Abstract
Worldwide, over 40% of children have iron deficiency anaemia, frequently associated with infections. Certain cytokines are involved in both immune activation/response to infection and iron transport/metabolism. We therefore assessed the relations among iron deficiency, cytokine production and lymphocyte activation markers in 142 hospitalized Malawian children. We examined peripheral blood lymphocyte antigens/cytokine production using four- colour flow cytometry and serum transferrin receptor (TfR) levels, an inverse measure of iron status unaffected by acute illness or infection, with an enzyme-linked immunosorbent assay. Wilcoxon rank sum tests and logistic regression analyses (LRA) were performed. Iron deficiency (TfR ≥10 μg/ml) versus TfR < 10 μg/ml, was associated with higher percentages of lymphocytes producing: (a) induced or spontaneous IL-6 (medians: induced, 15·9% for iron-deficient children versus 8·8% for iron-replete children, P = 0·002; spontaneous, 24·4% versus 13·0%, P <0·001) and (b) induced IFN-γ (medians:18·4% versus 12·4%, P = 0·006). The percentages of CD8+ T cells spontaneously producing IL-6 and of all lymphocytes producing induced TNF-α and IFN-γ in the same cell had the strongest relationships to iron deficiency (b = + 0·0211, P = 0·005 and b = +0·1158, P = 0·012, respectively, LRA) and were also positively related to the co-expression of the T cell activation markers HLA DR and CD38. Severe iron deficiency (TfR ≥30 μg/ml) was associated with the percentage of lymphocytes producing induced IL-4 (medians: 0·5% versus 1·6%, P <0·010). The cytokine patterns associated with iron deficiency in our study would preserve iron stores but also preferentially retain the activation capabilities of T cells, albeit not necessarily other immune cells, until a critical level of iron depletion is reached.
Keywords: cytokines, IL-4, IL-6, iron deficiency, lymphocyte activation, transferrin receptor
Introduction
Worldwide, an estimated 500–600 million people have iron deficiency anaemia; the problem is especially prevalent and severe in children and pregnant women in developing countries [1]. The clinical and social implications of this disorder are profound and include decreased physical strength and performance, impaired neurological function and increased infectious disease-related morbidity and mortality [2]. These effects are not necessarily counteracted by iron supplementation during acute illness and, in fact, may be worsened by supplementation during active infection with malaria parasites or iron-dependent organisms [2]. Many effects of iron deficiency on the susceptibility and response to infection occur secondarily to immune dysfunction. Reported immune defects include decreased cell-mediated immunity, mitogen responsiveness and natural killer cell activity [3–7]. Neutrophil phagocytosis and B lymphocyte function are reported to be generally intact [4,5], but lymphocyte cidal activity is decreased [3,5].
A number of parameters can be used individually or in combination to assess the status of an individual's iron stores, including serum ferritin, iron and total iron binding capacity, erythrocyte protoporphyrin, haemoglobin, haematocrit, mean red cell volume and red blood cell distribution width. Haemoglobin, haematocrit and erythrocyte protoporphyrin assessments require little blood but are functionally late indicators of iron deficiency and can be affected by intercurrent infection, inflammation, acute phase reactants or illnesses. Ferritin is the most specific indicator of depleted iron stores but plateaus at increasing levels of iron deficiency, is affected by haemolysis, and is an acute-phase reactant requiring a C-reactive protein for correct interpretation [8]. Serum iron and total iron binding capacity are not as sensitive as ferritin and have similar disadvantages. Serum transferrin receptor (CD71) is a cellular transmembrane protein that binds transferrin. It is found in highest concentrations on the surfaces of cells requiring large amounts of iron, e.g. placenta, bone marrow, liver and spleen. Soluble transferrin receptor is found in plasma after proteolytic cleavage of its extracellular component. Serum transferrin receptor levels are a sensitive indicator of iron deficiency, are not affected by haemolysis, infection, acute or chronic disease or inflammation and do not plateau with increasing levels of deficiency [9]. When iron stores are depleted, the serum transferrin receptor level is associated with the severity of iron deficiency and is unaffected by underlying acute or chronic infection or illness [10–14]. Serum transferrin receptor is not an acute-phase reactant and thus does not require assessment of C-reactive protein levels for interpretation [8,9,15].
Cytokines are important mediators of cellular immune activity, with the micro-environmental cytokine profile determining the balance between, and the intensity of, cellular and humoral immune responsiveness. Little is known concerning the effects of clinical iron deficiency on cytokines, although it has been reported that the in vitro production of interleukin (IL)-2 by lymphocytes of iron-deficient children may be impaired [10]. We examined hospitalized children's cell-specific, ex vivo and in vitro cytokine production in relation to both lymphocyte surface activation markers and serum transferrin receptor levels, to assess the effects of iron deficiency on the cytokine network.
