Abstract
Helicobacter pylori colonizes the gastric epithelial surface and induces epithelial cells to increase production of the neutrophil attractant IL-8. Little is known about the role of the gastric epithelium in regulating mucosal T cell trafficking. We therefore characterized constitutive and regulated epithelial expression of the CXC chemokines IP-10, I-TAC and Mig, which specifically attract CXCR3 expressing CD4+ T cells. Human gastric epithelial cell lines (AGS, Kato III, NCI) were used to characterize the constitutive and regulated expression of three CXC chemokines in response to IFN-γ, TNF-α and different H. pylori preparations. Chemokine mRNA and protein production were measured by RT-PCR and ELISA. Gastric epithelial cells constitutively expressed mRNA for IP-10, Mig and I-TAC. IFN-γ in combination with TNF-α strongly induced secretion of those chemokines. Soluble or membranous fractions of H. pylori significantly inhibited IFN-γ/TNF-α induced epithelial cell IP-10 and Mig production. Gastric epithelial cells may contribute to mucosal T cell trafficking. The capacity of H. pylori products to inhibit IP-10 and Mig secretion may explain, at least in part, the failure to induce protective immunity against this bacterium and the ability of H. pylori to affect the presentation of the local inflammation.
Keywords: gastric epithelial cells, chemokines, H. pylori, IP-10, Mig, I-TAC
INTRODUCTION
Helicobacter pylori infection is an important risk factor for chronic gastritis, peptic ulcer, gastric carcinoma and gastric B-cell lymphoma [1,2]. Although the pathological mechanisms involved in H. pylori-induced inflammation are not yet completely defined, there is accumulating evidence that activated neutrophils play an important role in the pathogenesis [1,2]. Gastric epithelial cells produce and secrete IL-8, a potent chemotactic and activating factor for leucocytes, in response to H. pylori infection both in vivo and in vitro [3]. Indeed, gastric mucosal levels of IL-8 are increased, and its activity correlates with histological severity in patients with H. pylori-induced gastritis [4] as well as cagA status [5]. Prolonged IL-8 production by gastric epithelial cells during H. pylori infection might result in the recruitment of leucocytes to infected tissues and therefore may be important in the regulation of inflammatory and immune processes in response to this bacterium [6].
Despite increasing evidence for involvement of T lymphocytes in H. pylori-associated gastritis, little is known about the signals that regulate T lymphocyte recruitment within the gastric lesions. Lymphocyte recruitment into tissues is a multistep process involving adhesion molecules and chemokines [7]. Chemokines are secreted basic proteins (6–14 kDa) subdivided into four families based on the relative position of their cysteine residues (CC, CXC, C, CXC3). CXC chemokines further fall into two classes based on the presence or absence of a NH2-terminal sequence Glutamic acid–Leucine–Arginine (ERL). The ERL-containing CXC chemokines, such as IL-8, chemoattract neutrophils, whereas for the non-ELR CXC chemokines, three of them – IP-10 (IFN-inducible protein 10), Mig (monokine induced by IFN-γ) and I-TAC (IFN-inducible T cell α chemoattractant) – are IFN-γ inducible and potently chemoattract activated T lymphocytes [8]. All three chemokines signal through a common receptor, CXCR3, expressed by memory (CD45R0+) T cells [9], preferentially of the Th1 subset, and by natural killer cells, but not by monocytes or neutrophils [10,11]. Recent studies have colocalized CD4+ T lymphocytes and IFN-γ in animal models and H. pylori-infected patients with chronic gastritis and MALT-lymphoma, indicating the dominance of a Th1-type response [12,13]. Moreover, IFN-γ has been shown to induce expression of CXC3 receptors on lymphocytes [10].
