Abstract
Enterovirus infections are a potential environmental trigger of the autoimmune process leading to clinical type 1 diabetes. It has been suggested that the risk of virus-induced beta-cell damage might be connected with a defect in humoral immune responsiveness to enteroviruses. In the present study we assessed whether such a defect in IgG responsiveness to coxsackievirus B4 antigen existed in young children who developed diabetes-associated autoantibodies during prospective observation from birth until the age of 18 months. IgG levels and maturation of antibody avidity were analysed in 21 children with autoantibodies and 41 control children who had experienced an equal number of enterovirus infections and were additionally matched for age, sex and HLA-DQB1 risk alleles for type 1 diabetes but had not produced diabetes-associated autoantibodies. IgG levels to coxsackievirus B4 were high in cord serum reflecting the presence of maternal antibodies. Mean IgG levels gradually decreased but began to increase after the age of 6 months, showing no significant difference between autoantibody positive and control children. The avidity of antibodies was strong in cord serum and decreased gradually during the first year of life when maternal antibodies disappeared. The avidity indices, which varied considerably from child to child, did not differ between the autoantibody-positive and -negative subjects. In conclusion, our data suggest that children affected by a beta-cell damaging autoimmune process show normal responses to coxsackievirus B4 antigens.
Keywords: antibody, avidity, enterovirus, type 1 diabetes
Introduction
Type 1 diabetes (insulin-dependent diabetes mellitus, IDDM) is caused by progressive loss of pancreatic beta cells leading to insulin deficiency and hyperglycaemia. The risk of the disease is genetically determined, but environmental factors influence the induction and progression of the beta-cell damaging autoimmune process. A number of possible environmental triggers and accelerators have been suggested, including nutritional factors and infectious diseases [1–3].
Enteroviruses, particularly certain coxsackievirus B (CBV) serotypes, have been connected to the pathogenesis of type 1 diabetes in animal and human studies [2,4]. Increased frequency of enterovirus antibodies and enterovirus RNA has been observed in the peripheral circulation of patients with newly diagnosed type 1 diabetes [5–8], and on one occasion a CBV4 strain was isolated from the pancreas of a child who died at the clinical presentation of type 1 diabetes [9]. An increased frequency of enterovirus infections has also been reported in prediabetic children several years before the onset of clinical type 1 diabetes [10–12]. In addition, maternal enterovirus infections during pregnancy have been associated with an increased risk of type 1 diabetes in the offspring [11,13,14].
Enterovirus infections are particularly severe in individuals with defects in the humoral immune system. Agammaglobulinaemic patients suffer from chronic enterovirus infections. A milder immune defect may delay the eradication of the virus and predispose to complications such as beta-cell damage. The antibody response, e.g. to mumps virus vaccinations and natural virus infections, appears to be weak in patients with type 1 diabetes [15,16]. The aim of this study was to evaluate whether any defect could be detected in the humoral immune responsiveness against enterovirus antigen in young prediabetic children who were followed from birth and began to develop diabetes-associated autoantibodies during prospective observation. We have shown previously that these children had more enterovirus infections than autoantibody-negative control subjects [10]. In the present study both enterovirus antibody levels and the maturation of the antibody avidity were analysed in autoantibody positive children and compared to those in control children who were matched for the number of enterovirus infections to find out if autoantibody-positive children have a defect in humoral immune responsiveness which could make them susceptible to enterovirus infections.
Maturation of antibody avidity is one of the primary characteristics of B-cell memory representing the strength with which a multi-valent antibody is bound to a multi-valent antigen, and aberrant avidity maturation suggests abnormalities in the regulation of immune responsiveness. Studies using various inbred mouse strains have shown that the maturation of antibody avidity is regulated genetically and may vary between different individuals [17–19]. The cellular immune system, together with cytokines and the Th1/Th2 balance, may play an important role in this regulation and, for example, interferon-gamma has been shown to augment avidity maturation [20–22]. High avidity antibodies are more effective in virus neutralization [23,24], which is important for immunity against enterovirus infections.
