF and IL-4 for 6 d followed by TNF-α for 24 h in the presence or absence of different concentrations of rSEB vaccine (0·03 µm to 3 µm) in a 96-well microtitre plate. After 7 days of culture media were removed, and the wells were washed gently to remove residual cytokines. Cells were cultured with autologous (memory and naïve) T-cells (4 × 105). After day 6 of culture, cells were pulsed with [3H]thymidine for 12 h and proliferation was measured by incorporation of [3H]thymidine. Combination treatment with GM-CSF, IL-4 and TNF-α, ▴; IL-15 treatment, ▪; without cytokine treatment (control), U25CF. Data were expressed as means of triplicate determinations ± s.e.m.
Chemokine production by mature DC
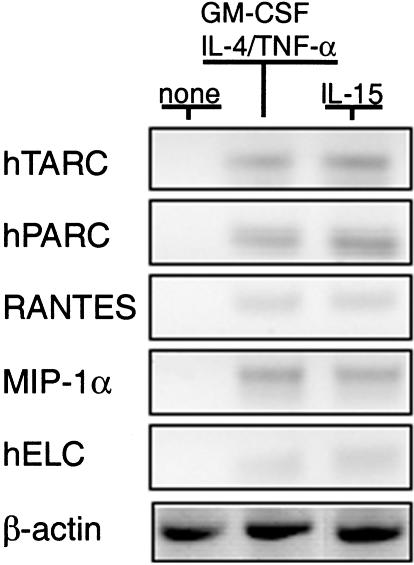
The transformation to DC is accompanied by the acquisition of chemokine receptors and increased release of chemokines. It was possible that the independent activation pathways of IL-15 and GM-CSF may have resulted in distinguishable patterns of chemokine and receptor expression. To address this issue, we examined the transcriptional activation of chemokine genes and release of chemokines into culture supernatants. The relative levels of gene transcription for pulmonary and activation regulated chemokine (PARC), thymus and activation-regulated chemokine (TARC), regulated on activation of normal T cell expressed and secreted (RANTES), macrophage inflammatory protein (MIP-1α), macrophage chemotactic protein (MCP-1) and human EBI1-ligand chemokine (hELC) (Fig. 6) were similar for IL-15 and GM-CSF activated DC. In contrast, chemokine genes of monocytes were not activated prior to culture (Fig. 6). Significantly more inflammatory chemokines, of the CC family, which includes MIP-1α, MCP-1, RANTES, were produced by IL-15-induced DC (Table 1) compared to DC induced by the combination of GM-CSF, IL-4 and TNF-α. In addition, IL-15-induced DC secreted the greatest amounts of the CXC chemokines IL-8 and MCP-1 (Table 1). The constitutive and DC-specific chemokines, such as PARC and TARC, were also up-regulated in mature DC when cultured with IL-15.
Fig. 6.
Inflammatory and constitutive chemokines production by mature DC. Transcriptional activation of chemokine genes was measured by RT-PCR and mRNA from CD14+ monocytes before culture or after incubation with either IL-15 alone for 7 d or GM-CSF and IL-4 for 6 d and 24 h stimulation with TNF-α. A total of 30 PCR cycles were used. Amplification of β-actin from the same RNA samples was used a control.
Table 1.
Comparison of chemokines secreted by mature dendritic cells
| RANTES* | MIP-1α | IL-8 | MCP-1 | |
|---|---|---|---|---|
| Media alone† | < 13·1 | < 31·2 | < 62·5 | < 31·2 |
| No cytokines‡ | 249 | 700 | 24286 | 7789 |
| GM-CSF, IL-4 and TNF-α | 575 | 993 | 8724 | 3089 |
| IL-15 | 3438 | 2000 | 25858 | 8278 |
Concentrations in pg/ml.
Negative control (without cells and cytokine).
Cells without cytokines.
