Abstract
The primary aim of this work was to survey normal urothelium and transitional cell carcinoma (TCC) for the presence of T lymphocytes expressing the intraepithelial, CD103+ phenotype. This antigen defines the αEβ7-integrin. The adhesive counter-receptor for αEβ7 is E-cadherin, which is down-regulated during cancer progression. The secondary aim was to determine the pattern of distribution of CD103+ lymphocytes in relation to E-cadherin expression in bladder cancer. Cryostat sections of normal bladder and TCC were treated with antibodies specific for human CD103, CD3, CD8 and E-cadherin. Visualization was performed by immunoperoxidase or alkaline phosphatase development with light and confocal microscopy. Dual staining and serial sections were used to assess the relationship between these antigens. Four samples of normal bladder and 26 TCC samples were assessed. Occasional T lymphocytes (CD3+) were seen in normal urothelium and lamina propria. In the urothelium the majority of these T lymphocytes (71%) were also CD8+ and of these 68% expressed the CD103 marker. In the lamina propria 62% of the T lymphocytes were CD8+ and 56% of these expressed the CD103 marker. In carcinomas significantly greater numbers of CD103+ T lymphocytes were present in the surrounding stroma rather than infiltrating the carcinomas (P = 0·0006). Of those T lymphocytes infiltrating the tumours, 71% were CD8+ and of these 58% expressed CD103. In the surrounding stroma 52% of lymphocytes were CD8+ and 82% of this subset expressed CD103. Infiltration by CD103+ lymphocytes was not related to the intensity of E-cadherin expression. T lymphocytes of the CD103+ phenotype are present in normal urothelium where they may play a role in immunosurveillance. Rather than infiltrating into carcinomas, these cells predominate in the surrounding stroma which could suggest a failure of immune function.
Keywords: urothelium, bladder cancer, αEβ7, CD103, E-cadherin
INTRODUCTION
Continuity of the bladder lumen with the external environment predisposes the urothelium to infection. Other mucosal tissues, including the gut and lung, share this vulnerability but are known to host an extensive population of intraepithelial lymphocytes (IEL). These T lymphocytes which are typically CD8+CD4−, specifically express the αEβ7-integrin, which is a heterotypic adhesive counter-receptor for E-cadherin [1]. The αEβ7-integrin is defined by CD103, which is expressed by less than 2% of peripheral blood lymphocytes (PBL). However, following activation, this antigen can be induced to high levels on the majority of PBL by the presence of transforming growth factor β1 (TGFβ1) [2]. Previous studies have demonstrated the presence of TGFβ1 in both normal urothelium and TCC [3].
E-cadherin is almost exclusively expressed by epithelial cells, forming homotypic bonds between adjacent cells. It has been proposed that interaction between the αEβ7-integrin and E-cadherin serves to retain the IEL population within the epithelium. These lymphocytes are thus ideally situated to provide immune surveillance for epithelial tissues. Despite the vulnerability of the urothelium to infection, the phenotype of the lymphocytes within this tissue has not been defined.
In addition to the potential for infection, more than 90% of human cancers are epithelial in origin. It has been suggested recently that IEL might also play a role in immune surveillance for developing carcinomas [2]. Bladder cancer is one of the few solid cancers to be treated routinely with immunotherapy. Intravesical treatment of superficial TCC with bacille Calmette–Guèrin (BCG) vaccine initiates lymphocyte infiltration and reduces both disease progression and recurrence [4]. Bladder cancer therefore provides a relevant model for investigation of tumour immunobiology.
In many epithelial tumours, including TCC of the bladder, reduced expression of the cell adhesion molecule E-cadherin has been correlated with increased cancer invasion and metastasis [5–7]. Clearly, the loss of E-cadherin expression will also disrupt the potential for adhesive interaction with IEL, which may result in the failure of local, anticancer immune surveillance. It has been observed in human pancreatic cancer that IEL are predominantly distributed in the surrounding stroma [8]. The authors suggest that this lack of tumour infiltration may be due to the loss of E-cadherin expression.
Given the proposed importance of IEL for immune surveillance of epithelial tissues, the distribution of the αEβ7-integrin within normal bladder tissue was studied. As bladder cancer is uniquely sensitive to BCG-based immunotherapy, the distribution of IEL within bladder cancer specimens was also surveyed and the results were compared to the local expression of E-cadherin.
