Abstract
Polyarthritis may result from the haematogenous distribution of arthritogenic effector lymphocytes that emerge in the efferent lymph and pass through the thoracic duct (TD) to the circulation. We therefore examined whether TD cells collected from rats in the late prodrome of adjuvant-induced arthritis (AA) could transfer polyarthritis adoptively and whether these cells included a subpopulation of arthritogenic cells that could be identified phenotypically. Unfractionated TD cells collected from donor rats 9 days after adjuvant inoculation were injected intravenously into normal syngeneic recipients in numbers equivalent to the overnight harvest from a single donor. TD cell subpopulations, equivalent in number to proportions in the same inoculum, were prepared by negative selection. Unfractionated TD cells transferred polyarthritis without in vitro stimulation or conditioning of recipient animals. Abrogation of arthritogenicity by depletion of α/β TCR+ cells showed that the polyarthritis was transferred by T cells. Negatively selected CD4+ but not CD8+ TD cells transferred AA. An arthritogenic subpopulation of CD4+ T cells, enriched by either negative or positive selection, expressed the activation markers CD25 (IL-2 receptor alpha), CD71 (transferrin receptor), CD134 (OX40 antigen) and MHC class II. Cells expressing these markers were more numerous in TD lymph from arthritic rats than in lymph from normal rats and they included the majority of large CD4+ T cells. Thus, arthritogenic effector T cells bearing activation markers are released into the central efferent lymph in the late prodrome of AA. Recruitment of these arthritogenic cells to synovium probably determines the polyarticular pattern of AA.
Keywords: adoptive transfer, adjuvant arthritis, rats, CD4-positive T-lymphocytes, thoracic duct
INTRODUCTION
Activated T cells appear to be involved centrally in the pathogenesis of rheumatoid arthritis (RA) [1]. In synovium of RA patients, the majority of activated CD4+ T cells are thought to be recruited from the blood as ‘pre-activated’ cells [2–4] that may function as effector cells following their entry into the synovium [5,6]. However, the evidence that supports this mechanism is indirect. Because activated lymphocytes in the blood enter from the efferent lymph, we have examined the behaviour of thoracic duct (TD) lymphocytes from rats with adjuvant-induced arthritis (AA) after intravenous injection into syngeneic recipients. We have shown previously [7] that activated lymphocytes (‘lymphoblasts’, defined experimentally as lymphocytes in cell cycle), obtained from the TD lymph of donor rats with AA, accumulate in inflamed joints preferentially compared with lymphoblasts from normal rats. Small numbers of lymphoblasts from arthritic donors but not from normal donors, also accumulate in normal synovium. These results imply the presence of a ‘joint-finding’ population of lymphocytes in the TD of rats with AA. Since intravenous injection of TD lymphocytes mimics their physiological delivery into the venous side of the circulation, the observed recruitment of activated lymphocytes from arthritic donor rats into the synovium of normal recipient rats suggests that these cells may be significant in the pathogenesis of polyarthritis.
The pathogenic significance of activated lymphocytes is also suggested by experiments which show that AA can be transferred adoptively by Con A activated lymphocytes isolated from the spleen or draining lymph nodes of rats injected with complete Freund's adjuvant (CFA) [8], and by CD4+ T cell lines and clones cultured with the mycobacterial antigens [9,10]. However, in vitro stimulation of cells introduces uncertainty as to whether the disease-causing lymphocytes generated in vitro express the same surface molecules as their counterparts in vivo. Information about the activated state of migratory, arthritogenic lymphocytes is important because molecules with adhesive, cytokine receptor or co-stimulatory functions are likely to influence the recruitment of cells into synovial tissue, their effector function and their fate [3,4].
It is significant, therefore, that we have been able to transfer AA using a physiological dose of donor cells, equivalent to the overnight output of TD cells from a single arthritic donor rat. These cells require no deliberate stimulation in vitro to express arthritogenicity. In the only similar study [11], successful transfer of AA required an inoculum of 109 cells pooled from multiple donors and the phenotype of the disease transferring subpopulation was not characterized. The donor TD lymph contained, therefore, fully competent arthritogenic cells at the time of harvest. Because the cells required no ex vivo stimulation, we could explore for the first time the surface markers expressed by these physiologically circulating effector cells in vivo. The arthritogenic cells demonstrated by adoptive transfer of disease are contained within a subpopulation of CD4+ T cells that expresses multiple markers of activation (MHC Class II, Il-2R, CD71, CD134).
MATERIALS AND METHODS
Rats
Female inbred specific pathogen-free Dark Agouti (DA) rats were obtained from either the Animal Resource Centre (Perth, Western Australia) or the Gilles Plains Animal Resource Centre (Adelaide). Rats in each experiment were all from the same source and from the same weaning. During the experimental period they were provided with standard rat chow and water ad libitum, and housed in conventional conditions at the Veterinary Services Division, Institute of Medical and Veterinary Science, Adelaide. All animal studies were approved by the Institute of Medical and Veterinary Science Animal Ethics Committee and the University of Adelaide Animal Ethics Committee.
Induction of adjuvant arthritis
AA was induced in 7-week-old rats by subcutaneous injection of 0·1 ml of CFA at the base of the tail. The adjuvant consisted of incomplete Freund's adjuvant (Difco, MI, USA) to which was added 10 mg/ml heat-killed Mycobacterium tuberculosis H37RA (Difco). Essentially all DA rats develop polyarthritis using this protocol. Rats receiving this treatment were used as lymphocyte donors and will be referred to as ‘arthritic donors’, although early signs of polyarthritis were evident in only about half of rats at the time of TD cannulation.
Immunological reagents
The monoclonal antibodies (MoAbs) used in this study were of mouse origin. Monoclonal antibodies R73 (anti-αβ T cell receptor [12]), W3/25 (anti-CD4 [13]), OX8 (anti-CD8 [14]), OX33 (against a B cell-specific isoform of CD45 [15]), OX6 (anti-MHC Class II [16]), OX39 (CD25 (IL-2 Rα) [17]), OX26 (anti-CD71 (transferrin receptor), [18], OX40 (anti-CD134 [17]), OX41 (anti-SIRP antigen [19]) and OX62 (anti-αE2 integrin) [20] were gifts from Dr D. W. Mason and Dr A. N. Barclay, MRC Cellular Immunology Unit, Oxford, UK. The MoAb WT.5 (anti-CD11b (Mac-1 α chain) [21] was purchased from the European Collection of Cell Cultures, Centre for Applied Microbiology and Research, Salisbury, Wiltshire, UK. The MoAb MARK-1 (anti-κ light chains [22]), was produced by hybridoma cells provided by Dr H. Bazin (Université Catholique de Louvain, Brusselles, Belgium). Isotype matched control MoAbs were 1B5 (IgG1, against Giardia intestinalis), which was made in our laboratory (G.M., unpublished) and 1D4·5 (IgG2a, against Salmonella typhimurium [23]), which was a gift from Dr L. K. Ashman, Leukaemia Haemopoiesis Laboratory, Hanson Centre for Cancer Research, Adelaide, Australia. Monoclonal antibodies were used as neat culture supernatants from their respective hybridomas. Fluorescein isothiocyanate (FITC)-conjugated goat antimouse immunoglobulin (GAM-Ig-FITC) was obtained from Pharmingen, San Diego, CA, USA and used at a concentration of 10 µg/ml.