Since gastric epithelial cells have receptors for TNF-α and IFN-γ, and those cytokines are known to be potent inducers of IP-10, Mig and I-TAC production in several other cell types [14–16], we investigated whether gastric epithelial cells may produce IFN-γ-inducible chemokines that chemoattract T cell populations known to be important for mediating pathologic inflammation in the stomach. We further characterized the influence of H. pylori bacterial lysates in terms of expression of the CXC chemokines IP-10 and Mig in gastric epithelial cells.
MATERIALS AND METHODS
Reagents list
The recombinant human (rh) IFN-γ and TNF-α, anti-IP-10 (1 µg/µl), anti-Mig (1 µg/µl), as well as biotinylated antibodies against IP-10 and Mig used in the ELISA protocol and rh IP-10 (20 ng/µl) and rh Mig (20 ng/µl) were obtained from R & D Systems (Wiesbaden, Germany). Streptavidine horse radish peroxidase conjugate was obtained from Amersham Pharmacia Biotech (Freiburg, Germany). Annexin-V FITC was purchased from Bender Medsystems (Vienna, Austria), Ig isotype and propidium-iodide (PI) were puchased from BD PharMingen (La Jolla, San Diego, CA, USA)
Bacterial strain, epithelial cell lines and culture conditions
For in vitro experiments we used the cagA-positive H. pylori strain NTCT 11637 (DMSZ, Braunschweig, Germany). Bacteria were grown on columbia base agar containing 4% defibrinated horse blood (Oxoid, Wesel, Germany) and one tube of H. pylori selective supplement (Oxoid, Wesel, Germany) in 500 ml media. Supplement contains vancomycine, trimethoprime, cefusoldin and amphotericine B. Plates were freshly prepared and cooled down before use. The plates were incubated in a microaerophilic atmosphere containing 80% N2, 15% CO2, 5% O2 at 37°C. Culture conditions were achieved by using an Anoxomat WS8080-System (MART, Lichtenvoorde, The Netherlands). The plates were incubated by use of a pressure resistant incubation vessel system. Cells were harvested after 5 d of culture and subsequently resuspended in Brucella Broth (BB) Bouillion (B & D, Heidelberg, Germany) containing 8% FCS and H. pylori selective supplement. Quantification was performed by optical density measurements on a spectralphotometer (Ultraspect III, Pharmacia Biotech, Freiburg, Germany). Bacteria were diluted in BB-FCS to an OD550 = 1 and used for further investigations.
The human gastric carcinoma cell lines AGS, Kato III and NCI were obtained from the American Type Culture Collection (Rockville, MD, USA). The cell line AGS was cultured in HAM's F12 media (Gibco, Deisenhofen, Germany) supplemented with 10% fetal bovine serum (FBS) and 1% antibiotics. Kato III and NCI were cultured in RPMI (Gibco) containing 20% FBS and 1% antibiotics. Cells were cultured at 37°C in 5% CO2 saturated atmosphere.
Preparing soluble and membranous fractions of H. pylori
For the production of soluble and membranous fractions of H. pylori a cell suspension in PBS was sonicated for 20 min and 50% intensity (Sonorex Super RK255, Bandelin) and centrifuged for 30 min at 4°C. The supernatant was characterized as soluble fraction and the pellet dissolved in PBS as membranous fraction. Protein concentration was measured with the Biuret method (Bio-Rad Protein-Assay, Bio-Rad Laboratories GmbH, München, Germany).
Stimulation protocol
Confluent monolayers of human gastric cell lines were stimulated with TNF-α and IFN-γ (50 ng/ml each) alone or in combination. Negative controls were performed in each experimental setting without stimulation. Supernatants were harvested, centrifuged and collected for chemokine measurements by ELISA. For bacterial infection confluent epithelial monolayers were stimulated with IFN-γ and TNF-α alone or in combination for 12 h. Supernatants were removed and cells washed four times in PBS. The membranous and soluble fractions of H. pylori cells were used at a protein concentration of 50 µg/ml, if nothing else is stated. After 12 and 24 h of incubation time, supernatants were collected, centrifuged and analysed for Mig, IP-10 and IL-8 production. For each experiment duplicates were assessed.