Methods
Patients
We enrolled 149 children (< 13 years old) admitted to the Lilongwe Central Hospital, Lilongwe, Malawi, Africa from July 28 August 18 1998 into a study of the immune correlates of bloodstream infections; 142 had serum available for assessment of transferrin receptor levels. For each patient, epidemiological data and a medical history were obtained, and a physical examination was performed by one of the investigators. At admission, blood was drawn for cultures and cellular immune and intracellular cytokine testing. All children admitted to the hospital during the enrolment period were included in the study, irrespective of their temperature at admission, since infected children do not necessarily present with fever. Of the children, 54% were male, 25% were seropositive for the human immunodeficiency virus (HIV), nine had malaria parasitaemia and 18 had positive blood cultures (Salmonella spp. (n = 13) and Gram-negative rods (n = 5)). The children's mean age was 3 years and median age was 2 years. Admission temperatures ranged from 35·1°C to 41·0°C. Up to three symptoms/findings were recorded per child; these included systemic symptoms associated with negative blood cultures (n = 53), gastroenteritis (n = 30), respiratory symptoms (n = 26), suspected meningitis (n = 6), localized infection (n = 13), malnutrition (n = 9) or clinical anaemia (n = 14). Following advice from the institutional review boards, healthy children were not included in the study. The study protocol was approved by the institutional review boards of the Centers for Disease Control and Prevention (CDC) and the Malawian Health Sciences Research Committee; informed consent was obtained from all participants or their guardians.
Laboratory procedures
Serum transferrin receptor levels
Levels were assessed using an enzyme-linked immunosorbent assay with polyclonal and monoclonal antibodies against purified transferrin receptor (Ramco Laboratories, Inc., Houston, TX, USA) [14,16]. [Use of all trade names and commercial sources is for identification only and does not imply endorsement by the Public Health Service or the US Department of Health and Human Services.] The analytical detection limit for this assay is 0·07 μg/ml. The reference range has not been defined on a population level, but has been reported in various study groups as 2·9–8·3 μg/ml overall, 4·5–11·1 μg/ml for infants, and 4·7–9·2 μg/ml for 11–12-year-old males [14,16,17]. Herein, iron deficiency was defined as being present if the serum transferrin receptor level was ≥10 μg/ml and severe iron deficiency if the transferrin receptor was ≥30 μg/ml.
Whole blood cultures
These were performed by inoculating 5 ml of blood aseptically into each of two BACTEC™ MYCO/F LYTIC (MFL) bottles (Becton Dickinson Microbiology Systems, Cockeysville, MD, USA). MFL bottles were then cultured on site at 35°C. The MFL bottles were read for evidence of growth using an ultraviolet lamp (λ = 365 nm) to detect fluorescence of the indicator at the bottom of the bottle. Both MFL bottles were examined twice in the first 24 h following incubation for signs of growth, daily for the next 7 days, then weekly for 8 weeks or until growth was observed. Preliminary identification of bacteria and fungi were made onsite using standard microbiological tests. All blood culture bottles and all bacteria, fungi or mycobacteria isolates that were isolated at the study site were transported to the Clinical Microbiology Laboratory at Duke University Medical Center in the United States, where mycobacterial and fungal cultures were processed and the identities of bacterial and fungal isolates that had already been isolated in Malawi were confirmed. These culture techniques readily detect pathogenic bacteria, fungi and mycobacteria species [18].
Diagnosis of malaria
Thick and thin smears were performed at admission. A smear was considered positive if any Plasmodium falciparum asexual parasites were seen on examination of peripheral blood smears (thick films and the tails of thin films) by a single, experienced individual.
Diagnosis of HIV
HIV antibody testing was performed at study enrolment, using enzyme-linked immunosorbent assay (ELISA) test kits (Murex Diagnostics Inc., Norcross, GA, USA). HIV-2 has not been reported in Malawi. As in most developing nations, HIV-infected people in this study were not receiving antiviral therapies, nor being monitored for changes in CD4 counts or HIV viral RNA levels.
Stimulation of cytokine production
Two ml of heparinized blood were either stimulated for 5 h at 37°C with phorbol 12-myristate 13-acetate (PMA) (200 ng/ml) (Sigma Chemical Co., St Louis, MO, USA) and ionomycin (4 μg/ml) (Sigma) in the presence of brefeldin-A (40 μg/ml) (Sigma) and RPMI 1640 with l-glutamine (induced cytokine expression), or retained in identical media without PMA and ionomycin but with brefeldin-A (spontaneous cytokine expression) [18]. No serum was added to the cultures. After washing, the red blood cells were lysed with ammonium chloride solution and lymphocytes were permeabilized and fixed using Ortho Permeafix (Ortho Diagnostic Systems, Inc., Raritan, NJ, USA). After processing, samples were shipped at 4–8°C to CDC for further analysis, performed within 4 weeks of blood having been drawn. Work in our laboratory has shown that cytometric results are stable under these conditions for at least 2 months (data not shown).
Flow cytometric reagents
The surface antigens assessed in this study were those shown in our laboratory to be stable with this permeabilization/fixation protocol (data not shown), i.e. using these techniques, we had comparable results for the surface-related antigens when staining was done either pre- or postpermeabilization. Fluorescein isothiocyanate (FITC)-conjugated, phycoerythrin (PE)-conjugated, peridinin chlorophyll protein (PerCP)-conjugated or allophycocyanin (APC)-conjugated, murine monoclonal antibodies were obtained from the following sources: (a) Becton Dickinson Immunocytometry Systems/PharMingen (BD/PMG), San Jose, CA, USA (CD8-FITC and -PE [clone SK1], CD3-PerCP and -APC [clone SK7], CD4-APC [clone SK3], CD45-FITC [clone 2D1], CD19-APC [clone SJ25C1], CD14-PE [clone MφP9], CD16-PE [clone B73·1], IL-4-PE [clone 8D4-8], IL-8-PE [clone G265-8], IL-10-PE [clone JES3–9D7], HLA DR-FITC [clone L243] and CD38-PE [clone HB7], (b) Research and Diagnostics, Minneapolis, MN, USA (IL-6-PE [clone 1927·311]), (c) Immune Source, Reno, NV, USA (CD8-APC [clone KL.12], IL-2 APC [R-56·2], TNF-α-FITC [clone DTX.34] and IFN-γ-APC [clone 13.TR]) and (d) Sigma (microtubulin (MT) [clone DM1A]) custom conjugated to FITC by CalTag, South San Francisco, CA, USA. Isotype controls were obtained from BD/PMG. All antibodies used had been pretested for stability to permeabilization/fixation protocol.