Materials and methods
Subjects
In this nested case–control study the case and control children were recruited from The Finnish Type 1 Diabetes Prediction and Prevention (DIPP) study, which is a large prospective trial started in 1994. All infants born at the university hospitals in the cities of Turku, Oulu and Tampere are screened after parental consent for HLA-DQB1 risk alleles for type 1 diabetes and the children with increased genetic risk, i.e. either the high-risk HLA-DQB1 *02/*0302 or the moderate-risk *0302/x genotype (x referring to alleles other than *0301 or *0602), are sequentially observed from birth. The Ethics Committees of the participating hospitals have approved the study.
The first 21 children (10 boys, 11 girls), who turned persistently positive for one or more of the autoantibodies associated with type 1 diabetes, were included in the group of cases. Clinical type 1 diabetes has been diagnosed so far in eight of these children. In the last follow-up samples 12 of the remaining case children have all developed two or more of the diabetes-associated autoantibodies: islet cell antibodies (ICA), insulin autoantibodies (IAA), glutamic acid decarboxylase antibodies (GADA) or antibodies to the IA-2 protein (IA-2 A). One child was positive for ICA only.
Case children were born between November 1994 and June 1997. Serum samples were collected at birth (cord blood) and subsequently with an interval varying from 3 to 6 months. Altogether 109 serum samples were available. Mean follow-up time was 16 (range 6–18) months. Nine cases (43%) carried the HLA-DQB1*02/*0302 genotype while the remaining 12 case children (57%) had the *0302/x genotype.
One to three control children matched for time of birth, gender, number of enterovirus infections and HLA-DQB1 genotype were chosen for each case child from the same DIPP follow-up cohort. The control children were adjusted for the number of enterovirus infections according to diagnostic increases (more than twofold) in CBV4 IgG. The control group comprised 41 children from whom altogether 192 samples were available, with a mean follow-up period of 17 (range 9–18) months. During the follow-up none of the control children has turned positive for ICA.
Enterovirus antibodies
IgG class antibodies were measured by EIA against purified coxsackievirus B4 (CBV4) as described [10]. Antibody avidity was analysed with the addition of a urea treatment, which is a modification of the urea denaturation procedure used previously to diagnose acute virus infections on the basis of low antibody avidity [25]. In this assay urea is used as a denaturating agent due to its ability to disrupt weak hydrophobic antigen–antibody bonds [26].
The wells of microtitre plates (Nunc Immunoplate, Nunc, Glostrup, Roskilde, Denmark) were coated with CBV4 antigens at a concentration of 1 µg/ml in carbonate buffer, pH 9·4. After overnight incubation at room temperature, the wells were washed with PBS and 0·005% Tween 20, blocked using 0·1% BSA in PBS and the wells were dried. Serum samples were analysed in 1/500 and 1/2000 dilutions in PBS supplemented with 1% bovine serum albumine and 0·005% Tween 20 (100 µl/well). Duplicate plates were made for all serum samples. After incubation for 60 min at 37°C, one plate was washed with PBS-Tween alone and the other plate was washed with PBS-Tween containing 6 mol/l urea and then once more with PBS-Tween. Binding of antibodies was documented using a 60-min incubation at 37°C with peroxidase-conjugated antihuman IgG (P214, Dako, Copenhagen, Denmark). Plates were then washed three times and the substrate (1,2-phenylenediamine dihydrochloride and H2O2 in citrate buffer) reaction was terminated after 30 min incubation at 37°C with 1 N H2SO4. The optical density (O.D. 492) was measured with a Victor 1420 multi-label counter (PerkinElmer Life Sciences Wallac, Turku, Finland). For each specimen, the O.D. of the urea-treated well was compared with the O.D. of the reference well. This quotient was expressed as an avidity index (AI): AI = O.D. (assay with urea wash)/O.D. (assay without urea) × 100. In the EIA analyses a twofold or greater increase in IgG antibody levels against CBV4 between two subsequent serum samples was considered to indicate an enterovirus infection.