Collection of thoracic duct (TD) lymph
Cannulation of the abdominal TD was performed as described previously [24]. TD lymph was collected overnight at room temperature into flasks containing 5 ml of PBS containing 25 U/ml of preservative-free heparin. Arthritic donors were cannulated usually on day 9 after injection of CFA (the day of anticipated onset of clinical disease) but in a few experiments, TD lymph was collected from donors cannulated on day 8.
Mesenteric lymphadenectomy
The mesenteric lymph nodes were removed from 5-week-old rats by blunt dissection. These rats (MLNX rats) were allowed to recover for 6 weeks before TD cannulation to harvest lymph enriched for DC (MLNX-TDL) [25].
Enrichment of dendritic cells
Enrichment of dendritic cells (DC) from MLNX-TDL or TD lymph from arthritic rats was performed by single step density separation. The density gradient was prepared from a stock solution of Nycodenz® (Nycomed, Pharma, Oslo, Norway; 30·55 g/100 ml of water). This stock was diluted in a solution containing 0·147 m NaCl, 0·004 m KCl, 0·005 m EDTA and buffered with KH2PO4 (0·78 mm)/K2HPO4 (1·30 mm) and HEPES (7·27 mm) at pH 7·2 (EDTA-BSS) [26]. In order to obtain optimal yields of DC, the diluted Nycodenz® (Nycodenz®-EDTA-BSS) was adjusted to a density of 1·068 g/ml at 4°C (calculated gravimetrically and checked using a refractometer) and an osmolarity of 340 mOsm (measured using an Advanced™ Micro-Osmometer Model 3MO; Advanced Instruments Inc., MA, USA) [27]. Cells from MLNX-TDL or TD lymph were washed twice in RPMI + 2% FCS (RPMI-FCS) and resuspended at approximately 0·5 × 108 cells/ml in 4 ml of a 1 : 1 mixture of RPMI-FCS and Nycodenz®-EDTA-BSS. The suspension was underlaid with 8 ml of Nycodenz®-EDTA-BSS, overlaid with 2 ml of EDTA-BSS-2% FCS and centrifuged at 1912 g for 10 min at 4°C with no brake. The low density fraction was recovered in the upper 12 ml of the gradient and washed once in a total volume of 50 ml of RPMI-FCS at 544 g for 10 min at 4°C, before resuspending in RPMI-FCS for labelling with MoAb.
Adoptive transfer of TD lymphocytes
TD lymphocytes from lymph pooled from several donors were collected by centrifugation (350 g for 7 min) at room temperature and washed twice in RPMI-FCS. In some experiments, unfractionated cells were injected intravenously in 2 ml of the same medium over a period of 1 min into normal 7-week-old recipient rats. In other experiments, specific lymphocyte subsets were enriched by positive or negative selection prior to injection (see below). In most transfers, donor cells were divided equally between recipient rats, each receiving the equivalent of the overnight output of lymphocytes (or of a particular subpopulation) from a single donor.
Assessment of severity of arthritis
Recipients of adoptive transfers were observed daily under anaesthesia (halothane or isofluorane plus nitrous oxide) for the development of polyarthritis. Severity of polyarthritis was measured by attributing a score for each paw as follows: 0 (no evidence of arthritis), 1 (single focus of redness or swelling), 2 (two or more foci of redness or swelling), 3 (confluent but not global swelling) or 4 (severe global swelling) for each paw. The ‘joint score’ for each rat is the sum of the scores obtained from the four paws (maximum 16).
Depletion of specific subsets of TD lymphocytes
Depletion of specific subpopulations from TD lymphocytes was achieved by sequential incubations with mouse antirat MoAbs followed by M450 Dynabeads (Dynal, A.S., Oslo, Norway) armed with sheep antimouse immunoglobulin G (ShAM-IgG). Cells were incubated with neat culture supernatants (or mixtures) containing 0·01 m sodium azide for 40–60 min on ice and washed three times before incubation with Dynabeads (1·0–1·5 beads per cell) for 20–40 min on a rotator at 4°C. Washes and incubations with Dynabeads were performed in RPMI + 2% FCS + 0·01 m azide (RPMI-FCS-Az). After removal of cells bound to beads by three cycles of a Dynal MPC-6 magnet, the cells remaining in the supernatant were washed twice in RPMI-FCS and allowed to reach room temperature before intravenous injection. CD4+ T cells were obtained by depletion of CD8+ T cells (MoAb OX8) and B cells (MoAbs OX33 and Mark-1). For purification of CD8+ T cells, MoAb W3/25 was used to deplete CD4+ in combination with MoAbs OX33 and Mark-1 for B cells. Activated cells were removed from purified CD4+ T cells by further incubation with one or more of the MoAbs against MHC class II (OX6), CD25 (OX39), CD71 (OX26) and CD134 (OX40) and an additional round of magnetic bead depletion. Non-lymphoid cells in TD lymph were depleted using the MoAbs against αE2 integrin (OX62), SIRP (OX41) and CD11b (WT5). For control depletions, the mouse antirat MoAbs were replaced by isotype-matched mouse MoAbs of irrelevant specificity.
Positive selection of TD lymphocyte subpopulations
To select activated cells positively, CD4+ T cells (prepared as described above) were labelled with MoAbs against MHC class II, CD25 and CD71 and captured with a Dynal RAM IgG1 CELLection kit, according to the manufacturers' instructions. The beads were used at a ratio of 3–6 beads per target cell and were collected, with attached cells, by two cycles of a Dynal MPC-6 magnet. Those cells remaining in the supernatant (i.e. negatively selected) were washed in RPMI-FCS and allowed to reach room temperature prior to intravenous injection. The beads with the positively selected cells were resuspended, transferred to a fresh tube and washed using the MPC-6 magnet in RPMI-FCS-Az (two cycles) and RPMI-FCS (two cycles). After resuspension at 2·5 × 108 beads/ml, they were incubated with DNase (4 µl of a 50-U/µl solution per 1 × 107 Dynabeads) at 37°C for 15 min to cleave the DNA linker. The released beads were removed by four cycles on the MPC-6 magnet and the cells were resuspended in RPMI-FCS for intravenous injection.
Flow cytometric analysis of lymphocyte subpopulations
The cell surface phenotype and the purity of isolated subpopulations were determined by flow cytometry. Cells were incubated for 1 h on ice with mouse antirat MoAbs (approximately 1 × 106 cells per 100 µl of neat culture supernatant containing 0·01 m azide), washed twice in PBS−2% FCS−0·01 m azide (PBS-FCS-Az) and incubated with GAM-Ig-FITC (diluted in 10% normal rat serum) for a further 1 h on ice in the dark. The cells were then washed twice in PBS-FCS-Az and fixed with 1% formalin (v/v) in PBS containing 2% glucose (w/v) and 0·02% azide (w/v). Control preparations, in which the primary antibody was replaced by washing buffer or by an isotype-matched mouse MoAb of irrelevant specificity, were included in each analysis. Labelled cells were analysed using a coulter® profile ii® or a coulter® epics® xlmcl flow cytometer and system ii™ v. 3 software. Cell aggregates were excluded by gating on events with a constant peak height versus peak area ratio.