MATERIALS AND METHODS
Tissue samples
Cryostat sections were cut from snap frozen specimens of bladder tumours obtained from patients undergoing trans-urethral resection of tumour (TURT). Normal urothelium, obtained from cystectomy specimens and an organ transplant donor, were also used.
Materials
The following monoclonal antibodies were used: anti-CD103 (Dilution 1 : 25, Clone Ber-ACT8, Isotype IgG1), anti-CD3 (1 : 50, UCHT-1, IgG1), anti-CD8 (1 : 50, C8/144B, IgG1, DAKO, Ely, Camb., UK), anti-E-cadherin (1 : 10, 67A4, IgG1, Immunotech, Luton, Beds., UK), Isotype control (1 : 25 and 1 : 10, IgG1, DAKO). The secondary antibody used was biotinylated rabbit antimouse IgG (1 : 500, DAKO). Antibodies were diluted in 20% normal rabbit serum (NRS; Gibco-BRL, UK) in Tris-buffered saline (TBS; pH 7·6) Normal rabbit serum was used as a protein-rich medium to maintain optimal condition of tissue sections. Sections were washed between stages with TBS.
Immunostaining
Sections were fixed in cold acetone for 10 min and treated with 0·3% H2O2 in sodium azide to minimize endogenous peroxidase activity. Endogenous biotin was also blocked (DAKO blocking kit). Non-specific binding of immune reagents was prevented by treatment with NRS for 2 h at 4°C. Sections were incubated at 4°C with anti-CD103 overnight. The secondary antibody was applied for one hour at room temperature and the sections were treated with streptavidin–biotin–peroxidase complex for 30 min. Nickel-enhanced diaminobenzidine (DAB) was used for visualization. This technique was also used to stain serial sections for CD3 and CD8 expression. The second primary antibody, anti-E-cadherin, was added and the sections incubated overnight at 4°C. A further one-hour incubation with biotinylated rabbit antimouse antibody was followed by incubation at room temperature with streptavidin–biotin–alkaline phosphatase complex for 30 min. The sections were developed using Vector red, with levamisole to inhibit endogenous alkaline phosphatase. Sections were prepared in duplicate and the first of each pair was counterstained with Mayer's haematoxylin, dehydrated, cleared with xylene and mounted in DPX. The second of each pair of slides was mounted in water-based medium without counterstain for confocal microscopy. For each section the controls consisted of nonimmune mouse IgG1 at the same dilution as the corresponding primary antibodies with the same secondary antibody and development technique.
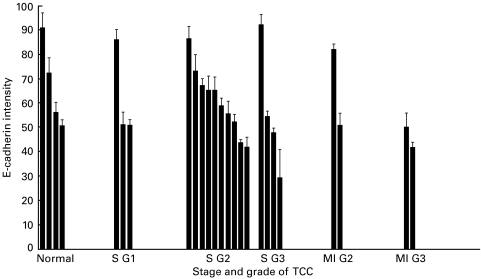
Counting was performed at ×400 magnification using light microscopy. For each section, CD103+ cells were counted in three fields consisting of stroma and three fields consisting of TCC. A mean of each group of three fields was taken for the purposes of statistical analysis. Two observers performed counts independently, blinded to each others results. Although individual counts varied between observers the overall statistical relationship between fields was maintained. Student's paired two-tailed t-test was used to analyse the difference between the numbers of CD103+ cells infiltrating TCC compared to the stromal tissue. A ratio of CD103+ cells infiltrating tumours compared to those infiltrating stroma was calculated and used to assess the relationship to E-cadherin intensity. The proportion of CD3 and CD8 cells also expressing CD103 was assessed by counting cells in corresponding areas of serial sections. Scanning confocal microscopy was used to measure the intensity of E-cadherin staining for each tumour. The immunofluorescence procedure was performed using Vector red as described above and the sections analysed by scanning laser confocal microscopy as described previously [9], with minor modifications. Fluorescence images were collected using an MRC-600 confocal imaging system with krypton/argon laser (Bio-Rad) implemented on a Nikon Optiphot microscope. The single channel mode was based on the laser line at 488 nm with the confocal aperture, gain and black-level settings kept constant. Optical sectioning was performed by scanning across the XY axis at 3 μm increments in the Z-axis and a ‘Z-series’ image was constructed from digitally stored serial images. Quantitative information was obtained using COMOS software applying histogram analysis. A ‘colour banding’ technique allowed exclusion of unstained features such as defects in tissue sections and noncancerous areas. The intensity of E-cadherin expression was calculated by multiplying the pixel intensity by the percentage of the selected area covered by tumour. The background fluorescence was minimal and was excluded by subtraction of the intensity of the isotype control from the E-cadherin intensity. This calculation was repeated for three fields in each section to generate a mean intensity and standard error of the mean (Fig. 2).