Neutralizing antibodies were analysed against the six coxsackievirus B serotypes using a plaque neutralization assay as described [27].
Autoantibody analyses
Antibodies against islet cells (ICA), glutamic acid decarboxylase (GADA) and the protein tyrosine phosphatase-related IA-2 molecule (IA-2 A) were analysed as described previously [28,29]. Insulin autoantibody (IAA) levels were measured with a recently described microassay [30]. The detection limit of ICA was 2·5 Juvenile Diabetes Foundation units (JDFU). The cut-off limits for positivity for IAA, GADA and IA-2 A were set at the 99th percentile (1·56 RU for IAA, 5·36 RU for GADA and 0·43 RU for IA-2 A) in more than 370 non-diabetic Finnish children and adolescents. All samples resulting in an autoantibody level between the 97·5th and 99·5th percentiles were reanalysed to confirm antibody negativity or positivity.
HLA analysis
The HLA-DQB1 alleles associated with increased type 1 diabetes risk or protection were determined from the cord blood samples as described [31].
Statistical analysis
Student's paired and unpaired t-tests, when appropriate, were used to estimate the significance of differences between two populations.
Results
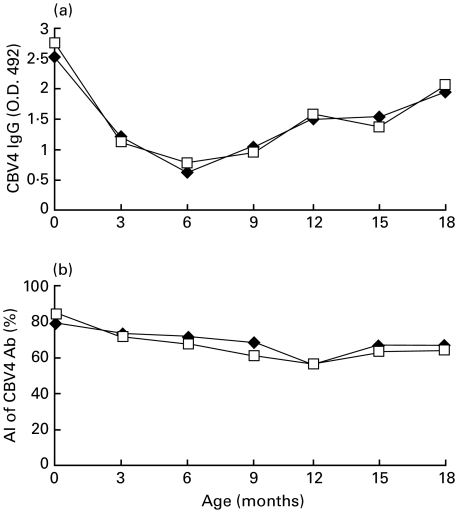
The mean IgG class antibody levels to coxsackievirus B4 (CBV4) were high in cord blood and decreased gradually during the first months of life until the concentrations started to increase after the age of 6 months. No difference was found in antibody levels between the case and control children at any age (Fig. 1a).
Fig. 1.
(a) Mean absorbance values of IgG antibodies against coxsackievirus B4 in case (♦) and control (□) children during the follow-up. (b) Mean avidity indices (AI) of IgG class antibodies against coxsackievirus B4 in case (♦) and control (□) children. The values are expressed in percentages. CBV4: coxsackievirus B4.
The average avidity index (AI) values of CBV4 IgG did not differ between case and control subjects (Fig. 1b). In cord blood the average AI values were 79% in cases and 84% in controls when analysed using 1/500 serum dilution (n.s.). AI values then gradually decreased and the lowest values were observed at the age of 12 months (55% in cases and 56% in controls, n.s.). This decrease was slightly stronger in controls than in cases but not significantly so. Subsequently, AI values remained relatively stable showing only a minor increase towards the end of the follow-up (Fig. 1b). The results of AI assays obtained using 1/2000 serum dilution were closely similar to the findings described above (data not shown).