Flow cytofluorometry
All staining was performed after permeabilization, fixation and shipment to CDC at room temperature for 30 min in the dark. Staining was followed by a buffered saline wash. Four-colour cytofluorometry was performed using a FACSort or FACSCalibur flow cytometer and CellQuest software (BD/PMG). Between 50 000 and 80 000 ungated events were collected from each tube in the panel. Lymphocytes were defined on the basis of forward and side scatter; monocytes, on a wide scatter gate based on forward and side scatter of stimulated and unstimulated CD14+ cells, but not on CD14 positivity per se. As is routinely performed in cytometry, specific lymphocyte types were defined using multiple parameters, e.g. CD3+ cells with a lymphocyte scatter pattern were defined as CD3+ lymphocytes. In analyses, the percentages of lymphocytes expressing CD4, HLA DR, CD38, CD45RA and CD45RO were based on data for unstimulated cells.
Analytical techniques
For each participant, analyses were performed using multiple parameters to define and assess the following cell types: all lymphocytes, CD3+ lymphocytes, CD3+ CD8+ lymphocytes, CD3+ CD8 lymphocytes, CD3+ CD16/56+ lymphocytes, CD3 CD16/56+ lymphocytes, CD19+ (B) lymphocytes and/or monocytes. We also examined the ratios of the percentage of CD3+ lymphocytes producing IL-10 to the percentage producing each of the other cytokines and similar ratios for monocytes.
Statistical techniques
Comparisons of continuous data between dichotomized categories were made using non-parametric techniques, including Wilcoxon rank sum and Kruskal–Wallis tests and logistic regression analyses. Transferrin receptor levels were dichotomized in two ways: (a) < 10 μg/ml versus ≥10 μg/ml (iron deficiency) and (b) < 30 μg/ml versus ≥30 μg/ml (severe iron deficiency). Proportions were compared using Fisher's exact or Pearson chi-square tests. Spearman's rank correlation coefficients (rs) were computed to assess correlations between immune parameters and transferrin receptor levels as a continuous variable. Linear regression analyses were used to assess relationships among activation markers (CD38 and HLA DR) on unstimulated cells, transferrin receptor levels, and specified cellular cytokines. The significance level was set at P <0·025; data not provided herein did not reach that level of significance on any type of analysis.
Results
Participant characteristics
Children with iron deficiency did not differ significantly from those without deficiency in regard to gender, age, mortality, HIV-seropositivity, blood culture-positivity or malaria smear-positivity (Table 1). For children with positive blood cultures, the type of organism did not differ between those with and without iron deficiency (data not shown). The proportion of lymphocytes expressing CD4 did not vary by the presence, absence or level of iron deficiency (Table 1); this was true for both HIV-seropositive and HIV-seronegative children (data not shown). The difference in haematocrit did not reach significance, nor was it significant for the malaria-negative or -positive children. Clinically, those with any iron deficiency (transferrin receptor levels ≥10 μg/ml) were less likely to present with a bulging fontanelle, suggestive of meningitis (P = 0·019), and more likely to have organomegaly (P = 0·007) which, as recorded, included but did not differentiate hepatomegaly and splenomegaly (Table 1). Of the seven children with serum transferrin receptor levels ≥30 μg/ml, three had symptoms suggestive of sickle cell crisis, one had Salmonella spp. bacteraemia, two had gastroenteritis and/or pneumonia and one had malaria.
Table 1.
Characteristics of children tested for serum transferrin receptor levels, by level
| Transferrin receptor level | |||
|---|---|---|---|
| Characteristica | ≤ 10 μg/ml (n = 54) | 10–≤ 30 μg/ml (n = 81) | ≥ 30 μg/ml (n = 7) |
| % Male | 49 | 53 | 86 |
| Age in years | |||
| Mean | 3·5 | 3·0 | 3·5 |
| Median | 2·8 | 1·8 | 1·0 |
| Range | 0–13 | 0–12 | 0–15 |
| % died | 8 | 5 | 0 |
| % malaria-positive | 4 | 6 | 14 |
| % blood culture-positive | 11 | 12 | 12 |
| % HIV-positive | 28 | 26 | 0 |
| % of lymphocytes expressing CD4 | |||
| Mean | 32 | 32 | 29 |
| Median | 31 | 34 | 33 |
| Range | 6–64 | 4–56 | 10–43 |
| Haematocrit (%) | |||
| Mean | 34·5 | 35·4 | 25·4 |
| Median | 33·7 | 36·4 | 29·3 |
| Range | 11·0–65·7 | 8·3–68·0 | 11·4–34·4 |
| % with organomegaly | 17 | 40 | 29 |
| % with bulging fontanelle | 4 | 1 | 0 |
Data incomplete for various individuals: gender, age, and blood culture results (n = 1 each), malaria status (n = 2), HIV status (n = 3), clinical findings (n = 8), haematocrit (n = 20), and mortality status (n = 42).