Discussion
Although GM-CSF combined with IL-4 and TNF-α is commonly used in the laboratory for generating mature DC from CD14+ precursors, the physiological relevance of this activation pathway in vivo remains to be established. Our results demonstrate that IL-15 causes the transformation of CD14+ monocytes to mature DC by a pathway that is independent of GM-CSF. These IL-15-induced cells are similar to myeloid DC generated by culture of CD14+ monocytes with GM-CSF, IL-4 and TNF-α. It is possible that this cytokine-mediated pathway for DC transformation is more physiologically relevant because IL-15 is produced in response to direct tissue injury or pathogen-derived components [29,30]. Further, the IL-15 gene was shown to be transcriptionally silent in monocytes cultured with GM-CSF and IL-4, whereas type 1 interferon or LPS induced significant production of IL-15 when added to these same cultures [31]. These results suggest that alternative pathways may amplify DC activation and recruitment by release of IL-15.
It is possible that alternative activation pathways may produce different types of DC from the same monocyte precursors. Discrete populations of NK and DC were recently shown [7] to differentiate from CD34+ haematopoietic progenitor cells cultured with IL-15. It was unclear if monocytes were an intermediate in the transformation of the CD34 cells to DC. However, the GM-CSF, IL-4, TNF-α and IL-15-driven activation pathways both appeared to result in equivalent DC in our study. For example, levels of MHC and costimulatory molecule expression on mature DC were the same for both cytokine-activated pathways. Further, it is known that inflammatory chemokines such as MIP-1α, RANTES are abundantly produced by activated DC, and other chemokine genes, such as hELC, hTARC and hPARC, are activated in mature DC [32,33]. The production of chemokines by mature DC facilitates the recruitment of other mononuclear cells and granulocytes, and also directs homing of DC from inflammatory sites to the T- and B-cell areas of secondary lymphoid organs. Our results indicate that these chemokine genes are quiescent in resting monocytes, whereas, IL-15-activated monocytes produce MCP-1, IL-8, and several other cytokines. Thus, with the possible exception of chemokine production, both DC-induction pathways examined appear to produce equivalent phenotypes of DC. Previous reports suggested that IL-8 was transiently expressed during maturation of DC, following stimulation with LPS, TNF-α or CD40 ligand, whereas MCP-1 secretion is more sustained [32,34]. These chemokines stimulate chemotaxis and adhesion of PMNs, monocytes and lymphocytes [35,36]. Presently, it is not clear if a difference in expression of the chemokines IL-8 and MCP-1 between IL-15- or the GM-CSF-induced DC pathways indicate potential differences in function or if the kinetics of secretion vary between these two maturation pathways. The expression of IL-15 and IL-15Rα in virtually all tissues contrasts sharply with the highly restricted expression of IL-2 and IL-2R and suggest that IL-15 may have a broader role in stimulating immune responses [29]. IL-15 enhances antigen-specific immunity against infectious pathogens [37–39] and is a survival factor for memory T cells [25,40]. Our results suggest that IL-15 and the combination of GM-CSF, IL4 and TNF-α are independent signals for DC maturation. In addition, the release of IL-15 by mature DC [31,41–43] may serve as a positive feed-back loop for expanding populations of professional APCs. Collectively, these observations indicate that activation of DC from CD14+ monocytes in response to IL-15 may be important for amplifying primary immune responses.
Acknowledgments
We thank Helen Rager and William Kopp for cytokine analyses, Beverly Dyas for technical support, Larry K. Ostby for photomicroscopy and Paul Gibbs for statistical analyses.