ELISA
ELISA measurements for Mig and IP-10 were performed as described before [17]. Briefly, polysterol 96-well plates (Greiner, Solingen, Germany) were coated with monoclonal antibodies to IP-10 (1 : 250) and Mig (1 : 500) and incubated for 4 h at 37°C. Antibodies were blocked by an overnight incubation at 4°C with blocking solution (PBS, 5% dry milk as well as 0·1 Tween-20). As detection antibodies biotinylated goat anti-IP-10 (R & D systems) and anti-Mig (R & D systems) were diluted 1 : 500 and 1 : 800 in dilution buffer (PBS, 1% BSA and 0·1% Tween-20). Second step reagents were HRP-conjugated streptavidin (ECL, Amersham Pharmacia Biotech, Freiburg, Germany) diluted 1 : 4000 in dilution buffer. Bound HRP was visualized with TMB and H2O2 diluted in sodium acetate buffer, pH 6·0. The colour reaction was stopped by adding 50 µl of stop solution (1·2 m H2SO4) and absorbency was measured at 450 nm. Concentration of the chemokines was calculated from the standard curves using rh IP-10 and Mig. The specific sensitivity for both ELISA's was 50 pg/ml. Supernatant concentrations of IL-8 were assessed by a specific sandwich ELISA (Biosource, Camarillo, USA), according to the manufacture's protocol. All samples were analysed in duplicates.
RT-PCR analysis
RNA isolation was performed using standard methods (Stratagene, Amsterdam, The Netherlands), and total RNA was determined by measuring absorbency on a spectrophotometer (Eppendorf, Hamburg, Germany). Three µg of total RNA was reverse transcribed by MMLV reverse transcriptase (Gibco Life Technologies, Karlsruhe, Germany), and cDNA was amplified as already described [17]. The primers for I-TAC, Mig and IP-10 were as follows: I-TAC (sense 5′-GCT ATA GCC TTG GCT GTG ATA TTG TG-3′ and antisense 5′-CTG CCA CTT TCA CTG CTT TTA CC-3′) and for Mig (sense 5′-GTA TCT GAG GCA CAT GCT AG-3′ and antisense 5′-AAA GGC ACT GCA TTG TGG TAG G-3′) and IP-10 (sense 5′-AGT GGC ATT CAA GGA GTA CC-3′ and antisense 5′-ATC CTT GGA AGC ACT GCA TC-3′). The amplification profile for IP-10, Mig and I-TAC consisted of 27–35 cycles of a 45 s denaturation step at 95°C followed by 2·5 min annealing and extension at 60°C as described before [17]. Primers for IP-10, Mig and I-TAC did not amplify either murine genomic DNA or splenic cDNA. Negative control reactions had no added RNA in the RT and subsequent PCR amplification. PBMC isolated from the buffy coat of a healthy donor and stimulated overnight with 10 µg/ml PHA and 2 µg/ml of bacterial LPS were used as a source of cDNA for positive controls. Amplification products were run on 4% polyacrylamide gel electrophoresis and visualized by silver-staining.
Flow cytometric analysis
Necrotic membrane damage was excluded by trypan blue exclusion. Furthermore apoptosis and necrosis were evaluated by double-labelling with annexin V-FITC as well as measurement of propidium iodide (PI) uptake into nonpermeabilized cells and subsequent flow cytometry. Briefly, confluent monolayers of gastric epithelial cell lines (0·5–1 × 107 cells) were coincubated with different concentrations of living H. pylori or soluble and membranous fractions of H. pylori for 20 h. Cells were stained with annexin V-FITC and PI in PBS for 1 h at room temperature in the dark. FITC-conjugated murine MoAbs with unrelated specificity were always used as controls for unspecific binding of mAB. For positive controls, cells were permeabilized with Saponin 0·1% for 1 h. Two-colour FACS analysis was performed using a Becton Dickinson FACScan flow cytometer. Data acquisition and analysis were based on Lysis II software (Becton Dickinson, Heidelberg, Germany).