Cytokines related to iron deficiency
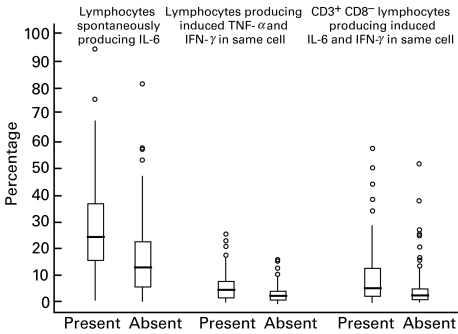
Iron deficiency was significantly related to the percentage of lymphocytes producing IFN-γ, IL-6 and IL-8 (Table 2 and Fig. 1). The median percentage of all lymphocytes, and of CD3+ lymphocytes, producing IFN-γ without stimulation was lower but the median percentage producing IFN-γ with PMA/ionomycin induction was higher in the iron-deficient children compared to the iron-replete children (Table 2). A high proportion of lymphocytes, including CD8 and CD8+ T lymphocytes, spontaneously produced IL-6, irrespective of the iron status. The median percentages of all lymphocytes, T lymphocytes and CD8 T lymphocytes spontaneously producing IL-6 and producing induced IL-6 were significantly higher in the iron-deficient compared to the non-deficient children (Table 2,Fig. 1). The median percentage of lymphocytes producing induced IL-8 was lower in the iron-deficient than the iron-replete children (Table 2). All these parameters were also significantly correlated with transferrin receptor levels (0·17| ≤ rss |≤ 0·27). In logistic regression analyses, the percentage of CD8+ T lymphocytes spontaneously producing IL-6 and the percentage of all lymphocytes producing induced TNF-α and IFN-γ in the same cell (Fig. 1) were most strongly related to iron deficiency (b = + 0·0211, P = 0·005 and b = +0·1158, P = 0·012, respectively). When both parameters were put into the same model, only the IL-6 parameter remained significant (data not shown). Further, when the IL-6 parameter was put into a regression model with transferrin receptor level as the dependent variable and the following additional independent variables, only IL-6 was significantly associated with iron deficiency: temperature, pulse, HIV serostatus, percentage of lymphocytes expressing CD4 and infection category (Salmonella bloodstream infection, Gram-negative bacteraemia, malaria or negative blood culture).
Table 2.
Lymphocyte cytokine production significantly associated with iron deficiency * by type of lymphocyte and cytokine(s)
| Iron deficiency | |||||
|---|---|---|---|---|---|
| Present | Absent | ||||
| Induced (I) or | Lymphocyte | (n = 86–88) | (n = 52–54) | Wilcoxon | |
| Cytokine(s) | spontaneous (S) | type | (median % of cells producing the cytokine[s]) | P-value | |
| IFN-γ | I | All | 18·4 | 12·4 | 0·006 |
| IFN-γ | S | All | 0·4 | 0·7 | 0·007 |
| IFN-γ | S | CD3+ | 0·5 | 0·8 | 0·008 |
| IFN-γ and TNF-α | I | All | 4·5 | 2·2 | 0·006 |
| IL-6 | I | All | 15·9 | 8·8 | 0·002 |
| IL-6 | S | All | 24·4 | 13·0 | < 0·001 |
| IL-6 | I | CD3+ | 18·2 | 10·0 | 0·009 |
| IL-6 | S | CD3+ | 23·6 | 16·3 | 0·004 |
| IL-6 | I | CD3+ 8 | 12·5 | 6·4 | 0·004 |
| IL-6 | S | CD3+ 8 | 17·7 | 8·8 | < 0·001 |
| IL-6 | S | CD3+ 8+ | 36·0 | 22·8 | 0·005 |
| IL-6 and IFN-γ | I | All | 6·3 | 3·3 | 0·002 |
| IL-6 and IFN-γ | I | CD3+ | 10·0 | 5·2 | 0·003 |
| IL-6 and IFN-γ | I | CD3+ 8 | 5·6 | 2·6 | 0·006 |
| IL-8 | I | All | 7·2 | 10·6 | 0·008 |
Serum transferrin recepter level ≥ 10 μ g/ml (iron deficiency present) versus < 10 μ g/ml (iron deficiency absent).
Fig. 1.
Cell-specific cytokines related to the presence or absence of iron deficiency. Iron deficiency was defined as being present when the serum transferrin receptor level was ≥10 μg/ml. Boxes include medians (lines) and values between the 25th and 75th percentiles. Lines extend to the furthest value within ±1·5 × the interquartile range from the 25th and 75th percentiles; outliers are presented as circles.
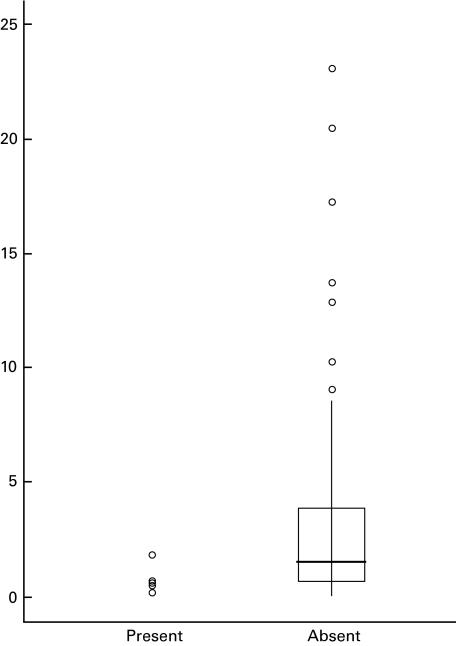
The only immune finding significantly associated with serum transferrin receptor levels ≥30 μg/ml was the percentage of lymphocytes producing induced IL-4 (medians 0·5%, n = 7 versus 1·6%, n = 132, P = 0·0097)(Fig. 2). This difference remained when the three children with symptoms suggestive of sickle crisis were removed from the analysis (P = 0·0090). We next examined the relationship between IL-4 and less severe iron deficiency. This relationship was not present throughout all transferrin receptor levels (rs = 0·002, n = 139, P = 0·980). We further examined the relationship between IL-4 and the cytokines associated with lower levels of iron deficiency, i.e. IFN-γ, TNF-α, IL-6 and IL-8. The percentage of lymphocytes producing induced IL-4 was not related to the percentage of CD8-positive cells spontaneously producing IL-6 but was correlated with the percentage of lymphocytes producing induced TNF-α and IFN-γ in the same cell (rs = 0·277, n = 138, P = 0·001).