REFERENCES
- 1.Steinman RM. The dendritic cell system and its role in immunogenicity. Annu Rev Immunol. 1991;9:271–6. doi: 10.1146/annurev.iy.09.040191.001415. [DOI] [PubMed] [Google Scholar]
- 2.Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245–52. doi: 10.1038/32588. 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 3.Caux C, Dezutter-Dambuyant C, Schmitt D, Banchereau J. GM-CSF and TNF-alpha cooperate in the generation of dendritic Langerhans cells. Nature. 1992;360:258–61. doi: 10.1038/360258a0. [DOI] [PubMed] [Google Scholar]
- 4.Sallusto F, Lanzavecchia A. Efficient presentation of soluble antigen by cultured human dendritic cells is maintained by granulocyte/macrophage colony-stimulating factor plus interleukin-4 and down regulated by tumor necrosis factor-α. J Exp Med. 1994;179:1109–18. doi: 10.1084/jem.179.4.1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhou LJ, Tedder TF. CD14+ blood monocytes can differentiate into functionally mature CD83+ dendritic cells. Proc Natl Acad Sci USA. 1996;93:2588–92. doi: 10.1073/pnas.93.6.2588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Saunders D, Lucas K, Ismaili J, Wu L, Maraskovasky E, Dunn A, Shortman K. Dendritic cell development in culture from thymic precursor cells in the absence of granulocyte/macrophage colony stimulating factor. J Exp Med. 1996;184:2185–96. doi: 10.1084/jem.184.6.2185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bykovskaia SN, Buffo M, Zhang H, et al. The generation of human dendritic and NK cells from hemopoietic progenitors induced by interleukin-15. J Leuk Biol. 1999;66:659–66. doi: 10.1002/jlb.66.4.659. [DOI] [PubMed] [Google Scholar]
- 8.Chapuis F, Rosenzwajg M, Yagello M, Ekman M, Biberfeld P, Gluckman JC. Differentiation of human dendritic cells from monocytes in vitro. Eur J Immunol. 1997;27:431–44. doi: 10.1002/eji.1830270213. [DOI] [PubMed] [Google Scholar]
- 9.Cella M, Sallusto F, Lanzavecchia A. Origin, maturation and antigen presenting function of dendritic cells. Curr Opin Immunol. 1997;9:10–6. doi: 10.1016/s0952-7915(97)80153-7. [DOI] [PubMed] [Google Scholar]
- 10.Grouard G, Rissoau MC, Figueria L, Durrand I, Banchereau J, Liu YJ. The enigmatic plasmacytoid T cells develop into dendritic cells with interleukin 3 (IL-3) and CD40 ligand. J Exp Med. 1997;195:1101–12. doi: 10.1084/jem.185.6.1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tagaya Y, Bamford RN, DcFilippis A, Waldmann TA. IL-15: a pleiotropic cytosine with diverse receptor/signaling pathways whose expression is controlled at multiple levels. Immunity. 1996;4:329–36. doi: 10.1016/s1074-7613(00)80246-0. [DOI] [PubMed] [Google Scholar]
- 12.Grabstein KH, Eiseman J, Shanebeck K, et al. Cloning of T cell growth factor that interacts with the β chain of the interleukin-2 receptor. Science. 1994;264:965–8. doi: 10.1126/science.8178155. [DOI] [PubMed] [Google Scholar]
- 13.Giri JG, Ahdieh M, Eisemann J, et al. Utilization of the β and γ chains of the IL-2 receptor by the novel cytokine IL-15. EMBO J. 1994;13:2822–30. doi: 10.1002/j.1460-2075.1994.tb06576.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Giri JG, Kumaki S, Ahdieh M, et al. Identification and cloning of a novel IL-15 binding protein that is structurally related to the alpha chain of the IL-2 receptor. EMBO J. 1995;14:3654–63. doi: 10.1002/j.1460-2075.1995.tb00035.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Anderson DM, Kumaki S, Ahdieh M, et al. Functional characterization of the human interleukin-15 receptor alpha chain and close linkage of IL-15Rα and IL2Rα genes. J Biol Chem. 1995;270:29862–9. doi: 10.1074/jbc.270.50.29862. [DOI] [PubMed] [Google Scholar]
- 16.Mrozek E, Anderson P, Caligiuri MA. Role of interleukin-15 in the development of human CD56+ natural killer cells from CD34+ hematopoietic progenitor cells. Blood. 1996;87:2632–40. [PubMed] [Google Scholar]
- 17.Waldmann TA, Tagaya Y. The multifaceted regulation of interleukin-15 expression and role of this cytokine in NK cell differentiation and host response to intracellular pathogens. Annu Rev Immunol. 1999;17:19–24. doi: 10.1146/annurev.immunol.17.1.19. [DOI] [PubMed] [Google Scholar]
- 18.Shreedhar V, Moodycliffe AM, Ulrich SE, Bucana C, Kripke ML, Flores-Romo L. Dendritic cells require T cells for functional maturation in vivo. Immunity. 1999;11:625–36. doi: 10.1016/s1074-7613(00)80137-5. [DOI] [PubMed] [Google Scholar]
- 19.Wong BR, Josien R, Lee SY, Sauter B, Li HL, Steinman RM, Choi Y. Trance (tumor necrosis factor [TNF]-related activation-induced cytokine), a new TNF family member predominantly expressed in T-cells, is a dendritic cell specific survival factor. J Exp Med. 1997;186:2075–80. doi: 10.1084/jem.186.12.2075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Saikh KU, Conlon K, Ulrich RG. A dendritic cell from peripheral blood exhibits a rapid increase and decrease in antigen processing that coincides with TNF-α linked apoptosis. Abstract. Keystone symposia, Santa Fe, NM, March 7–13.