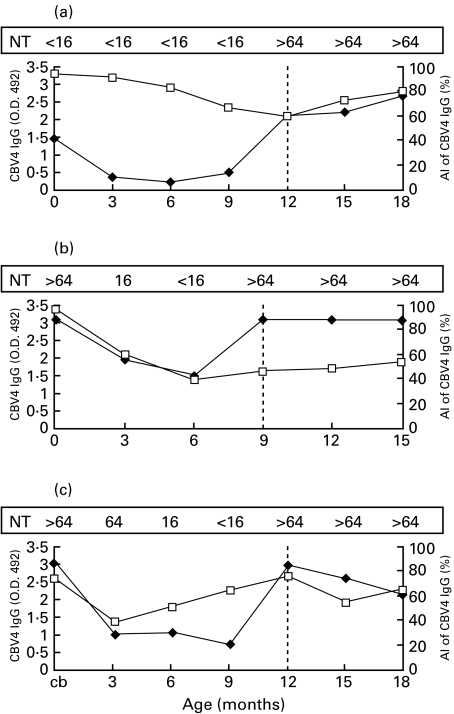
Altogether 50 infections were recognized according to significant increases in CBV4 IgG levels including 16 infections in case children and 34 infections in control children (0·8 infections per child in both groups). Avidity indices, which were analysed selectively from those samples with signs of acute enterovirus infections (significant increase in IgG levels) did not differ between case and control children (mean AI values 59% and 56%, respectively). The patterns of AI values observed in individual subjects during the follow-up varied greatly, while some children had constantly very low avidity index values, remaining low several months after confirmed enterovirus infections. Figure 2 shows AI values in three individual children whose CBV4 infection was confirmed by a significant increase in the serum neutralizing antibodies against CBV4.
Fig. 2.
Different patterns of CBV4 antibody levels (♦) and avidity values (□) in three study subjects with a confirmed CBV4 infection (a–c). The vertical dotted lines indicate the time of coxsackievirus B4 infection, i.e. the samples showing significant increase in the titre of neutralizing antibodies against CBV4. NT: The titre of antibodies against coxsackievirus B4 in the plaque neutralization test; AI: avidity index; CBV4: coxsackievirus B4.
Discussion
Protection against enterovirus infections as well as the eradication of acute infection is based mainly on a humoral immune response against the virus. This is reflected by the increased frequency of complications of enterovirus infections in patients with agammaglobulinaemia who can also be affected by chronic enterovirus infections secreting the virus for years. As enterovirus infections have been implicated in the pathogenesis of type 1 diabetes, we hypothesized that a defect in the humoral immune responsiveness against enterovirus antigens could be associated with type 1 diabetes, making these individuals more susceptible to enterovirus infections. This defect could be reflected by poor antibody responsiveness or defective maturation of antibody avidity.
The maturation of antibody avidity is one of the key characteristics of humoral immunity. Low avidity of antibodies is characteristic for the acute phase of infection while high avidity indicates past infection. The shift from low to high avidity occurs usually 100–150 days after the infection [32]. The maturation of antibody avidity is influenced by various factors which regulate the balance of the immune system. For example, Th1-type cytokines IL-2 and interferon-gamma have been suggested to induce the avidity maturation [33]. Type 1 diabetes is an autoimmune disease where a Th1-type immune response is perceived to be important [34,35]. Antibody avidity is also influenced by genetic factors which are associated with a tendency to either high or low avidity antibody responses in a given individual [17]. These genetic factors have not been identified but they may be located in the immunoglobulin gene region.
In this study the age-dependent trends of coxsackievirus IgG levels followed a pattern, which is typical for many virus antibodies. High IgG levels were observed in cord blood reflecting the presence of maternal antibodies. Antibody levels decreased rapidly after birth, reaching the lowest levels at the age of 6 months, whereafter the levels started to increase again. This is in line with our previous observations, showing that enterovirus infections are already frequent during the first year of life when they are mainly subclinical [27].
The average avidity index (AI) values decreased during the first year of life. This probably reflects gradual disappearance of high-avidity maternal antibodies from the child's circulation during the first 6 months of life. AI values subsequently remained relatively stable showing only a minor increasing trend towards the end of the follow-up.