Statistical analyses
Differences in the severity of polyarthritis observed in rats receiving different treatments (different doses of cells, different preparations of cells) were tested for significance using a mixed model analysis of variance, with treatments and day after cell transfer designated as fixed effects. Random effects were incorporated into the model to explain individual variability between rats in developing and recovering from polyarthritis [28].
RESULTS
TD lymphocyte subsets in donor rats
Lymphocyte subsets in TD lymph were enumerated by flow cytometry with gates set to include all viable mononuclear cells. In arthritic donor rats, the proportions α/β TCR+ T cells (64%) and B cells (33%) together constituted 97% of TD cells, while the sum of the proportions of CD4+ (51%) and CD8+ (13%) cells together accounted numerically for all α/β TCR+ cells (n = five pools of TD lymph, 2–4 rats per pool). T lymphocytes expressing the γ/δ TCR and cells expressing CD11b (macrophages) constituted less than 2% of TD cells. Similar proportions of lymphocyte subsets were seen in TD lymph from normal rats.
TD cells transfer polyarthritis
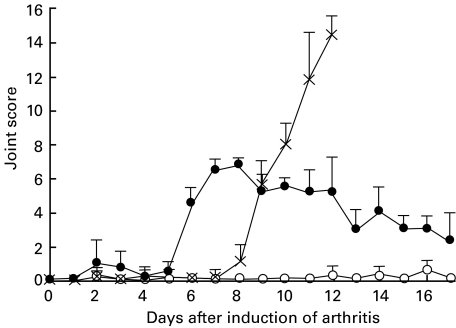
The arthritogenic activity of migratory lymphocytes was investigated by the adoptive transfer of TD lymphocytes collected from donor rats in the late prodrome of AA (‘arthritic donor rats’). Donor cells were pooled, washed and injected intravenously into normal syngeneic recipients. Each recipient received the equivalent of the average overnight output of cells (approximately 3 × 108 cells) from one donor. A representative experiment from a series of seven transfers is shown in Fig. 1, in which the time-course of adoptively transferred disease is compared with that of actively induced AA. The incidence of adoptively transferred polyarthritis was 100% (19/19), while transfers of TD lymphocytes from normal donors did not result in polyarthritis. The adoptively transferred polyarthritis followed a biphasic course with an onset, typically 2–4 days (range 1–7 days) after cell transfer, marked by foci of redness and oedema around the paw joints. This first phase was usually mild and sometimes resolved within 4–5 days after cell transfer. In the second phase, commencing at about day 6, the number of inflamed joints and the degree of oedema increased, leading usually to more extensive swelling associated with the larger joints. Most of the recipients also developed arthritis affecting one or two intervertebral joints of the tail. The inflammation in the paws reached a peak by days 8–10, following which the erythema and oedema receded, leaving induration that resolved gradually over several weeks. During the period of regression, fresh areas of inflammation appeared occasionally, but these remained focal and resolved within a few days. Beyond this period, rats which recovered from adoptively transferred polyarthritis showed no clinical or radiographic evidence of residual arthropathy (not shown).
Fig. 1.
Induction of polyarthritis in DA rats by adoptive transfer of thoracic duct (TD) lymphocytes from arthritic donors. Lymphocytes used for adoptive transfer were pooled from TD lymph collected from groups of four donor rats cannulated 9 days after subcutaneous injection with either CFA (arthritic donors) or PBS (normal donors). Normal syngeneic recipients were injected in a tail vein with the equivalent of the average overnight output (approximately 3 × 108 TD lymphocytes) of cells from one donor. These cells were drawn from either arthritic (n = 4) or normal (n = 4) donor pools. The course of adjuvant-induced arthritis (AA) in DA rats injected at day 0 with complete Freund's adjuvant (CFA) (n = 6) is shown for comparison. Rats receiving CFA were euthanased on or before day 14, in accordance with ethical agreements on disease severity. Joint scores are shown as the mean ± s.d. •, Arthritic donor thoracic duct lymphocytes; ○, normal donor thoracic duct lymphocytes; ×, adjuvant-induced arthritis.
α/β TCR negative thoracic duct cells do not transfer polyarthritis
To test whether α/β TCR− cells in TD lymph could transfer polyarthritis, α/β TCR+ cells were depleted from pooled TD cells from arthritic donors using the MoAb R73. The number of the remaining cells transferred to each normal recipient was equivalent to the output of α/β TCR− cells from the TD of a single donor rat. Transfers of 1 × 108 α/β TCR− (94–96% pure) TD cells failed to induce polyarthritis, in contrast to control cells (treated with an isotype matched MoAb of irrelevant specificity substituted for the depleting antibody) from the same donor pool (Fig. 2a). These results indicate clearly that α/β TCR+ cells are necessary for the transfer of polyarthritis. They also indicate that the other cell types in the inoculum, which are mainly B cells but may also include small numbers of γ/δ TCR+ cells, macrophages, dendritic cells and neutrophils, are not able to transfer the disease.
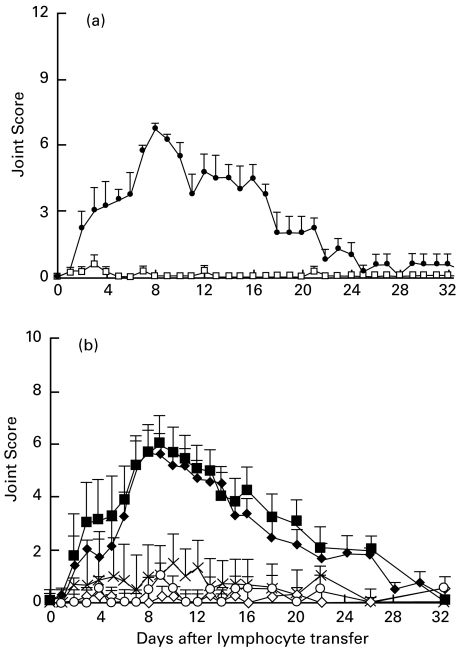
Fig. 2.
(a) Depletion of α/β TCR+ cells abrogates adoptive transfer of polyarthritis by thoracic duct (TD) cells. Pooled TD lymphocytes from arthritic donors were depleted of α/β TCR+ T cells by the use of immunomagnetic beads and a MoAb against the α/β-TCR (n = 4) prior to transfer. A separate aliquot of cells was subjected to the same procedure, except for the use of the isotype-matched control MoAb (n = 4). Joint scores (mean ± s.e.m.) represent the combined results from two separate experiments. •, Control MoAb depleted; □, α/β TCR depleted. (b) Induction of polyarthritis by adoptive transfer of CD4+ T lymphocytes. CD4+ T cells were purified by negative selection from pooled thoracic duct (TD) lymphocytes from arthritic donor rats and injected intravenously into normal syngeneic recipients at the following doses; a1 × 106 (n = 2); a5 × 106 (n = 4); a1 × 107 (n = 6); b5 × 107 (n = 8); b1 × 108 (n = 20). ♦, 1×108 CD4+ T cells; ▪, 5×107 CD4+ T cells; ×, 1×107 CD4+ T cells; ◊, 5×106 CD4+ T cells; ○, 1×106 CD4+ cells. For each group, joint scores are shown as the mean ± s.e.m. Data are combined from 18 separate experiments. Comparisons between transfers of different doses of cells were made using mixed model analysis (see Materials and methods). The course of polyarthritis was significantly different only for those doses (a, b) not bearing a common letter (P < 0·001) (as shown above).