Analysis of data
In the statistical calculations the data were treated as being nonparametric, and results were analysed by the two-tailed Wilcoxon's signed rank test. Results are presented as mean ± 95% confidence interval, unless stated otherwise. P-values <0·05 were considered significant.
RESULTS
Constitutive and regulated expression of Mig, IP-10 and I-TAC mRNA by human gastric epithelial cell lines
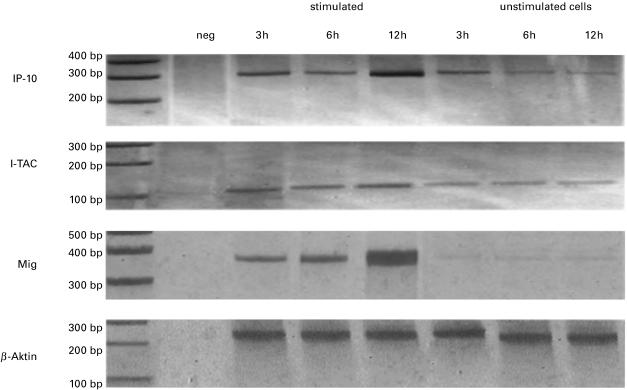
Non-stimulated gastric epithelial cell lines (Kato III, AGS, NCI) constitutively expressed detectable levels of mRNA for IP-10, Mig and I-TAC (Fig. 1). Since gastric epithelial cells have receptors for IFN-γ and TNF-α and as these cytokines have been shown to up-regulate the expression of Mig, IP-10 and I-TAC mRNA by human monocytes, neutrophils [15], astrocytes [18] and bronchial epithelial cells [19], we next examined the regulated mRNA expression of these chemokines in human gastric epithelial cells in response to TNF-α and IFN-γ stimulation. We found an increased expression of I-TAC, Mig and IP-10 starting 2–3 h after stimulation with IFN-γ in combination with TNF-α, and reaching maximal levels by 8–12 h (Fig. 1). No significant effect was observed employing IFN-γ and TNF-α alone, not even with concentrations as high as 100 ng/ml.
Fig. 1.
Constitutive and regulated expression of IP-10, I-TAC and Mig in the gastric epithelial cell lines Kato III. Total RNA was isolated from stimulated and unstimulated confluent monolayers of cell lines after 3, 6 and 12 h as indicated. RT-PCR cDNA transcripts were generated and amplified using oligonucleotide primers specific for IP-10, Mig and I-TAC. RT-PCR reactions were also performed in the absence of RNA as a negative control. Amplification products were run on 4% polyacrylamide-gel electrophoresis, visualized by silver-staining and photographed. Representative results from one of three experiments are shown.
Secretion of Mig and IP-10 by human gastric epithelial cell lines
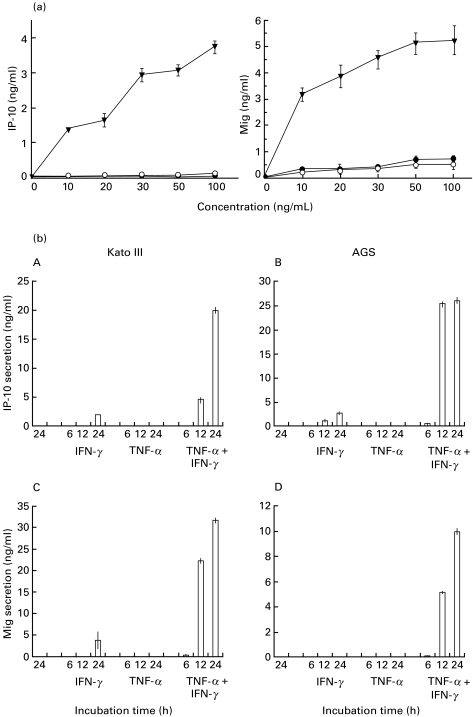
We turned our attention to the question whether Mig and IP-10 mRNA expression was accompanied by secretion of those chemokines. Gastric epithelial cells were stimulated with different concentrations of IFN-γ and TNF-α and protein secretion was measured in culture supernatants by ELISA. In all gastric cell lines, IFN-γ and TNF-α synergistically increased IP-10 and Mig secretion in a dose- (Fig. 2a) and time-dependent manner (Fig. 2b). In the absence of IFN-γ and TNF-α, gastric epithelial cells secreted <50 pg/ml of IP-10 and Mig. In all cell lines investigated, a slight up-regulation of IP-10 secretion could be observed after stimulation with IFN-γ alone, while TNF-α had no effect on IP-10 secretion. Mig secretion could only be detected in Kato III cells upon stimulation with IFN-γ alone and was not observed when cells were stimulated with TNF-α alone (Fig. 2b).