Fig. 2.
Percentage of lymphocytes producing induced IL-4, by the presence or absence of severe iron deficiency. Severe iron deficiency was defined as being present when the serum transferrin receptor level was ≥30 μg/ml. Individual data points are provided for those with severe iron deficiency (n = 7). For the non-deficient, the box includes the median (line) and values between the 25th and 75th percentile. Lines extend to the furthest value within ±1·5 × the interquartile range from the 25th and 75th percentiles; outliers are presented as circles.
Lymphocyte activation markers (unstimulated cells)
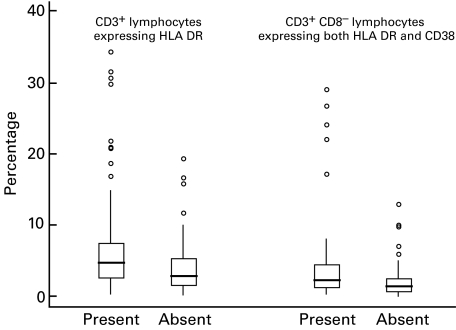
The median percentage of T cells expressing HLA DR and the median percentage of CD8 T cells expressing both HLA DR and CD38 were significantly higher in those with iron deficiency compared to those without deficiency (Fig. 3). Correlations were significant between these activation markers and transferrin receptor levels (rs = + 0·194, P = 0·021 and rs = + 0·232, P = 0·006) but were even stronger with the percentage of CD8+ cells spontaneously producing IL-6 (rs = + 0·430, P <0·001 and rs = + 0·470, P <0·001) and the percentage of lymphocytes producing induced TNF-α and IFN-γ in the same cell (rs = + 0·365, P <0·001 and rs = + 0·397, P <0·001). In linear regression analyses using log-transformed dependent variables, the two cytokine-related parameters (percentage of CD8+ cells spontaneously producing IL-6 and the percentage of lymphocytes producing induced TNF-α and IFN-γ in the same cell) were significantly related to the activation markers (P-values ≤0·001); transferrin receptor levels were not. In addition, in these analyses the percentage of CD8+ T cells co-expressing HLA DR and CD38 was significantly related to CD8+ T cells spontaneously producing IL-6 (P < 0·001) and the percentage of lymphocytes producing induced TNF-α and IFN-γ in the same cell (P = 0·024). The percentage of lymphocytes producing induced IL-4 was not related to any activation parameter, for either the entire study group or those with severe iron deficiency (data not shown). However, there were only seven individuals in the latter group.
Fig. 3.
Activation markers related to the presence or absence of iron deficiency, by cell type. Iron deficiency was defined as being present when the serum transferrin receptor level was ≥10 μg/ml. Boxes include medians (lines) and values between the 25th and 75th percentiles. Lines extend to the furthest value within ±1·5 × the interquartile range from the 25th and 75th percentiles; outliers are presented as circles.
Additional analyses
Six children were suspected of being in sickle cell crisis, based on clinical findings. For these six, no immune finding was significantly related to serum transferrin receptor levels. We next compared two groups of children: 12 children with serum transferrin receptor levels ≥10 μg/ml and haematocrits < 30% and nine children with serum transferrin receptor levels < 10 μg/ml and haematocrits ≥30%. For this analysis, children were included only if they had negative blood cultures, white blood cell counts < 15000/ml and oral temperatures < 39°C. Again, the median percentage of lymphocytes and T lymphocytes producing IL-6, IL8 and the combination of IL-6 and IFN-γ were significantly different between the two groups of children, in patterns very similar to those presented above (data not shown). The pattern in the difference in median percentage of lymphocytes producing induced TNF-α and IFN-γ in the same cell was similar to that discussed above but the difference did not reach significance (4·3% versus 2·7%). We next removed the 11 individuals with haematocrits > 50%; the results did not change in any substantive fashion. Finally, we analysed children with organomegaly (n = 42) separately from those without organomegaly (n = 100). The IL-6 and IFN-γ findings were statistically significant only for those without organomegaly, although trends consistent with the data presented above were found in both groups. The TNF-α findings were significant only in those without organomegaly but again, statistically insignificant trends in a similar direction were found in those with organomegaly (data not shown). For those without organomegaly, the percentages of lymphocytes and B lymphocytes producing IL-4 were lower in those with serum transferrin levels ≥30 μg/ml compared to those with levels < 30 μg/ml (medians: all lymphocytes, 0·4%, n = 5 versus 1·6%, n = 95, P = 0·005; B lymphocytes, 0·1%, n = 5 versus 1·1%, n = 94, P = 0·018), with similar but statistically insignificant trends found for those with organomegaly (1·2%, n = 2 versus 1·6%, n = 39 and 0·5%, n = 2 versus 0·9%, n = 40, respectively).