CD4+ T cells transfer polyarthritis
To determine whether the CD4+ T cell subset contained the arthritogenic cells, CD4+ T cells prepared by negative selection from pools of TD cells from arthritic donors were transferred to normal recipients. After depletion of B cells and CD8+ cells, only 4% of the remaining cells had depleting MoAbs attached to them (Table 1,Fig. 3a). The remaining 96% of cells were therefore pristine and essentially all of them expressed CD4 and the α/β TCR (Table 1). These purified CD4+ T cells were able to transfer polyarthritis in a dose dependent manner (Fig. 2b). At the lowest doses (1 × 106 and 5 × 106 cells), single phalangeal joints showing redness without swelling were observed occasionally. Their significance is uncertain, particularly as lesions of this nature were seen occasionally in normal rats. However, doses of 1 × 107 cells induced clinically significant arthritis in five of six recipients, while transfers of 5 × 107 or 1 × 108 CD4+ T cells (i.e. less than the overnight harvest of CD4+ cells from one donor) induced arthritis in all recipients. In addition, two experiments, each using paired transfers of either purified CD4+ T cells or unseparated cells containing an equivalent number of CD4+ T cells, produced indistinguishable polyarthritis (data not shown).
Table 1.
Cell surface phenotype of CD4+ T cells selected negatively from thoracic duct cells of normal rats and of arthritic rats and of subsets prepared by depletion using monoclonal antibodies against activation markers*
| Percentage of positive cells† | ||||||
|---|---|---|---|---|---|---|
| MoAbs used to deplete CD4+ T cells obtained from arthritic donors | ||||||
| MoAbs used for analysis | Normal CD4+ T cells‡ | 1B5 (Control antibody) (n = 11)§ | OX6, OX39, OX26, OX40 (n = 3) | OX6, OX39 (n = 2) | OX39 (n = 4) | OX6 (n = 6) |
| Control MoAb | 1 | 4 ± 2 | 2 ± 2 | 1 ± 0·1 | 2 ± 1 | 2 ± 2 |
| R73 | 98 | 100 ± 1 | n.d.** | 98 ± 2 | 100 ± 1 | 100 ± 0·3 |
| W3/25 | 98 | 99 ± 1 | 99 ± 0·3 | 98 ± 2 | 99 ± 0·2 | 100 ± 0·3 |
| OX8 + OX33 + Mark-1 | 1 | 4 ± 3 | 3 ± 2 | 1 ± 0·2 | 3 ± 1 | 2 ± 2 |
| OX6 (against MHC Class II) | 3 | 15 ± 4 | 3 ± 2 | 1 ± 0·4 | 8 ± 1 | 2 ± 1 |
| OX26 (against CD71) | 4 | 14 ± 4 | 4 ± 2 | 3 ± 0·1 | 7 ± 1 | 6 ± 3 |
| OX39 (against CD25) | 6 | 17 ± 3 | 2 ± 2 | 1 ± 0 | 3 ± 1 | 9 ± 2 |
| OX40 (against CD134) | 5 | 10 ± 3 | 3 ± 3 | 3 ± 4 | 5 ± 2 | 5 ± 2 |
| OX6 + OX39 | n.d. | 20 ± 5 | n.d. | 1 ± 0·1 | n.d. | n.d. |
| OX6 + OX26 + OX39 + OX40 | 8 | 24 ± 5 | 6 ± 2 | 4 ± 1 | 10 ± 3 | 11 ± 4 |
CD4+ T cells were prepared by negative selection from thoracic duct (TD) lymph from arthritic donor rats (except in the case of column headed ‘normal’), as described in Materials and methods. Arthritic donor cells were then subjected to further depletion as indicated, using MoAbs against activation markers (either singly or in combinations). The resulting cells were analysed by flow cytometry. Specificities of MoAb used are detailed in Materials and methods.
Mean percentage positive cells (± s.d.)
CD4 + T cells were prepared by negative selection from TD lymph from two normal rats and pooled.
Not done.
Number of pools of TD lymph analysed (two rats per pool).
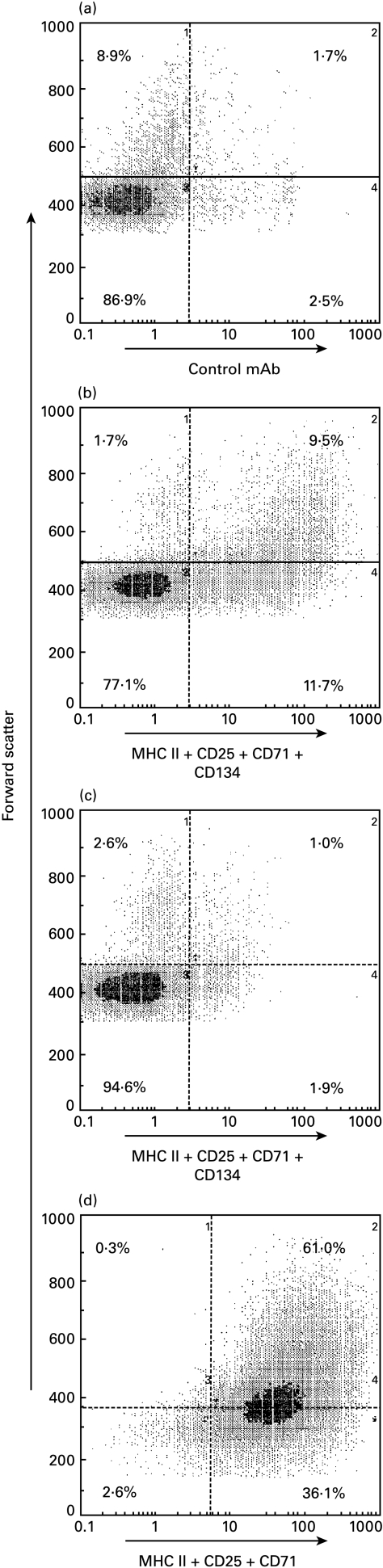
Fig. 3.
The surface antigen phenotype of purified thoracic duct (TD) CD4+ T cells and of the same cells after a secondary depletion of cells expressing the activation markers MHCII, CD25, CD71 and CD134 using immunomagnetic beads and a cocktail of the MoAbs against these markers. The cells were analysed by flow cytometry and the fluorescent intensity is shown plotted against forward light scatter, an index of cell size. TD lymphocytes pooled from four arthritic donor rats were depleted of B cells and CD8+ cells (see Materials and methods). The purified CD4+ T cells were stained with (a) negative control MoAb 1B5 or (b) a mixture of MoAbs against MHC II, CD25, CD71 and CD134. (c) Cells remaining after depletion of those cells expressing the above activation markers (see Materials and methods). The remaining cells were re-stained with the mixture of depleting antibodies. (D). Flow cytometric analysis of CD4+ T cells selected positively on the basis of their activated phenotype. CD4+ T cells were prepared by negative selection (see Materials and methods) from TD lymphocytes pooled from four arthritic donor rats. They were then stained with a mixture of MoAbs against MHCII, CD25 and CD71. After positive selection of the labelled cells using a Dynal rabbit antimouse IgG1 CELLection kit (see Materials and methods), they were re-stained with the same mixture of MoAbs.