Fig. 2.
(a) Dose-dependent secretion of IP-10 and Mig by gastric epithelial cells. Confluent monolayers of NCI cell lines were stimulated with increasing concentrations of either TNF-α or IFN-γ or both in combination (• IFN-γ; ○ TNF-α; ▾ TNF-α + IFN-γ). Supernatants were harvested after 18 h and chemokine-secretion was measured by ELISA. Similar results were obtained using AGS and NCI cell lines. Values are expressed as mean ± CI. Representative results from one of four independent experiments are shown. (b) Time-dependent secretion of IP-10 and Mig by gastric epithelial cells. Confluent monolayers of AGS and Kato III cells were stimulated for different times (as indicated) with either IFN-γ 50 ng/ml or TNF-α 50 ng/ml alone or both cytokines in combination. Supernatants were harvested after 6, 12 and 18 h and chemokine concentrations were measured by ELISA. Values are expressed as mean ± CI. Representative results from one of four experiments are shown.
Effects of living H. pylori on epithelial IL-8, Mig and IP-10 secretion
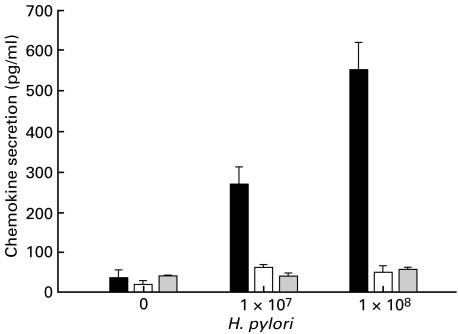
To determine the effect of living bacteria on IP-10 and Mig secretion, different concentrations (1 × 107 and 1 × 108) were coincubated with gastric epithelial cells for 24 h. IL-8 was included as positive control as H. pylori is well known to induce IL-8 secretion in gastric epithelial cells. As Fig. 3 shows H. pylori dose-dependently induces IL-8 secretion in gastric epithelial cells, while IP-10 and Mig secretion remained unchanged. Longer incubation periods had no further effect on chemokine secretion (data not shown).
Fig. 3.
Dose-dependent increase in IL-8 but neither IP-10 nor Mig secretion by living H. pylori (▪ IL-8 secretion; □ IP-10 secretion;  Mig secretion). Confluent monolayers of AGS cell line (2 × 106 cells) were coincubated with different concentrations of living H. pylori for 20 h. Supernatants were harvested, centrifuged and analysed for chemokine-secretion by ELISA. Values are expressed as mean ± CI. Representative results from one of four experiments are shown.
Mig secretion). Confluent monolayers of AGS cell line (2 × 106 cells) were coincubated with different concentrations of living H. pylori for 20 h. Supernatants were harvested, centrifuged and analysed for chemokine-secretion by ELISA. Values are expressed as mean ± CI. Representative results from one of four experiments are shown.