Discussion
In this study, we examined serum transferrin receptor levels in relation to the immune findings of hospitalized Malawian children. These levels do not increase until iron stores are exhausted and then increase in inverse proportion to the amount of tissue iron deficiency [13]. They have proved useful in the assessment of functional iron deficiency in the face of chronic disease, inflammatory disease and acute infection, including malaria [8,9,19 – 22]. We cannot be certain that the immune relationships we found were related solely to iron deficiency, rather than other, unexamined but associated nutritional deficiencies. However, these findings differed from those associated with vitamin A deficiency (manuscript in preparation), ruling against these results being due to generalized malnutrition per se rather than iron deficiency. Further, if any of these findings are related to unexamined deficiencies, the levels and effects of these additional deficiencies would have to be closely correlated with those of iron, for example, a deficiency of another mineral requiring iron for its absorption. Thus, separation of these effects in a clinical setting would be extremely difficult.
Given this caveat, we found several cytokine patterns associated with iron deficiency, all of which are consistent with data reported from various in vitro models. First and most straightforward, the percentage of lymphocytes producing induced IL-8 was lower in the iron-deficient than the iron-replete, consistent with these patients having a weaker proinflammatory response to stimulation. In one study using human lung epithelial cells, bioavailable iron induced cellular production of IL-8 [23]. Thus, our IL-8 finding could be directly related to the lower levels of bioavailable iron in iron-deficient patients' sera.
Secondly, we found chronic iron deficiency to be most strongly associated with the induced production of IL-6, of IFN-γ and of two type 1 cytokines by individual cells (IFN-γ and TNF-α). Iron deficiency was also strongly related to the spontaneous production of IL-6 by lymphocytes. The IL-6 finding was statistically strongest for CD8+ T cells, but the effects were also found in CD8 cells. Further, in logistic regression analyses including, as independent variables, the IL-6 finding, pulse rate, temperature, HIV status, the percentage of lymphocytes expressing CD4 and a parameter representing the type of acute infection, only the IL-6 parameter was related to iron deficiency.
Of note, in these acutely ill hospitalized children, the median percentages of cells producing IL-6 were consistently higher without stimulation than with stimulation; this was the case for both the iron-deficient and the iron-replete. This strongly suggests that, irrespective of iron status, the peripheral blood lymphocytes of many of these children were already stimulated in vivo. Further stimulation with PMA/ionomycin moved these in vivo-stimulated cells to apoptosis and/or death. We have not seen this in healthy individuals (data not shown). In this general milieu of in vivo stimulation, the percentage of cells spontaneously producing IFN−γ was lower, but the percentage producing IFN-γ with stimulation was higher in the iron-deficient than in the iron-replete. Therefore, although the iron-deficient children had a lower percentage of lymphocytes producing IFN-γ in vivo (i.e. spontaneously), they had a higher percentage capable of – or still capable of – producing IFN-γ with in vitro stimulation.
Surface transferrin receptor densities are highest on metabolically active cells, especially erythroid precursors and the placenta [22]. IL-6 has been shown to increase transferrin receptor density on hepatocytes and thus potentiate preferential iron transport to the liver [24,25]. IL-6 also increased ferritin synthesis and decreased transferrin synthesis when injected intraperitoneally into rats [24]. In our study, a greater proportion of lymphocytes in the peripheral blood of the iron-deficient children produced IL-6. This would be an appropriate physiological response to maximize the supply of limited amounts of iron to sites where it is most critically needed, i.e. the liver and bone marrow. Additional IL-6 effects include an increase in haematopoietic progenitors, induction of terminal B lymphocyte and macrophage differentiation, and induction of cytotoxic T cell differentiation [26,27].
Surface transferrin receptor expression is a necessary step in lymphocyte and monocyte activation, occurring after the expression of surface IL-2 receptors. In one study, TNF-α had effects similar to those of IL-6 when it was provided to a human hepatoblastoma cell line [25]; however, TNF-α decreased transferrin receptor expression on a human monocyte line [28]. In this same experiment, IFN-γ did not affect transferrin receptor expression on monocytes, but both TNF-α and IFN-γ were associated with an increase in mRNA for the H chain of ferritin and increased cell uptake of transferrin-associated iron [28]. In a trial of erythropoietin in patients with multiple organ dysfunction, both TNF-α and IFN-γ were associated with a decreased release of iron stores [29]. In experiments using a murine macrophage cell line, IFN-γ, with lipopolysaccharide induction, suppressed ferritin production and decreased the density of transferrin receptors, necessary for uptake of transferrin-associated iron [30]. While these results differ somewhat, they both suggest that the cytokine response to iron deficiency directs available iron to tissue sites, even at the expense of providing monocytes with the iron needed for their activation. Further, IL-6 in particular preferentially directs iron to hepatocytes, rather than other cells, including monocytes and macrophages. In our study, we did not evaluate cellular expression of transferrin receptors; however, we did not find any relationships between serum transferrin receptor levels and cytokine production, also a marker of activation, by peripheral blood monocytes.