- 21.Ma A, Boone DL, Lodolce JP. The pleiotropic functions of interleukin 15: not so interleukin 2-like after all. J Exp Med. 2000;191:753–5. doi: 10.1084/jem.191.5.753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fehniger TAYuH, Cooper MA, Suzuki K, Shah MH, Caligiuri MA. IL-15 co-stimulates the generalized Shwartzman reaction and innate immune IFN-γ production in vivo. J Immunol. 2000;164:1643–7. doi: 10.4049/jimmunol.164.4.1643. [DOI] [PubMed] [Google Scholar]
- 23.Agostini C, Zambello R, Facco M, et al. CD8 T-cell infiltration in extravascular tissues of patients with human immunodeficiency virus infection: Interleukin-15 up modulates co-stimulatory pathways involved in the antigen-presenting cells–T-cell interaction. Blood. 1999;93:1277–86. [PubMed] [Google Scholar]
- 24.Zhang X, Sun S, Hwang I, Tough DF, Sprent J. Potent and selective stimulation of memory-phenotype CD8+ T cells in vivo by IL-15. Immunity. 1998;8:591–9. doi: 10.1016/s1074-7613(00)80564-6. [DOI] [PubMed] [Google Scholar]
- 25.Ku CC, Murakami M, Sakamoto A, Kappler J, Marrack P. Control of homeostasis of CD8+ memory T-cells by opposing cytokines. Science. 2000;288:675–8. doi: 10.1126/science.288.5466.675. [DOI] [PubMed] [Google Scholar]
- 26.Ortaldo JR, Winkler-Pickett RT, Yagita H, Young HA. Comparative studies of CD3- and CD3+CD56+ cells: Examination of morphology, functions, T cell receptor rearrangement, and pore forming protein expression. Cell Immunol. 1991;136:486–95. doi: 10.1016/0008-8749(91)90369-m. [DOI] [PubMed] [Google Scholar]
- 27.Kita H, Ohnishi T, Okubo Y, Weiler D, Abrams JS, Gleich GJ. Granulocyte/Macrophage colony-stimulating factor and interleukin 3 release from human peripheral blood eosinophils and neutrophils. J Exp Med. 1991;174:745–8. doi: 10.1084/jem.174.3.745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ulrich RG, Olson MA, Bavari S. Development of engineered vaccines effective against structurally related bacterial superantigens. Vaccine. 1998;16:1857–64. doi: 10.1016/s0264-410x(98)00176-5. 10.1016/s0264-410x(98)00176-5. [DOI] [PubMed] [Google Scholar]
- 29.Reis e Sousa C, Sher A, Kaye P. The role of dendritic cells in the induction and regulation of immunity to microbial infection. Curr Opin Immunol. 1999;11:392–9. doi: 10.1016/S0952-7915(99)80066-1. 10.1016/s0952-7915(99)80066-1. [DOI] [PubMed] [Google Scholar]
- 30.Salio M, Cerundolo V, Lanzavecchia A. Dendritic cell maturation is induced by mycoplasma infection but not by necrotic cells. Eur J Immunol. 2000;30:705–8. doi: 10.1002/1521-4141(200002)30:2<705::AID-IMMU705>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 31.Santini SM, Lapenta C, Logozzi M, Parlato S, Spada M, Pucchio TD, Belardelli F. Type I Interferon as a powerful adjuvant for monocyte-derived dendritic cell development and activity in vitro and in Hu-PBL-SCID mice. J Exp Med. 2000;191:1777–88. doi: 10.1084/jem.191.10.1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sallusto F, Palermo B, Lenig D, et al. Distinct patterns and kinetics of chemokine production regulate dendritic cell function. Eur J Immunol. 