Effects of soluble and membranous fractions of H. pylori on epithelial Mig and IP-10 secretion
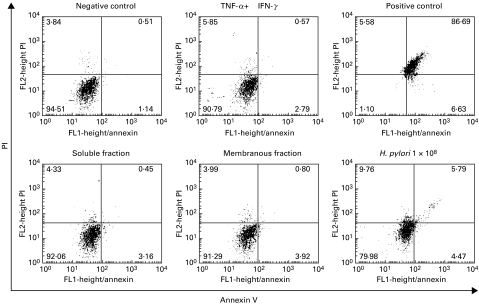
Helicobacter pylori preparations have been shown to differ from living bacteria in their potential to modulate cytokine production [20–22]. We therefore performed further experiments to determine the impact of different H. pylori preparations on chemokine secretion by gastric epithelial cell lines. Soluble and membranous fractions of H. pylori were generated as described in Materials and methods. Gastric epithelial cells were stimulated with INF-γ and TNF-α (50 ng/ml each) for 20 h and additionally with soluble or membranous fractions of H. pylori sonicates. Chemokine secretion was measured in supernatants by ELISA measurement. In agreement with previous studies [23] we found a strong IL-8 production by gastric epithelial cells in response to TNF-α. Fractions of H. pylori neither alone nor in combination with TNF-α and IFN-γ stimulated IL-8 secretion. In contrast, both membranous and soluble fractions of H. pylori induced a marked decrease of Mig and IP-10 concentration in supernatants of gastric epithelial cells, as measured by ELISA (Table 1). Apoptosis and necrosis were excluded by FACS-analysis (Fig. 4).
Table 1.
Inhibition of Mig and IP-10 but not IL-8 by membranous (MF) and soluble fractions (SF) of H. pylori
| Chemokine secretion (ng/ml) | |||
|---|---|---|---|
| Stimulation with | IP-10 | Mig | IL-8 |
| TNF (50 ng/ml) | n.d. | n.d. | 2·318 ± 0·264 |
| IFN (50 ng/ml) | n.d. | n.d. | 0·227 ± 0·06 |
| IFN + TNF (50 ng/ml each) | 25·46 ± 1·23 | 13·23 ± 0·87 | 2·362 ± 0·047 |
| SF (500 µg/ml) | n.d. | n.d. | 0·274 ± 0·15 |
| MF (500 µg/ml) | n.d. | n.d. | 0·132 ± 0·07 |
| IFN + TNF + SF (50 µg/ml) | 4·47 ± 0·33 | 4·195 ± 0·64 | 2·301 ± 0·033 |
| IFN + TNF + SF (100 µg/ml) | 3·009 ± 0·36 | 3·984 ± 0·45 | 2·524 ± 0·78 |
| IFN + TNF + SF (500 µg/ml) | 2·77 ± 0·42 | 3·307 ± 0·21 | 2·282 ± 0·195 |
| IFN + TNF + MF (50 µg/ml) | 9·62 ± 0·53 | 5·29 ± 0·34 | 2·535 ± 0·006 |
| IFN + TNF + MF (100 µg/ml) | 2·7 ± 0·41 | 2·23 ± 0·39 | 2·884 ± 0·124 |
| IFN + TNF + MF (500 µg/ml) | 1·54 ± 0·23 | 0·82 ± 0·32 | 3·002 ± 0·142 |
Stimulated gastric epithelial cells were coincubated with membranous and soluble fractions of H. pylori in concentrations of 50, 100 and 500 µg/ml. After 20 h of stimulation supernatants were harvested and centrifuged. ELISA measurements were performed as described in Materials and methods. Values are expressed as mean ± CI. Representative results from one of four experiments are shown. n.d. = not determined.
Fig. 4.