The pattern of AI values varied considerably from child to child. In some children AI values increased after serologically confirmed enterovirus infections, as expected, while in other children AI values remained constantly at very low levels. In general the avidity of antibodies was quite low in these young infants (mean AI being 69% at the age of 6 months and 65% at the age of 18 months). This may reflect the fact that enterovirus infections are very frequent in this age group, as children acquire series of infections of varying enterovirus serotypes (over 60 serotypes altogether). The low avidity values in young infants clearly contrast with the relatively high avidity of maternal antibodies in cord blood (mean AI in cord blood 82%), suggesting that maternal antibodies represent mature immunity induced by past enterovirus infections. Another possibility is that the low avidity of enterovirus antibodies in these very young infants is due to their immature immune system.
In conclusion, humoral immune responsiveness to coxsackievirus B4 antigen, as characterized by IgG levels and avidity maturation, did not differ between autoantibody-positive and autoantibody-negative children. As the case and control children were matched for the number of enterovirus infections, the results suggest that the regulation of humoral immune responsiveness against enterovirus antigens is not disturbed in prediabetic children. However, because the case and control subjects were also matched for HLA-DQB1 risk alleles for type 1 diabetes, we can not exclude the possibility that the HLA genes conferring high risk for diabetes could be associated with abnormal humoral immune responsiveness to enterovirus antigens.
Acknowledgments
This work was supported by the Emil Aaltonen foundation, the Jalmari and Rauha Ahokas Foundation, the Finnish Medical Foundation, the Juvenile Diabetes Foundation International (grants 395019 and 197114 to H.H., grant 197032 to M.K. and grant 4–1998–274 to O.S.), the Academy of Finland, the Sigrid Juselius Foundation, the Medical Reseach Council, the Foundation for Diabetes Research in Finland and the Medical Research Funds of Tampere University Hospital, Oulu University Hospital and Turku University Central Hospital. We gratefully acknowledge the technical assistance of Eeva Jokela, Anne Karjalainen, Inkeri Lehtimäki, Anne Suominen, Sari Valorinta, Päivi Salmijärvi, Tuovi Mehtälä, Susanna Heikkilä and Riitta Päkkilä.
REFERENCES
- 1.Yoon J-W. The role of viruses and environmental factors in the induction of diabetes. Curr Top Microbiol Immunol. 1990;164:95–123. doi: 10.1007/978-3-642-75741-9_6. [DOI] [PubMed] [Google Scholar]
- 2.Yoon J-W. A new look at viruses in type 1 diabetes. Diabetes Metab Rev. 1995;11:83–107. doi: 10.1002/dmr.5610110202. [DOI] [PubMed] [Google Scholar]
- 3.Åkerblom HK, Knip M. Putative environmental factors in type 1 diabetes. Diabetes Metab Rev. 1998;14:31–67. doi: 10.1002/(sici)1099-0895(199803)14:1<31::aid-dmr201>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 4.Szopa TM, Titchener PA, Portwood ND, et al. Diabetes mellitus due to viruses − some recent developments. Diabetologia. 1993;36:687–95. doi: 10.1007/BF00401138. [DOI] [PubMed] [Google Scholar]
- 5.Clements GB, Galbraith DN, Taylor KW. Coxsackie B virus infection and onset of childhood diabetes. Lancet. 1995;346:221–3. doi: 10.1016/s0140-6736(95)91270-3. [DOI] [PubMed] [Google Scholar]