CD8+ T cells do not transfer polyarthritis
CD8+ T cells were prepared from TD lymph by depletion of CD4+ cells and B cells. Transfers of 4 × 107 CD8+ cells to each of two recipients failed to induce polyarthritis (data not shown). This dose of CD8+ T cells is equivalent to the number of CD8+ T cells in TD lymph collected overnight from a single donor. Similar numbers of CD4+ T cells from the same pool of TD lymph induced polyarthritis in a single recipient. This dose of CD4+ T cells represents only approximately 25% of the number contained in TD lymph collected overnight from an arthritic donor.
Depletion of accessory cells does not inhibit the transfer of polyarthritis
Together, the results reported above indicated that the cells in TD lymph that were capable of transferring arthritis were probably CD4+ T cells. Although α/β TCR− TD lymphocytes were unable to transfer arthritis adoptively, it remains possible that antigen-laden accessory cells could have a synergistic effect with CD4+ T cells in the pathogenesis of the adoptively transferred disease. Macrophages or dendritic cells (DC), which express CD4 in rats [29,30], could also contaminate negatively selected CD4+ T cells prepared by selective removal of B cells and CD8+ T cells. An attempt was made therefore to deplete non-lymphoid cells using a mixture of the MoAbs OX62 (anti-αE2 integrin, expressed on rat DC), OX41 (against SIRP antigen expressed on rat macrophages, polymorphonuclear leucocytes and a subset of DC) and WT5 (antirat CD11b) [31]. Cells from the pseudoafferent lymph of mesenteric lymphadenectomized (MLNX) rats, which contains large numbers of DC [25,32], were treated in parallel and the efficacy of the depletion was assessed by flow cytometry, comparing aliquots (1 × 108 cells) of undepleted and depleted cells that were enriched for low-density cells (presumed DC, 20, 31]. Depletion with the above mixture of MoAbs reduced the proportion of large OX62+, OX41+, WT5+ cells present in the low density fraction of pseudo-afferent lymph cells from approximately 17% to 2%. Cells expressing these markers in the low density fraction of TD cells from the arthritic donors were difficult to measure accurately by flow cytometry (∼1%). Cells with DC morphology were observed in the low density fraction of non-depleted arthritic donor TD lymph by microscopy at a frequency of approximately 0·6%. These cells were not detected after depletion with the antibody cocktail. Adoptive transfer of the depleted preparations (1·5 × 108 cells in each of two recipient rats) exhibited unimpaired ability to transfer arthritis, compared to two recipients of the same dose of control TD lymphocytes from the same arthritic donor pool that had been exposed to the isotype-matched control MoAb and immunomagnetic bead treatment (mean maximum joint scores were 11 ± 2 for both recipients of both control and depleted cells).
Depletion of activated CD4+ T cells abrogates adoptive transfer of arthritis
The presence in the TD lymph from arthritic rats of CD4+ T cells that are competent to transfer polyarthritis provides an opportunity to define the state of activation of these cells in vivo. In the rat, markers which define activated subpopulations of CD4+ T cells include MHC Class II [33,34], CD25 [17], CD134 [17] and CD71 [18]. MoAbs against each of these markers stained 10–17% of CD4+ T cells from the TD lymph of arthritic rats when used singly and approximately 24% when used as a mixture, implying that there is co-expression of these markers on many of the cells (Table 1,Fig. 3b). The stained population included the majority of large CD4+ T lymphocytes (cells with high forward scatter relative to the majority of lymphocytes) (Fig. 3b). The population of CD4+ T cells staining for activation markers in arthritic rats was approximately twofold (CD134) to fivefold (MHC Class II) larger than in normal rats (Table 1).
In order to determine whether the arthritogenic cells belonged to the activated CD4+ T cell population, CD4+ T cells were prepared from pooled arthritic donor TD lymph by depletion of B cells and CD8+ cells and then subjected to a second depletion using a mixture of the MoAbs against CD25, CD71, CD134 and MHC Class II molecules. The depletion removed all cells that stained brightly with these MoAbs (including large lymphocytes) leaving approximately 6% or less of weakly staining cells (Table 1,Fig. 3c). In three separate experiments, in which each recipient received the equivalent of the purified cells from a single donor, transfers of CD4+ T cells which had been depleted of cells bearing activation markers showed only minimal capacity to induce polyarthritis, when compared with CD4+ T cells from the same donor pool treated with the isotype-matched control MoAb (Fig. 4a).
Fig. 4.
Depletion of an activated population abrogates adoptive transfer of polyarthritis by thoracic duct (TD) CD4+ T cells. Purified CD4+ T lymphocytes were prepared from pools of TD lymph from arthritic donor rats by negative selection. They were further depleted of cells expressing activation markers and transferred by intravenous injection to normal syngeneic recipients. MoAbs directed against activation markers were used either singly or in combination or were replaced by the isotype-matched control MoAb (see below). Equal numbers of cells remaining after the secondary depletion with immunomagnetic beads were transferred to recipients in each experiment. The number of cells transferred represented approximately the yield obtained by each procedure from the overnight output of one donor rat and ranged from 5 × 107 to 1 × 108. (a) Transfers of CD4+ T lymphocytes remaining after depletion with a control MoAb (n = 6) or a mixture of MoAbs OX6, OX39, OX26 and OX40 against MHCII, CD25, CD71 and CD134, respectively (n = 5). Joint scores (mean ± SEM) represent the results from three separate experiments. Mixed model analysis showed the curves to be significantly different for days 5–17 (P < 0·0001). •, Control MoAb depleted; ○, anti-MHCII + anti-CD25+ anti-CD71 + anti-CD134 depleted. (b) Transfers of CD4+ T lymphocytes remaining after depletion with either control MoAb IB5(n = 19), MoAb OX39 against CD25 (n = 8), MoAb OX6 against MHC II (n = 12) or a mixture of the MoAbs against MHCII and CD25 (n = 4). •, Control MoAb depleted; ◊, anti-CD25 depleted; ×, anti-MHCII depleted; □, anti-MHCII + anti-CD25 depleted. Data are combined from 10 separate experiments. Comparisons between transfers of cells from each depletion protocol were made using mixed model analysis (see Materials and methods). The course of polyarthritis was significantly different (P < 0·0001) only for the preparations and periods not bearing a common letter (a, b, c) as designated in the following; •, depletion with negative control MoAb, adays 2–30; ◊, depletion of CD25+ cells, bdays 2–5, 20–30, cdays 6–19; ×, depletion of MHC class II+ cells, bdays 2–30; □, depletion of cells expressing CD25 and/or MHC class II,b days 2–30.
To define further which activation markers were associated with the arthritogenic subpopulation, CD4+ T cells were depleted of cells bearing activation markers by using MoAbs either singly or in combination. When cells were depleted using either MoAb OX26 (against CD71) or MoAb OX40 (against CD134), which detect antigens that are expressed at low copy number, only about half of the respective target cell populations were removed. Recipients of these cells developed arthritis of similar severity to that observed with transfers of cells depleted with the isotype-matched control MoAb (data not shown). Depletion of cells expressing MHC class II and/or CD25 (MoAb OX6 and/or MoAb OX39) was more complete (Table 1) and led to a significant reduction in the arthritis (Fig. 4b). Depletion only of cells expressing CD25 (MoAb OX39) was less effective in reducing the adoptively transferred arthritis than depletion of cells expressing MHC Class II (Fig. 4b, days 6–19, P <0·0001). This latter depletion was as effective in preventing transfer of arthritis as depletion with the mixture of MoAb OX6 and MoAb OX39 or depletion using the mixture of four MoAbs (Fig. 4a, b). These findings indicate that of the four activation markers, MHC Class II expression is associated most closely with arthritogenicity. However, depletion of either all cells expressing MHC Class II or all cells expressing CD25 reduced by approximately 50% the proportion of cells expressing each of the remaining three antigens (Table 1). These findings show that there is considerable overlap in the expression of the various activation markers and that the arthritogenic subpopulation is likely to express different combinations of these markers.