Determination of necrosis and apoptosis by propidium iodide (PI) and annexin V staining. Necrotic membrane damage as well as apoptosis of gastric epithelial cells cultured for 20 h in medium or in the presence of TNF-α and IFN-γ alone or in combination with membranous or soluble fractions (50 µg/ml) as well as living H. pylori (1 × 108) was excluded by double staining with PI and annexin V. Gastric epithelial cells showed an increase in annexin V and PI staining after incubation with living H. pylori for 20 h, but remained negative for PI and annexin V concerning soluble or membranous fractions. Gastric epithelial cells permeabilized with 0·1% saponin served as positive control. Data are presented as percentage of gated cells.
DISCUSSION
Chronic H. pylori infection is characterized by a pleiomorphic inflammatory cell infiltrate, composed of large numbers of polymorphonuclear and mononuclear cells in the epithelium and underlying lamina propria [2]. Because in addition to neutrophils predominantly Th1 cells are thought to play a central role in the pathogenesis of gastric inflammation in H. pylori infection [24], this study focused on the mediators that are important for the selective recruitment of these effector cells. In the present study, we demonstrate that human gastric epithelial cells produce IP-10, Mig and I-TAC at the mRNA and protein levels. These chemokines, all induced by IFN-γ, are known to be strong ligands for the CXCR3 receptor on T cells. Because the CXCR3 receptor is predominantly expressed on Th1 cells, it seems likely that IP-10, Mig and I-TAC might contribute to the selective recruitment of Th1 cells in sites of inflammation with high IFN-γ production, e.g. H. pylori-positive gastritis [25].
In vivo, gastric infection with H. pylori induces the mucosal production of various cytokines in the host, including TNF-α and IFN-γ [26]. Recent reports have also shown that the number of IFN-γ-producing cells is increased in the stomach during chronic gastritis [2]. Our findings of high levels of IP-10, Mig and I-TAC production by gastric epithelial cells after stimulation with IFN-γ and TNF-α in vitro indicate that gastric epithelial cells have the potential to participate in mucosal T-cell recruitment during inflammatory responses. By chemoattracting IFN-γ-producing CD4+ T cells in close proximity to the epithelium, epithelial-derived IP-10, Mig and I-TAC, which themselves are up-regulated by IFN-γ, may activate a positive feedback loop. The net effect of this would be amplification of the mucosal Th1 response seen in H. pylori-associated diseases [24]. Although gastric epithelial cells themselves do not produce IFN-γ, intraepithelial T cells and/or IFN-γ-producing mucosal dendritic cells present may provide a regulatory signal to epithelial cells and thereby act as an initial stimulus for epithelial cell IP-10, Mig and I-TAC production.
In our experiments, we observed synergy between TNF-α and IFN-γ in the induction of epithelial Mig and IP-10 secretion, while TNF-α or IFN-γ alone had little or no effect. This data is consistent with a prior report on the transcriptional activation of the murine IP-10 gene by IFN-γ and TNF-α in mouse fibroblasts [27,28], but differs from studies in intestinal epithelium and normal bronchial epithelium, where IFN-γ alone was able to maximally induce IP-10, Mig and I-TAC mRNA expression [17,19].
In a recent study, IP-10 and Mig expression has been described in the gastric mucosa in vivo for the first time by employing in situ hybridization and immunohistochemistry [29]. Eck et al. showed in this study that IP-10 and Mig are expressed by endothelial cells of gastric mucosal vessels and by mononuclear cells at sites of T cell infiltration. In contrast to our work they could not detect any IP-10 and Mig expression by the gastric epithelium, while we found strong increase of IP-10 and Mig secretion by gastric epithelial cell lines in response to TNF-α and IFN-γ. Similar conflicting reports using in situ hybridization to localize the cellular origin of chemokines exist for IL-8. This cytokine was identified in macrophages, neutrophils and epithelial cells in active lesions of patients with inflammatory bowel disease, whereas other studies could not detect intestinal epithelial IL-8 mRNA expression [30–34].