Fig. 2.
Intensity of E-cadherin expression measured by scanning confocal microscopy, related to grade and stage of tumour. ‘S’ indicates superficial (Ta–T1) and ‘MI’ indicates muscle invasive disease (stage T2).
RESULTS
Sections were taken from four samples of normal urothelium and 26 samples of TCC of the bladder. Disease stage and grade for each case is given in Table 1.
Table 1.
Stage and histopathological grade of TCC cases included in the series
| G1 | G2 | G2/3 | G3 | |
|---|---|---|---|---|
| Ta | 3 | 2 | 0 | 1 |
| T1 | 0 | 5 | 4 | 2 |
| T2 | 0 | 1 | 2 | 2 |
Stage was assessed by clinical examination and pathological assessment of resection specimens. Stages Ta–T1 indicate superficial disease. Stage T2 and higher indicate muscle invasive disease. Grade was assessed by histopathological examination. Grade 1 (G1) indicates well differentiated. Grade 2 (G2) indicates moderately differentiated. Grade 3 (G3) indicates poorly differentiated. Cases graded G2/3 had a mixed pattern of differentiation. In four cases grading information was not available.
Normal urothelium
Normal transitional epithelium demonstrated membranous E-cadherin expression in a uniform fashion although intensity varied between cases. A few cells with the CD103+ phenotype were present, predominantly in the epithelium with occasional CD103+ lymphocytes also present in the lamina propria (Figs 1a, 1e(i) and Fig. 3). In the urothelium the majority of the T lymphocytes (mean 71·5% ± 19·4) were also CD8+ and of these 68% ± 14·4 expressed the CD103 marker. In the lamina propria 62·2% ± 16·8 of the T lymphocytes were CD8+ and 56·2% ± 19 of these expressed the CD103 marker.
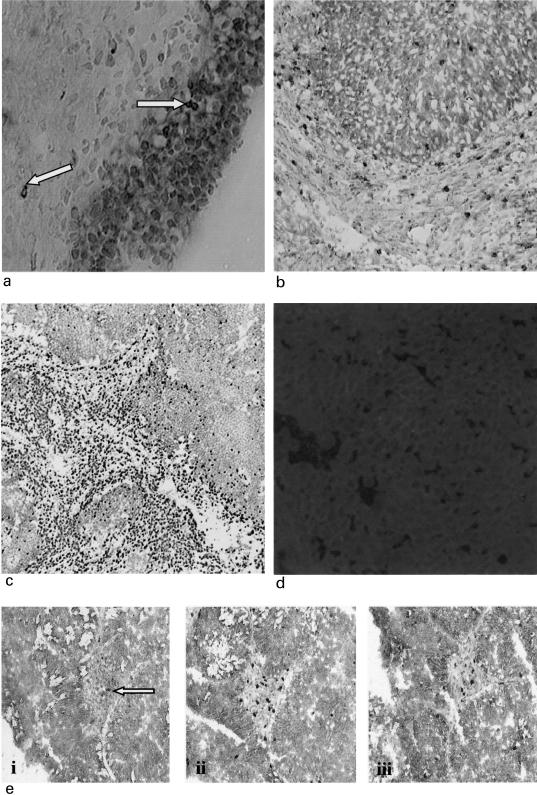
Fig. 1.
(a) Normal urothelium dual stained for E-cadherin (red) and CD103 (black). Urothelium strongly positive for E-cadherin. Occasional CD103+ cells in urothelium and lamina propria (× 400) indicated by arrows. (b) A moderately differentiated TCC with some CD103+ +ve cells (black) infiltrating E-cadherin positive tumour (red), but most clustered around the tumour in E-cadherin –ve stroma (× 200). (c) A poorly differentiated TCC demonstrating obvious clustering of CD3+ cells (black) in the stroma with minimal tumour infiltration (× 40). (d) Scanning confocal microscopy image showing E-cadherin expression in a TCC (× 200). (e) Serial sections stained with anti-CD103 (i), CD3 (ii) and CD8 (iii) indicating the relative proportions of each phenotype (× 100).