- 6.Andréoletti L, Hober D, Hober-Vandenberghe C, et al. Detection of coxsackie B virus RNA sequences in whole blood samples from adult patients at the onset of type 1 diabetes mellitus. J Med Virol. 1997;52:121–7. doi: 10.1002/(sici)1096-9071(199706)52:2<121::aid-jmv1>3.0.co;2-5. 10.1002/(sici)1096-9071(199706)52:2<121::aid-jmv1>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 7.Nairn C, Galbraith DN, Taylor KW, et al. Enterovirus variants in the serum of children at the onset of type 1 diabetes mellitus. Diabetic Med. 1999;16:509–13. doi: 10.1046/j.1464-5491.1999.00098.x. [DOI] [PubMed] [Google Scholar]
- 8.Chehadeh W, Weill J, Vantyghem M-C, et al. Increased level of interferon-α in blood of patients with insulin-dependent diabetes mellitus: relationship with coxsackievirus B infection. J Infect Dis. 2000;181:1929–39. doi: 10.1086/315516. 10.1086/315516. [DOI] [PubMed] [Google Scholar]
- 9.Yoon J-W, Austin M, Onodera T, et al. Isolation of a virus from the pancreas of a child with diabetic ketoacidosis. N Engl J Med. 1979;300:1173–9. doi: 10.1056/NEJM197905243002102. [DOI] [PubMed] [Google Scholar]
- 10.Lönnrot M, Korpela K, Knip M, et al. Enterovirus infection as a risk factor for β-cell autoimmunity in a prospectively observed birth cohort. The Finnish Diabetes Prediction and Prevention Study. Diabetes. 2000;49:1314–8. doi: 10.2337/diabetes.49.8.1314. [DOI] [PubMed] [Google Scholar]
- 11.Hyöty H, Hiltunen M, Knip M, et al. A prospective study of the role of coxsackie B and other enterovirus infections in the pathogenesis of IDDM. Diabetes. 1995;4:652–7. doi: 10.2337/diab.44.6.652. [DOI] [PubMed] [Google Scholar]
- 12.Hiltunen M, Hyöty H, Knip M, et al. Islet cell antibody seroconversion in children is temporally associated with enterovirus infections. J Infect Dis. 1997;175:554–60. doi: 10.1093/infdis/175.3.554. [DOI] [PubMed] [Google Scholar]
- 13.Dahlquist GG, Ivarsson S, Lindberg B, et al. Maternal enteroviral infection during pregnancy as a risk factor for childhood IDDM. A population-based case–control study. Diabetes. 1995;44:408–13. doi: 10.2337/diab.44.4.408. [DOI] [PubMed] [Google Scholar]
- 14.Dahlqvist G, Frisk G, Ivarsson SA, et al. Indications that maternal coxsackie B virus infection during pregnancy is a risk factor for childhood onset IDDM. Diabetologia. 1995;38:1371–3. doi: 10.1007/BF00401772. [DOI] [PubMed] [Google Scholar]
- 15.Hiltunen M, Hyöty H, Leinikki P, et al. Low mumps antibody levels induced by mumps–measles–rubella vaccinations in type 1 diabetic children. Diabetic Med. 1994;11:942–6. doi: 10.1111/j.1464-5491.1994.tb00251.x. [DOI] [PubMed] [Google Scholar]
- 16.Toniolo A, Conaldi PG, Garzelli C, et al. Role of antecedent mumps and reovirus infections on the development of type 1 (insulin-dependent) diabetes. Eur J Epidemiol. 1985;1:172–9. doi: 10.1007/BF00234091. [DOI] [PubMed] [Google Scholar]
- 17.Phillips C. Prostaglandin E2 production is enhanced in mice genetically selected to produce high affinity antibody responses. Cell Immunol. 1989;119:382–92. doi: 10.1016/0008-8749(89)90252-9. [DOI] [PubMed] [Google Scholar]
- 18.Steward MW, Petty RE. Evidence for the genetic control of antibody affinity from breeding studies with inbred mouse strains producing high and low affinity antibody. Immunology. 1976;30:789–97. [PMC free article] [PubMed] [Google Scholar]
- 19.Katz FE, Steward MW. The genetic control of antibody affinity in mice. Immunology. 1975;29:543–8. [PMC free article] [PubMed] [Google Scholar]