Despite iron-deficient children having a cytokine pattern that would promote iron storage, we found coexpression of the T cell activation markers HLA DR and CD38 was higher in iron-deficient, compared to iron-replete, children. Further, this was statistically more strongly related to the cytokine profile, not to the iron status per se. Thus, our findings suggest that the effects of iron deficiency on lymphocytes differ from iron deficiency's effects on monocytes and macrophages. The cytokine patterns associated with iron deficiency in our study are probably directed at conserving tissue iron stores, but they appear to also permit T lymphocyte activation in the face of iron deficits. Interestingly, TNF-α and IFN-γ have been shown to act synergistically with IL-2 to generate human lymphokine-activated cytotoxic killer cells [31,32]. We found this combination of cytokines being produced in a single cell to be positively related to the percentage of T cells expressing activation markers. Thus, the cells producing this combination of cytokines with induction may potentiate T cell activation and even cytotoxic activity, despite the presence of iron deficiency.
Our third notable finding was that only one cytokine parameter was related to the most severe levels of iron deficiency: proportionately fewer lymphocytes produced IL-4 with induction. IL-4 has been shown to counteract the effects of IFN-γ on a murine macrophage cell line [30]. IFN-γ, with lipopolysaccharide induction, suppressed ferritin production and decreased the density of transferrin receptors (see above). When IL-4 was given prior to this induction, ferritin and transferrin receptor synthesis increased. In our study, with severe iron deficiency, a lower proportion of lymphocytes made induced IL-4 and no association was found between IL-4 and T-lymphocyte activation markers. This IL-4 effect, not found with milder levels of iron deficiency, may represent an additional mechanism to preferentially provide iron to tissue sites, now even at the expense of lymphocyte activation and function.
In summary, the lymphocyte-specific cytokine patterns for the iron-deficient children in our study are consistent with various cytokine findings from in vitro experiments examining cytokines and iron-related proteins under relatively controlled conditions. This consistency is remarkable, given the copresence of acute illness, active infection, ongoing HIV infection and/or chronic ailments in our patient group. The cytokine alterations associated with iron deficiency in our study would all be expected to preserve stored – especially hepatic – iron but were also be associated with T cell activation markers. Thus, preservation of iron stores is not at the cost of impairing T lymphocyte activation and function until the iron tissue deficit becomes severe.
Acknowledgments
The authors would like to acknowledge the gracious and thoughtful advice of Dr Mary Serdula, Division of Nutrition and Physical Activity, National Center for Chronic Disease Prevention and Health Promotion, CDC. Dr Joanne Mei (DLS, NCEH) supervised Roberta J. Jensen's careful application of the Ramco Laboratories transferrin receptor test kits to our samples. As is so often the case in our studies, Timothy A. Green PhD graciously assisted us with the figures and contributed clear, concise statistical advice. We would like to thank the nursing and medical staff of Lilongwe Central Hospital and the patients and their parents who so graciously and willingly cooperated in this study.
REFERENCES
- 1.DeMaeyer E, Adiels-Tegman M. The prevalence of anaemia in the world. World Health Stat Q. 1985;38:302–16. [PubMed] [Google Scholar]
- 2.Cook JD, Lunch SR. The liabilities of iron deficiency. Blood. 1986;68:803–9. [PubMed] [Google Scholar]
- 3.Chandra RK. Trace element regulation of immunity and infection. J A Coll Nutr. 1985;4:5–16. doi: 10.1080/07315724.1985.10720062. [DOI] [PubMed] [Google Scholar]
- 4.Van Heerden C, Oosthuizen R, van Wyk H, Prinsloo P, Anderson R. Evaluation of neutrophil and lymphocyte function in subjects with iron deficiency. S African Med J. 1981;59:111–3. [PubMed] [Google Scholar]
- 5.Dhur A, Galan P, Hercberg S. Iron status, immune capacity and resistance to infection. Comp Biochem Physiol A Mol Integr Physiol. 1989;94:11–9. doi: 10.1016/0300-9629(89)90776-7. [DOI] [PubMed] [Google Scholar]
- 6.Strauss RG. Iron deficiency, infections, and immune function: a reassessment. Am J Clin Nutr. 1978;31:660–6. doi: 10.1093/ajcn/31.4.660. [DOI] [PubMed] [Google Scholar]
- 7.Farthing MJ. Iron and immunity. Acta Paediatr Scand. 1989;361(Suppl.):44–52. doi: 10.1111/apa.1989.78.s361.44. [DOI] [PubMed] [Google Scholar]
- 8.Dennison HA. Limitations of ferritin as a marker of anemia in end stage renal disease. Anna J. 1999;26:409–14. [PubMed] [Google Scholar]
- 9.Feelders RA, Kuiper-Kramer EP, van Eijk HG. Structure, function and clinical significance of transferrin receptors. Clin Chem Lab Med. 1999;37:1–10. doi: 10.1515/CCLM.1999.001. [DOI] [PubMed] [Google Scholar]