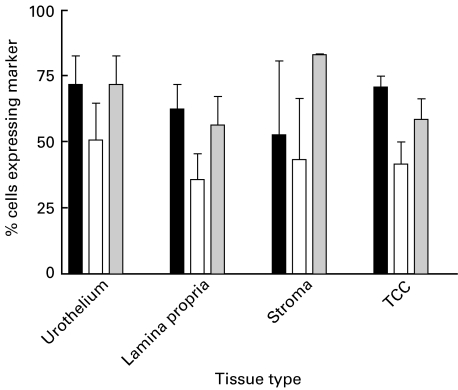
Fig. 3.
Pattern of distribution of CD103 positive cells (n = 26). Paired Student's two-tailed t test (P = 0·0006). ▪ % of CD3+ cells expressing CD8; □ % of CD3+ cells expressing CD103;  % of CD8+ cells expressing CD103.
% of CD8+ cells expressing CD103.
E-cadherin expression in TCC
E-cadherin staining was heterogeneous in the TCC specimens (Fig. 2) The poorly differentiated carcinomas tended to stain weakly for E-cadherin. Figure 1b shows a moderately differentiated TCC with more intense, widespread E-cadherin staining. A typical scanning confocal microscopy image of a moderately differentiated TCC is shown in Fig. 1d.
CD103+ lymphocyte infiltration of TCC
A few CD103+ cells were infiltrating the carcinomas; however, the majority of these cells tended to be in the peripheral parts of the TCC, close to the boundary with the surrounding stroma. Most of the CD103+ lymphocytes were clustered in the stroma surrounding the carcinomas (Fig. 1b,1e(i)). The ratio of CD103 counts in TCC compared to stroma were related to mean E-cadherin intensity for 22 cases of TCC. No statistically significant relationship was demonstrated. Analysis of the cell counts for the 26 samples of TCC demonstrated significantly more CD103+ cells clustered in the stroma around the TCC, compared to infiltrating the cancerous tissue (P = 0·0006). This relationship was seen in all but one of the sections analysed (Fig. 3), and was maintained when counting was performed by a second independent observer blinded to the results.
Phenotype of lymphocytes in TCC
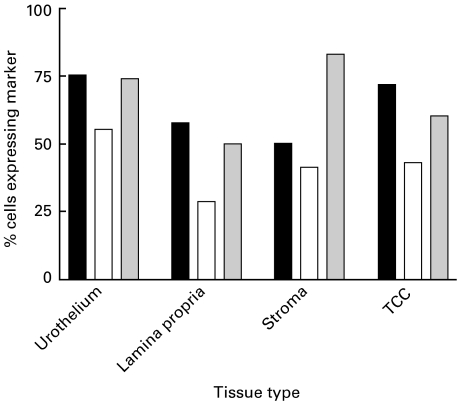
Figure 4 shows the proportions of CD3+ and CD8+ lymphocytes also expressing the CD103 marker, as assessed from serial sections of tissue (Fig. 1e). The pattern of expression was similar in normal urothelium and TCC. In carcinomas, significantly greater numbers of CD103+ T lymphocytes were present in the surrounding stroma rather than infiltrating the carcinomas (P = 0·0006). Of those T lymphocytes (CD3+) infiltrating the tumours, 70·8% ± 5·9 were CD8+ and of these 58·3% ± 11·8 expressed CD103. In the surrounding stroma 52·5% ± 11·8 of lymphocytes were CD8+ and 82·8% ± 0·7 of this subset expressed CD103.
Fig. 4.
Relative proportions of CD3+ T lymphocytes (TL) and CD8+ cytotoxic T lymphocytes (CTL) expressing CD103. Data generated from mean counts for TCC cases and normal tissue. ▪ % CD8+ TL; □ % CD103+ TL;  % CD103+ CTL.
% CD103+ CTL.