CD4+ TD lymphocytes expressing activation markers transfer polyarthritis
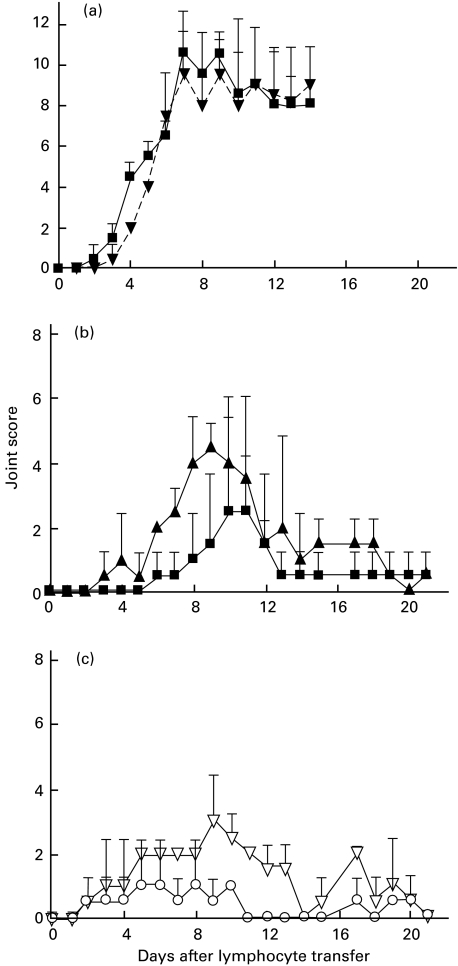
To investigate whether the activated CD4+ T lymphocytes in TD lymph from arthritic donors could transfer polyarthritis adoptively in the absence of the donor activation-marker negative CD4+ T cell population, purified CD4+ cells were prepared from pooled TD lymph from two arthritic donors and then cells were selected that expressed one or more of the markers CD25, CD71 and MHC class II molecules. The use of an antimouse IgG1 capture system (Dynal™) precluded inclusion of MoAb OX40 (IgG2a) in the mixture of selecting antibodies. The yield of positively selected cells was approximately 4% of the total CD4+ T cells, compared with the maximum theoretical yield of 22% (calculated from the proportion of cells expressing the target antigens in the purified preparation of CD4+ T cells) and the purity was approximately 97% (Fig. 3d). This activation-marker positive population was enriched for large cells. In the residual unselected cells, approximately 14% were labelled by the MoAb cocktail. Adoptive transfer of the activation-marker positive population (1 × 107 cells per rat) produced polyarthritis similar in severity to a dose of 3 × 108 cells from the residual fraction (Fig. 5a). In an additional experiment (Fig. 5b, c), significant polyarthritis was transferred by as few as 1 × 106 positively selected CD4+ T cells that expressed activation-markers, while more than 1 × 107 unselected CD4+ T cells from the same pool were required to induce disease. The adoptive transfer of polyarthritis by relatively small numbers of cells, selected positively on the basis of their activated phenotype, indicates that this population contains arthritogenic cells and that cotransfer of the majority activation-marker negative CD4+ T cell population is not necessary to induce disease.
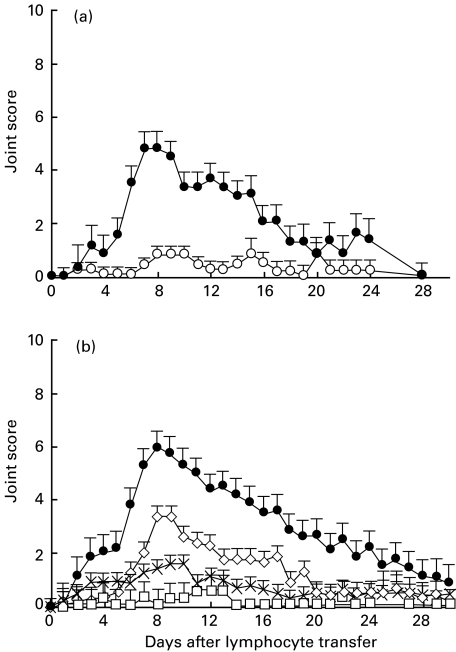
Fig. 5.
Adoptive transfer of polyarthritis by CD4+ T cells purified from TD lymph by positive selection using MoAbs against MHC II, CD25 and CD71. (a) CD4+ T cells were purified by negative selection from TD lymph, pooled from four arthritic donor rats and cells expressing one or more of the activation markers MHCII, CD25 and CD71 were selected positively using MoAbs OX6, OX39 and OX26, respectively (see Materials and methods). The positively selected (1 × 107 cells per rat, n = 2) or the residual (3 × 108 cells per rat, n = 2) fractions were transferred by intravenous injection to normal syngeneic rats. The proportions of cells expressing the activation markers in the fractions were 97% (see Fig. 3d) and 14% (not shown), respectively. Joint scores are shown as the mean ± s.d. (n = 2). ▪, 1×107 CD4+ T cells positively selected with anti-MHCII + anti-CD25 + anti-CD71; ▾, 3×108 residual CD4+ T cells. In a separate experiment (b) and (c), a comparison of potency was made between the complete CD4+ population of T cells from pooled TD lymph from arthritic donor rats and the activated subset purified from these cells by positive selection of cells expressing MHC II, CD25 and CD71. (b) Transfer of positively selected cells at doses of 1 × 106 and 1 × 107 cells per recipient. ▴, 1×107 CD4+ T cells positively selected with anti-MHCII + anti-CD25 + anti-CD71; ▪, 1×106 CD4+ T cells positively selected with anti-MHCII + anti-CD25 + anti-CD71; (c) Transfer of the CD4+ T cells (but without selection for activation marker-positive cells) at doses of 1 × 107 and 5 × 107 cells per rat. ▵, 5×107 CD4+ T cells; ○, 1×107 CD4+ T cells. Joint scores are shown as the mean ± s.d. (n = 2). Note that although the results using CD4+ T cells and the activated subset of CD4+ T cells are shown on separate panels, the cells were both derived from same donor pool.