- 10.Thibault H, Galan P, Selz F, et al. The immune response in iron-deficient young children: effect of iron supplementation on cell-mediated immunity. Eur J Pediatr. 1993;152:120–4. doi: 10.1007/BF02072487. [DOI] [PubMed] [Google Scholar]
- 11.Kling PJ, Roberts RA, Widness JA. Plasma transferrin receptor levels and indices of erythropoiesis and iron status in healthy term infants. J Pediatr Hematol/Oncol. 1998;20:309–14. doi: 10.1097/00043426-199807000-00005. [DOI] [PubMed] [Google Scholar]
- 12.Suominen P, Punnonen K, Rajamäki A, Irjala K. Serum transferrin receptor and transferrin receptor-ferritin index identify healthy subjects with subclinical iron deficits. Blood. 1998;92:2934–9. [PubMed] [Google Scholar]
- 13.Ahluwalia N. Diagnostic utility of serum transferrin receptors measurement in assessingiron status. Nutr Rev. 1998;56:133–41. doi: 10.1111/j.1753-4887.1998.tb01738.x. [DOI] [PubMed] [Google Scholar]
- 14.Ferguson BJ, Skikne BS, Simpson KM, Baynes RD, Cook JD. Serum transferrin receptor distinguishes the anemia of chronic disease from iron deficiency anemia. J Lab Clin Med. 1992;19:385–90. [PubMed] [Google Scholar]
- 15.Keevil B, Rowlands D, Burton I, Webb AK. Assessment of iron status in cystic fibrosis patients. Ann Clin Biochem. 2000;37:662–5. doi: 10.1258/0004563001899708. [DOI] [PubMed] [Google Scholar]
- 16.Cook JD, Skikne BS, Baynes RD. Serum transferrin receptor. Annu Rev Med. 1993;44:62–74. doi: 10.1146/annurev.me.44.020193.000431. [DOI] [PubMed] [Google Scholar]
- 17.Virtanen MA, Viinikka LU, Virtanen MK, et al. Higher concentrations of serum transferrin receptor in children than adults. Am J Clin Nutr. 1999;69:256–60. doi: 10.1093/ajcn/69.2.256. [DOI] [PubMed] [Google Scholar]
- 18.Jason J, Archibald L, McDonald C, et al. Immune determinants of organism and outcome in febrile hospitalized Thai patients with bloodstream infections. Clin Diagn Lab Immunol. 1999;6:73–8. doi: 10.1128/cdli.6.1.73-78.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mast AE, Blinder MA, Gronowski AM, Chumley C, Scott MG. Clinical utility of the soluble transferrin receptor and comparison with serum ferritin in several populations. Clin Chem. 1998;44:45–51. [PubMed] [Google Scholar]
- 20.Kuvibidila S, Mark JA, Warrier RP, Yu L, Ode D. Soluble transferrin receptor as an index of iron status in Zaïrian children with malaria. Am J Trop Med Hyg. 1995;98:373–8. [PubMed] [Google Scholar]
- 21.Kuiper-Kramer EP, Huisman CMS, van Raan J, van Eijk HG. Analytical and clinical implications of soluble transferrin receptors in serum. Eur J Clin Chem Clin Biochem. 1996;34:645–9. doi: 10.1515/cclm.1996.34.8.645. [DOI] [PubMed] [Google Scholar]
- 22.Skikne BS. Circulating transferrin receptor assay – coming of age [editorial; comment ] Clin Chem. 1998;44:7–9. [PubMed] [Google Scholar]
- 23.Smith KR, Veranth JM, Hu AA, Lighty JS, Aust AE. Interleukin-8 levels in human lung epithelial cells are increased in response to coal fly ash and vary with the bioavailability of iron, as a function of particle size and source of coal. Chem Res Toxicol. 2000;13:118–25. doi: 10.1021/tx9901736. [DOI] [PubMed] [Google Scholar]
- 24.Kobune M, Kohgo Y, Kato J, Miyazaki E, Niitsu Y. Interleukin-6 enhances hepatic transferrin uptake and ferritin expression in rats. Hepatology. 1994;19:1468–75. [PubMed] [Google Scholar]
- 25.Hirayama M, Kohgo Y, Kondo H, et al. Regulation of iron metabolism in HepG2 cells: a possible role for cytokines in the hepatic deposition of iron. Hepatology. 1993;18:874–80. doi: 10.1002/hep.1840180420. [DOI] [PubMed] [Google Scholar]
- 26.Le J, Vilèek J. Interleukin 6: a multifunctional cytokine regulating immune reactions and the acute phase protein response. Lab Invest. 1989;61:588–602. [PubMed] [Google Scholar]
- 27.Asano S, Okano A, Ozawa K, et al. In vivo effects of recombinant human interleukin-6 in primates: stimulated production of platelets. Blood. 1990;75:1602–5. [PubMed] [Google Scholar]
- 28.Fahmy M, Young SP. Modulation of iron metabolism in monocyte cell line U937 by inflammatory cytokines: changes in transferrin uptake, iron handling and ferritin mRNA. Biochem J. 1993;296:175–81. doi: 10.1042/bj2960175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gabriel A, Kozek S, Chiari A, et al. High-dose recombinant human erythropoietin stimulates reticulocyte production in patients with multiple organ dysfunction syndrome. J Trauma. 1998;44:361–7. doi: 10.1097/00005373-199802000-00023. [DOI] [PubMed] [Google Scholar]
- 30.Weiss G, Bogdan C, Hentze MW. Pathways for the regulation of macrophage iron metabolism by the anti-inflammatory cytokines IL-4 and IL-13. J Immunol. 1997;158:420–5. [PubMed] [Google Scholar]
- 31.Owen-Schaub LB, Gutterman JU, Grimm EA. Synergy of tumor necrosis factor and interleukin 2 in the activation of human cytotoxic lymphocytes: effect of tumor necrosis factor-α and interleukin 2 in the generation of human lymphokine-activated killer cell cytotoxicity. Cancer Res. 1988;48:788–92. [PubMed] [Google Scholar]
- 32.Itoh K, Shiba K, Shimizu Y, Suzuki R, Kumagai K. Generation of activated killer (AK) cells by recombinant interleukin 2 in collaboration with interferon-γ. J Immunol. 1985;134:3124–9. [PubMed] [Google Scholar]