- 20.Holland GP, Holland N, Steward MW. Interferon-gamma potentiates antibody affinity in mice with a genetically controlled defect in affinity maturation. Clin Exp Immunol. 1990;82:221–6. doi: 10.1111/j.1365-2249.1990.tb05430.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Warren RW, Murphy S, Davie JM. Role of lymphocytes in the humoral immune response. II. T cell-mediated regulation of antibody avidity. J Immunol. 1976;116:1385–90. [PubMed] [Google Scholar]
- 22.Szewczuk MR, Sherr DH, Siskind GW. Ontogeny of B lymphocyte function. VI. Ontogeny of thymus cell capacity to facilitate the functional maturation of B lymphocytes. Eur J Immunol. 1978;8:370–3. doi: 10.1002/eji.1830080514. [DOI] [PubMed] [Google Scholar]
- 23.Steward MW, Steensgaard J. Antibody affinityThermodynamic aspects and biological significance. Boca Raton, FL: CRC Press; 1983. [Google Scholar]
- 24.Olszewska W, Obeid OE, Steward MW. Protection against measles virus-induced encephalitis by anti-mimotope antibodies: the role of antibody affinity. Virology. 2000;1:98–105. doi: 10.1006/viro.2000.0285. [DOI] [PubMed] [Google Scholar]
- 25.Hedman K, Rousseau SA. Measurement of avidity of specific IgG for verification of recent primary rubella. J Med Virol. 1989;27:288–92. doi: 10.1002/jmv.1890270406. [DOI] [PubMed] [Google Scholar]
- 26.Kamoun PP. Denaturation of globular proteins by urea: breakdown of hydrogen or hydrophobic bonds? Trends Biochem Sci. 1988;13:424–5. doi: 10.1016/0968-0004(88)90211-3. [DOI] [PubMed] [Google Scholar]
- 27.Juhela S, Hyöty H, Lönnrot M, et al. Enterovirus infections and enterovirus specific T-cell responses in infancy. J Med Virol. 1998;54:226–32. doi: 10.1002/(sici)1096-9071(199803)54:3<226::aid-jmv14>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 28.Savola K, Bonifacio E, Sabbah E, et al. IA-2 antibodies – a sensitive marker of IDDM with clinical onset in childhood and adolescence. Diabetologia. 1998;41:424–9. doi: 10.1007/s001250050925. [DOI] [PubMed] [Google Scholar]
- 29.Savola K, Sabbah E, Kulmala P, et al. Autoantibodies associated with type 1 diabetes mellitus persist after diagnosis in children. Diabetologia. 1998;41:1293–7. doi: 10.1007/s001250051067. [DOI] [PubMed] [Google Scholar]
- 30.Williams AJ, Bingley PJ, Bonifacio E, et al. A novel microassay for insulin antibodies. J Autoimmun. 1997;10:473–8. doi: 10.1006/jaut.1997.0154. 10.1006/jaut.1997.0154. [DOI] [PubMed] [Google Scholar]
- 31.Ilonen J, Reijonen H, Herva E, et al. Rapid HLA-DQB1 genotyping for four alleles in the assessment of risk for IDDM in the Finnish population: Childhood Diabetes in Finland (DiMe) Study Group. Diabetes Care. 1996;19:795–800. doi: 10.2337/diacare.19.8.795. [DOI] [PubMed] [Google Scholar]
- 32.Narita M, Matsuzono Y, Takekoshi Y, et al. Analysis of mumps vaccine failure by means of avidity testing for mumps virus-specific immunoglobulin G. Clin Diagn Lab Immunol. 1998;5:799–803. doi: 10.1128/cdli.5.6.799-803.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rizzo LV, DeKruyff RH, Umetsu DT. Generation of B cell memory and affinity maturation. Induction with Th1 and Th2 T cell clones. J Immunol. 1992;148:3733–9. [PubMed] [Google Scholar]
- 34.Kroemer G, Hirsch F, González-García A, et al. Differential involvement of Th1 and Th2 cytokines in autoimmune diseases. Autoimmunity. 1996;24:25–33. doi: 10.3109/08916939608995354. [DOI] [PubMed] [Google Scholar]
- 35.Nicholson LB, Kuchroo VK. Manipulation of the Th1/Th2 balance in autoimmune disease. Curr Opin Immunol. 1996;8:837–42. doi: 10.1016/s0952-7915(96)80013-6. [DOI] [PubMed] [Google Scholar]