DISCUSSION
Although AA has been transferred adoptively by cultured cells, including CD4+ T cell lines and clones [8–10], the lineage and phenotype of the arthritogenic cells that circulate in vivo has not previously been investigated. Transfer of AA without mitogenic stimulation of donor cells in vitro has allowed us to investigate the phenotype of the arthritogenic subpopulation in TD lymph. By depleting donor cell subsets selectively, we have located the arthritogenic cells within a subset of CD4+ T cells bearing the activation markers MHC Class II, CD25, CD71 and CD134. The depletion experiments indicated that MHC Class II and CD25 are expressed by most if not all of the arthritogenic cells. The relationship of CD71 and CD134 expression to arthritogenicity could not be determined directly, because cells expressing these markers were not depleted completely by their respective MoAbs. Transfer of AA by cells selected positively from the CD4+ T cell fraction of TD cells on the basis of activation marker expression (MHC Class II, CD25, CD71) confirmed that the arthritogenic cells are located in the activated subset. Relatively small numbers of this purified subset of cells can transfer AA, suggesting that the subpopulation of large, activated CD4+ T cells includes fully competent arthritogenic effector cells. The lack of arthritogenicity of α/β TCR− cells (i.e. B cells and other potential APC) indicates that antigen-laden accessory cells are not responsible for the adoptive transfer of disease by TD lymphocytes, while the undiminished arthritogenicity of TD cells depleted substantially of macrophages and DC indicated that these cells are not essential to complement the arthritogenicity of the T cells.
Up-regulation of CD25 on T cells indicates recent stimulation of these cells through the TCR [2], while the expression of CD134, which in rats is restricted essentially to activated CD4+ T cells, is also a marker of recent activation [17]. The expression of transferrin receptor is not specific for T cells, but is only present on lymphoid cells when they are in cell cycle [18]. It is noteworthy that the large lymphocytes (lymphoblasts) were predominantly activation marker-positive and that large lymphocytes comprised nearly 50% of the CD4+ T cells that expressed activation markers. In both humans [2] and rats [33], up-regulation of MHC Class II molecules on T cells occurs relatively late after activation, suggesting that MHC Class II may be a marker of functionally mature effector T cells.
The proportion of CD4+ T cells that expressed the activation markers studied was increased at least twofold in rats with AA compared to normal rats. In our previous studies [7], the increase in output of TD lymphoblasts in arthritic rats was attributed to peripherally derived lymphoblasts. After intravenous injection, TD lymphoblasts obtained from arthritic donors accumulated in inflamed joints in greater numbers compared with lymphoblasts from normal rats. Small numbers of lymphoblasts from arthritic donors, but not from normal donors, were also recruited into normal synovium. Although we have not established a direct link between the arthritogenic cells and the TD lymphoblasts that accumulate in the joints, it seems likely that the increased proportion of activated CD4+ T cells observed in the TD lymph of arthritic rats arises from the lymph nodes draining the site of CFA injection and that some of these cells enter the joints, where they initiate inflammation.
In contrast to AA where, after a longer prodrome, the initial inflammatory lesions are followed rapidly by severe disease, the onset of the adoptively transferred disease tends to be biphasic. The presence of transitory inflammation as early as 24–48 h after the injection of donor cells suggests that some cells exert their effector functions rapidly. However, the onset of the more severe and sustained phase of the disease is delayed by a further 2–3 days. It is possible that the early phase of inflammation reflects the activities of a minority of fully activated effector cells in the transferred inoculum. From our studies on recruitment into synovium [7], we suggest that these cells may be the dividing T lymphoblasts in the population. They could act as ‘pioneer’ cells [35], which prepare the way for the recruitment and/or differentiation of other cells either from the inoculum or the host. Alternatively, the delayed onset of the more severe phase may reflect a requirement that some donor T cells receive further stimulation by antigen. This stimulation might be provided in naive recipients by local APC that present processed arthritogenic autoantigens.
In conclusion, the adoptive transfer of AA by T cells isolated freshly from efferent lymph provides a physiological model for the induction and dissemination of polyarthritis by migratory T cells. These studies highlight the potential importance of activated T cells in TD lymph in the pathogenesis of inflammatory diseases and thus have generic implications beyond features specific for AA. Further studies are in progress to examine the fate and function of the arthritogenic T cells after they enter synovium and their interactions with antigen presenting cells. A better understanding of these events could provide the basis for therapies that interfere with the recruitment of arthritogenic T cells to synovium and their pathogenic actions therein.
Acknowledgments
The authors thank Ms J. Bulau for excellent technical assistance, including corroboration of joint scores and assistance with intravenous cell transfer. We are grateful to Ms K. Willson, Department of Community Medicine, Royal Adelaide Hospital, for performing the statistical analyses. We also thank Debbie Fairchild for assistance in the preparation of this manuscript. This work was supported by a project grant from the National Health and Medical Research Council of Australia (GM, LGC, 970187), a grant-in-aid from the Arthritis Foundation of Australia (GM, LGC) and grants from the Royal Adelaide Hospital Research and Special Purposes Funds (GM, LGC)
REFERENCES
- 1.Fox DA. The role of T cells in the immunopathogenesis of rheumatoid arthritis: new perspectives. Arthritis Rheum. 1997;40:598–609. doi: 10.1002/art.1780400403. [DOI] [PubMed] [Google Scholar]
- 2.Iannone F, Corrigall VM, Kingsley GH, Panayi GS. Evidence for the continuous recruitment and activation of T cells into the joints of patients with rheumatoid arthritis. Eur J Immunol. 1994;24:2706–103. doi: 10.1002/eji.1830241120. [DOI] [PubMed] [Google Scholar]
- 3.Tak PP, Hintzen RQ, Teunissen JJM, et al. Expression of the activation antigen CD27 in rheumatoid arthritis. Clin Immunol Immunopathol. 1996;80:129–38. doi: 10.1006/clin.1996.0106. [DOI] [PubMed] [Google Scholar]
- 4.Gerli R, Pitzalis C, Bistoni O, et al. CD30+ T cells in rheumatoid synovitis: mechanisms of recruitment and functional role. J Immunol. 2000;164:4399–407. doi: 10.4049/jimmunol.164.8.4399. [DOI] [PubMed] [Google Scholar]
- 5.Feldmann M. What is the mechanism of action of anti-tumor factor necrosis factor-α antibody in rheumatoid arthritis? Int Arch Allergy Immunol. 1996;111:362–5. doi: 10.1159/000237393. [DOI] [PubMed] [Google Scholar]