1999;29:1671–25. doi: 10.1002/(SICI)1521-4141(199905)29:05<1617::AID-IMMU1617>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 33.Greaves DR, Wang W, Dairaghi DJ, et al. CCR6, a CC chemokine receptor that interacts with macrophage inflammatory protein 3 alpha and is highly expressed in human dendritic cells. J Exp Med. 1997;186:837–44. doi: 10.1084/jem.186.6.837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Snijders A, Kalinski P, Hilkens CMU, Kapsenberg ML. High-level IL-12 production by human dendritic cells requires two signals. Int Immunol. 1998;10:1593–8. doi: 10.1093/intimm/10.11.1593. 10.1093/intimm/10.11.1593. [DOI] [PubMed] [Google Scholar]
- 35.Carr MW, Roth SJ, Luther E, Rose SS, Springer TA. Monocyte chemoattractant protein 1 acts as a T-lymphocyte chemoattractant. Proc Natl Acad Sci USA. 1994;91:3652–6. doi: 10.1073/pnas.91.9.3652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Koch AE, Polverini PJ, Kunkel SL, et al. Interleukin-8 as a macrophage derived mediator of angiogenesis. Science. 1992;258:1798–01. doi: 10.1126/science.1281554. [DOI] [PubMed] [Google Scholar]
- 37.Khan IA, Kasper LH. IL-15 augments CD8+ T cell mediated against Toxoplasma gondii infection in mice. J Immunol. 1996;157:2103–8. [PubMed] [Google Scholar]
- 38.Nishimura H, Hiromats K, Kobayashi N, et al. IL-15 is a novel growth factor for murine gamma delta cells induced by Salmonella infection. J Immunol. 1996;156:663–9. [PubMed] [Google Scholar]
- 39.Jullien D, Sieling PA, Uyemura K, Mar ND, Rea TH, Modlin RL. IL-15, an immuno-modulator of T cell responses in intracellular infection. J Immunol. 1997;158:800–6. [PubMed] [Google Scholar]
- 40.Khan IA, Casciotti L. IL-15 prolongs the duration of CD8+ T cell-mediated immunity in mice infected with a vaccine strain of Toxoplasma gondii. J Immunol. 1999;163:4503–9. [PubMed] [Google Scholar]
- 41.Blauvelt A, Asada H, Klaus-Kovtun V, Altman D, Lucey DR, Katz SI. Interleukin-15 mRNA is expressed by human keratinocytes, Langherns cell, and blood-derived dendritic cells and is downregulated by UV-B radiation. J Invest Dermatol. 1996;106:1047–52. doi: 10.1111/1523-1747.ep12338641. [DOI] [PubMed] [Google Scholar]
- 42.Kuniyoshi JS, Kuniyoshi CJ, Lim AM, et al. Dendritic cell secretion of IL-15 is induced by recombinant huCD40LT and augments the stimulation of antigen-specific cytolytic T cells. Cell Immunol. 1999;193:48–58. doi: 10.1006/cimm.1999.1469. [DOI] [PubMed] [Google Scholar]
- 43.Jonuleit H, Weidemann K, Muller G, Degwert J, Hoppe U, Knop J, Enk AH. Induction of IL-15 messenger RNA and protein in human blood-derived dendritic cells; A role for IL-15 in attraction of T cells. J Immunol. 1997;158:2610–5. [PubMed] [Google Scholar]