DISCUSSION
We have demonstrated the presence of CD103+ lymphocytes within the normal human urothelium. These findings are consistent with the reported pattern of expression in the intestinal mucosa [10]. Huge numbers of lymphocytes reside in the mucosa of the human gut, possibly reflecting the constant exposure to exogenous antigen and the need for local immune surveillance. The CD103-expressing population consists of previously primed lymphocytes, poised to recognize antigen and to generate an effective immune response. An in vitro study of intestinal IEL demonstrated enhanced spontaneous cytotoxicity against epithelial tumour cell lines compared to peripheral blood lymphocytes [11]. Further this phenomenon was CD103-dependent and could be blocked with antibody to the αEβ7 integrin [12].
In the absence of inflammation or infection the corresponding population of the urothelium is relatively small but is ideally located to recognize pathogens and proliferate rapidly to produce a large population of cytotoxic T cells. In our series only 25–55% of IEL expressed the CD103 marker compared to 50–90% of gut IEL. However, in common with studies in gut there was a greater proportion of CD103+ lymphocytes in the urothelium compared to the lamina propria. Importantly, in all areas of normal and TCC tissue, 50–80% of cytotoxic T lymphocytes (CD8+) also expressed CD103. Lymphocytes of the CD103+ phenotype may play an important role in the recognition and killing of infected or malignant urothelial cells.
Our study shows heterogeneous expression of E-cadherin in TCC, although there is a trend towards lower expression in invasive and poorly differentiated tumours. The inconsistent correlation with stage and grade may reflect the small number of cases assessed. Previous larger studies of E-cadherin in bladder cancer have demonstrated that reduced expression was associated with bladder cancer progression [7]. This is likely to be due to a loss of adhesion between cancer cells, allowing greater mobility within tissues. It has been proposed that CD103+ lymphocytes may be involved in tumour immunosurveillance within epithelia [2] and that loss of E-cadherin may be a mechanism of immune escape [8].
We have demonstrated that more CD103+ lymphocytes were present in the stroma around cancerous tissue than actually infiltrating into TCC (P = 0·0006). In our study there was no correlation between intensity of E-cadherin staining and CD103 infiltration into TCC. This observation is consistent with previous evidence which suggests that CD103 is not simply a homing receptor for IEL. Its widespread distribution and preferential expression by the CD8+ population in the bladder mucosa and elsewhere, however, indicate an important role in epithelial immunity.
Our findings are consistent with a previous study of pancreatic cancer [8], although E-cadherin expression was not assessed in that report. The relative paucity of tumour infiltrating lymphocytes with the CD103 phenotype may indicate a failure of normal tissue surveillance. This observation may be the result of altered function of E-cadherin or dysregulation of the cytokines responsible for promoting differentiation to the CD103 phenotype. Further, there is an overall failure of lymphocytes identified by CD3 expression to infiltrate tumour tissues. An alternative explanation for the low numbers of CD103+ cells within the TCC is that other mechanisms of immune evasion are involved, such as down-regulation of MHC class I, immunosuppressive cytokine production [13] and tumour counterattack [14]. TGFβ1 has complex biological effects and has itself been implicated as an immunosuppressive cytokine potentially employed by tumours to effect immune escape. Its effects upon the function of cells with the CD103+ phenotype remain to be fully elucidated.
In conclusion, lymphocytes of the CD103+ phenotype are present in the normal and cancerous urothelium. These lymphocytes are clustered mainly in the surrounding stroma rather than infiltrating bladder carcinomas, suggesting a failure of the immune response. Loss of E-cadherin expression per se does not seem to be responsible for this pattern. It is likely that several mechanisms of immune evasion are responsible which may include defective E-cadherin function and an altered cytokine environment within cancers.
Acknowledgments
This work was supported by a Research Training Fellowship awarded to JC by the Northern and Yorkshire NHS Research and Development Executive. The authors would like to thank Sister Wendy Robson and Sister Teresa Mewes for assistance in collection of tissue samples and Dr Trevor Booth for assistance with scanning confocal microscopy.