- 6.Maurice MM, Van der Graaf WL, Leow A, Breedveld FC, Van Lier RAW, Verweij CL. Treatment with monoclonal anti-tumor necrosis factor α antibody results in an accumulation of Th 1 CD4+ T cells win the peripheral blood of patients with rheumatoid arthritis. Arthritis Rheum. 1999;42:2166–73. doi: 10.1002/1529-0131(199910)42:10<2166::AID-ANR18>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 7.Spargo LDJ, Hawkes JS, Cleland LG, Mayrhofer G. Recruitment of lymphoblasts derived from peripheral and intestinal lymph to synovium and other tissues in normal rats and rats with adjuvant arthritis. J Immunol. 1996;157:5198–207. [PubMed] [Google Scholar]
- 8.Taurog JD, Sandberg GP, Mahowald ME. The cellular basis of adjuvant arthritis. 1. Enhancement of cell-mediated passive transfer by concanavalin A and by immuno-suppressive pretreatment of the recipients. Cell Immunol. 1983;75:271–82. doi: 10.1016/0008-8749(83)90325-8. [DOI] [PubMed] [Google Scholar]
- 9.Holoshitz A, Naparstek Y, Ben-Nun A, Cohen IR. Lines of T lymphocytes mediate or vaccinate against autoimmune arthritis. Science. 1983;219:56–8. doi: 10.1126/science.6336851. [DOI] [PubMed] [Google Scholar]
- 10.Holoshitz A, Naparstek Y, Ben-Nun A, Cohen IR. Arthritis induced in rats by cloned T lymphocytes responsive to mycobacteria but not to collagen type II. J Clin Invest. 1984;73:211–5. doi: 10.1172/JCI111193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Whitehouse DJ, Whitehouse MW, Pearson CM. Passive transfer of adjuvant-induced arthritis and allergic encephalomyelitis in rats using thoracic duct lymphocytes. Nature. 1969;224:1322. doi: 10.1038/2241322a0. [DOI] [PubMed] [Google Scholar]
- 12.Hunig T, Wallny H-J, Hartley JK, Lawetzky A, Tiefenthaler G. A monoclonal antibody to a constant determinant of the rat T cell antigen receptor that induces T cell activation. Differential reactivity with subsets of immature and mature T lymphocytes. J Exp Med. 1989;169:73–86. doi: 10.1084/jem.169.1.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.White RA, Mason DW, Williams AF, Galfre G, Milstein C. T lymphocyte heterogeneity in the rat. Separation of functional subpopulations using a monoclonal antibody. J Exp Med. 1978;148:664–73. doi: 10.1084/jem.148.3.664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brideau RJ, Carter PB, McMaster WR, Mason DW, Williams AF. Two subsets of rat T-lymphocytes defined with monoclonal antibodies. Eur J Immunol. 1980;10:609–15. doi: 10.1002/eji.1830100807. [DOI] [PubMed] [Google Scholar]
- 15.Woollet GR, Barclay AN, Puklavec M, Williams AF. Molecular and antigenic heterogeneity of the rat leukocyte-common antigen from thymocytes and T and B lymphocytes. Eur J Immunol. 1985;15:168–73. doi: 10.1002/eji.1830150211. [DOI] [PubMed] [Google Scholar]
- 16.McMaster RW, Williams AF. Identification of Ia glycoproteins in rat thymus and purification from rat spleen. Eur J Immunol. 1979;9:426–33. doi: 10.1002/eji.1830090603. [DOI] [PubMed] [Google Scholar]
- 17.Paterson DJ, Jefferies WA, Green JR, et al. Antigens of activated rat T lymphocytes including a molecule of 50,000 Mr detected only on CD4 positive T blasts. Mol Immunol. 1987;24:1281–90. doi: 10.1016/0161-5890(87)90122-2. [DOI] [PubMed] [Google Scholar]
- 18.Jefferies WA, Brandon MR, Williams AF, Hunt SV. Analysis of lymphopoietic stem cells with a monoclonal antibody to the rat transferrin receptor. Immunology. 1985;54:333–41. [PMC free article] [PubMed] [Google Scholar]
- 19.Robinson AP, White TM, Mason DW. Macrophage heterogeneity in the rats as delineated by two monoclonal antibodies MRC OX-41 and MRC OX 42 the latter recognising complement receptor type 3. Immunology. 1986;57:239–47. [PMC free article] [PubMed] [Google Scholar]
- 20.Brenan M, Puklavec M. The MRC OX-62 antigen. A useful marker in the purification of rat veiled cells with the biochemical properties of an integrin. J Exp Med. 1992;175:1457–65. doi: 10.1084/jem.175.6.1457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tamatani T, Kitamura F, Kuida K, et al. Characterization of rat LECAM-1 (l-selectin) by the use monoclonal antibodies and evidence for the presence of soluble LECAM-1 in rat sera. Eur J Immunol. 1993;23:2181–8. doi: 10.1002/eji.1830230920. [DOI] [PubMed] [Google Scholar]
- 22.Bazin H, Xhurdebise L-M, Burtonboy G, Lebacq A-M, De Clercq L, Cormont F. Rat monoclonal antibodies. I. Rapid Purification from in vitro culture supernatants. J Immunol Meth. 1984;66:261–9. doi: 10.1016/0022-1759(84)90337-5. [DOI] [PubMed] [Google Scholar]
- 23.O'Connor CG, Ashman LK. Application of the nitrocellulose transfer technique and alkaline phosphatase conjugated anti-immunoglobulin for determination of the specificity of monoclonal antibodies to protein mixtures. J Immunol Meth. 1982;54:267–71. doi: 10.1016/0022-1759(82)90068-0. [DOI] [PubMed] [Google Scholar]
- 24.Ford WL. The preparation and labelling of lymphocytes. In: Weir DM, editor. Handbook of experimental immunology. Oxford: Blackwell Scientific Publications; 1978. 23.2. [Google Scholar]
- 25.Mayrhofer G, Holt PG, Papadimitriou JM. Functional characteristics of the veiled cells in afferent lymph from the rat intestine. Immunology. 1986;58:379–87. [PMC free article] [PubMed] [Google Scholar]
- 26.Vremec D, Shortman K. Dendritic cell subtypes in mouse lymphoid organs. J Immunol. 1997;159:565–73. [PubMed] [Google Scholar]
- 27.Boyum A, Lovhaug D, Tresland L, Nordlie EM. Separation of leucocytes: improved cell purity by fine adjustments of gradient medium density and osmolality. Scand J Immunol. 1991;34:697–712. doi: 10.1111/j.1365-3083.1991.tb01594.x. [DOI] [PubMed] [Google Scholar]
- 28.Laird NM, Ware JH. Random-effects models for longitudinal data. Biometrics. 1982;38:963–74. [PubMed] [Google Scholar]
- 29.Mayrhofer G, Pugh CW, Barclay AN. Distribution, ontogeny and origin in the rat of Ia-positive cells with dendritic morphology and of Ia antigen in epithelia, with special reference to the intestine. Eur J Immunol. 1983;13:112–22. doi: 10.1002/eji.1830130206. [DOI] [PubMed] [Google Scholar]
- 30.Barclay AN. The localisation of populations of lymphocytes defined by monoclonal antibodies in rat lymphoid tissues. Immunology. 1981;42:593–600. [PMC free article] [PubMed] [Google Scholar]
- 31.Huang FP, Platt N, Wykes M, Major JR, Powell TJ, Jenkins CD, MacPherson GG. A discrete subpopulation of dendritic cells transports apoptotic intestinal epithelial cells to T cell areas of mesenteric lymph nodes. J Exp Med. 2000;191:435–43. doi: 10.1084/jem.191.3.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pugh CW, MacPherson GG, Steer HW. Characterization of nonlymphoid cells derived from rat peripheral lymph. J Exp Med. 1983;157:1758–79. doi: 10.1084/jem.157.6.1758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Reizis B, Schramm C, Cohen IR, Mor F. Expression of major histocompatibility complex class II molecules in rat T cells. Eur J Immunol. 1994;24:2796–802. doi: 10.1002/eji.1830241133. [DOI] [PubMed] [Google Scholar]
- 34.Broeren CPM, Wauben MHM, Lucassen MA, et al. Activated T cells synthesize and express functional major histocompatibility Class II antigens. Immunology. 1995;84:193–201. [PMC free article] [PubMed] [Google Scholar]
- 35.Werdelin O, McClusky RT. The nature and the specificity of mononuclear cells in experimental autoimmune inflammation and the mechanisms leading to their accumulation. J Exp Med. 1971;133:1242–63. doi: 10.1084/jem.133.6.1242. [DOI] [PMC free article] [PubMed] [Google Scholar]