REFERENCES
- 1.Cepek KL, Shaw SK, Parker CM, Russell GJ, Morrow JS, Rimm DL, Brenner MB. Adhesion between epithelial cells and T lymphocytes mediated by E- cadherin and the alpha E beta 7 integrin. Nature. 1994;372:190–3. doi: 10.1038/372190a0. [DOI] [PubMed] [Google Scholar]
- 2.Kilshaw PJ, Karecla P. Structure and function of the mucosal T-cell integrin alpha E beta 7. Biochem Soc Trans. 1997;25:433–9. doi: 10.1042/bst0250433. [DOI] [PubMed] [Google Scholar]
- 3.Izadifar V, de Boer WI, Muscatelli-Groux B, Maille P, van der Kwast TH, Chopin DK. Expression of transforming growth factor beta1 and its receptors in normal human urothelium and human transitional cell carcinomas. Hum Pathol. 1999;30:372–7. doi: 10.1016/s0046-8177(99)90110-7. [DOI] [PubMed] [Google Scholar]
- 4.Brosman SA. Bacillus Calmette–Guerin immunotherapy. Techniques and results. Urol Clin North Am. 1992;19:557–64. [PubMed] [Google Scholar]
- 5.Bracke ME, Van Roy FM, Mareel MM. The E-cadherin/catenin complex in invasion and metastasis. Curr Top Microbiol Immunol. 1996;213:123–61. doi: 10.1007/978-3-642-61107-0_9. [DOI] [PubMed] [Google Scholar]
- 6.Richmond PJ, Karayiannakis AJ, Nagafuchi A, Kaisary AV, Pignatelli M. Aberrant E-cadherin and alpha-catenin expression in prostate cancer: correlation with patient survival. Cancer Res. 1997;57:3189–93. [PubMed] [Google Scholar]
- 7.Syrigos KN, Krausz T, Waxman J, et al. E-cadherin expression in bladder cancer using formalin-fixed, paraffin-embedded tissues: correlation with histopathological grade, tumour stage and survival. Int J Cancer. 1995;64:367–70. doi: 10.1002/ijc.2910640603. [DOI] [PubMed] [Google Scholar]
- 8.Ademmer K, Ebert M, Muller-Ostermeyer F, Friess H, Buchler MW, Schubert W, Malfertheiner P. Effector T lymphocyte subsets in human pancreatic cancer: detection of CD8+CD18+ cells and CD8+CD103+ cells by multi-epitope imaging. Clin Exp Immunol. 1998;112:21–6. doi: 10.1046/j.1365-2249.1998.00546.x. 10.1046/j.1365-2249.1998.00546.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Robertson H, Wheeler J, Morley AR, Booth TA, Talbot D, Kirby JA. Beta-chemokine expression and distribution in paraffin-embedded transplant renal biopsy sections: analysis by scanning laser confocal microscopy. Histochem Cell Biol. 1998;110:207–13. doi: 10.1007/s004180050283. 10.1007/s004180050283. [DOI] [PubMed] [Google Scholar]
- 10.Cerf-Bensussan N, Jarry A, Brousse N, Lisowska-Grospierre B, Guy-Grand D, Griscelli C. A monoclonal antibody (HML-1) defining a novel membrane molecule present on human intestinal lymphocytes. Eur J Immunol. 1987;17:1279–85. doi: 10.1002/eji.1830170910. [DOI] [PubMed] [Google Scholar]
- 11.Taunk J, Roberts AI, Ebert EC. Spontaneous cytotoxicity of human intraepithelial lymphocytes against epithelial cell tumors. Gastroenterology. 1996;94:69–75. doi: 10.1016/0016-5085(92)91785-3. [DOI] [PubMed] [Google Scholar]
- 12.Roberts AI, O'Connell SM, Biancone L, Brolin RE, Ebert EC. Spontaneous cytotoxicity of intestinal intraepithelial lymphocytes: clues to the mechanism. Clin Exp Immunol. 1993;94:527–32. doi: 10.1111/j.1365-2249.1993.tb08229.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Marincola FM, Jaffee EM, Hicklin DJ, Ferrone S. Escape of human solid tumours from T-cell recognition: molecular mechanisms and functional significance. Adv Immunol. 2000;74:181–273. doi: 10.1016/s0065-2776(08)60911-6. [DOI] [PubMed] [Google Scholar]
- 14.Igney FH, Behrens CK, Krammer PH. Tumor counterattack – concept and reality. Eur J Immunol. 2000;30:725–31. doi: 10.1002/1521-4141(200003)30:3<725::AID-IMMU725>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]