Furthermore, in this study we were able to show that soluble and membranous fractions of H. pylori but not living bacteria dose-dependently inhibited IFN-γ/TNF-α induced epithelial cell IP-10 and Mig production without changing the epithelial cell survival. Previous studies have already demonstrated that certain H. pylori preparations, e.g. urease, secreted proteins and bacterial DNA, can differ from living bacteria regarding their ability to modulate the local and/or systemic immune response [35–37]. Living H. pylori possess an immunosuppressive activity, either by the inhibition of antigen- or mitogen induced proliferative responses of PBMC and gastric epithelial cells [20] and/or by the inhibition of phagocytosis by monocytes and polymorphonuclear cells [38]. These inhibitory effects might be overcome by Th1-cytokines, which in turn are potent stimulators of the humoral immune response. Consequently, H. pylori infection and gastric inflammation might persist due to reduced influx of CD4+ cells preferentially of the Th1 subset. For this reason, it seems to be rational that H. pylori might have evolved strategies to minimize the immigration of activated Th1 cells thus explaining – at least in part – the failure to induce protective immunity against this bacterium.
In conclusion, the herein demonstrated cytokine-induced IP-10, Mig and I-TAC secretion by the gastric epithelium may represent an important element in the local gastric inflammatory immune response. The novel finding that H. pylori fractions are capable of suppressing the IP-10 and Mig protein production by the gastric epithelium is likely to represent a selective advantage by which this pathogen evades the host's immune response.
Acknowledgments
The authors thank Ivonne Klingenhagen and Elke Weber for excellent technical assistance. This work was supported by a grant from the Deutsche Krebshilfe/Dr Mildred Scheel Stiftung für Krebsforschung (N.L., 10–1396-Lü I).
REFERENCES
- 1.Genta RM, Lew GM, Graham DY. Changes in the gastric mucosa following eradication of Helicobacter pylori. Mod Pathol. 1993;6:281–9. [PubMed] [Google Scholar]
- 2.Genta RM. The immunobiology of Helicobacter pylori gastritis. Semin Gastrointest Dis. 1997;8:2–11. [PubMed] [Google Scholar]
- 3.Crabtree JE. Role of cytokines in pathogenesis of Helicobacter pylori-induced mucosal damage. Dig Dis Sci. 1998;43:46–55. [PubMed] [Google Scholar]
- 4.Shimoyama T, Everett SM, Dixon MF, et al. Chemokine mRNA expression in gastric mucosa is associated with Helicobacter pylori cagA positivity and severity of gastritis. J Clin Pathol. 1998;51:765–7. doi: 10.1136/jcp.51.10.765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ando T, Kusugami K, Ohsuga M, et al. Interleukin-8 activity correlates with histological severity in Helicobacter pylori-associated antral gastritis. Am J Gastroenterol. 1996;91:1150–6. [PubMed] [Google Scholar]
- 6.Crowe SE, Alvarez L, Dytoc M, et al. Expression of interleukin 8 and CD54 by human gastric epithelium after Helicobacter pylori infection in vitro. Gastroenterology. 1995;108:65–74. doi: 10.1016/0016-5085(95)90009-8. [DOI] [PubMed] [Google Scholar]
- 7.Nelson PJ, Krensky AM. Chemokines lymphocytes and viruses: what goes around, comes around. Curr Opin Immunol. 1998;10:265–70. doi: 10.1016/s0952-7915(98)80164-7. [DOI] [PubMed] [Google Scholar]
- 8.Baggiolini M, Dewald B, Moser B. Human chemokines: an update. Annu Rev Immunol. 1997;15:675–705. doi: 10.1146/annurev.immunol.15.1.675. [DOI] [PubMed] [Google Scholar]
- 9.Qin S, Rottman JB, Myers P, et al. The chemokine receptors CXCR3 and CCR5 mark subsets of T cells associated with certain inflammatory reactions. J Clin Invest. 1998;101:746–54. doi: 10.1172/JCI1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sallusto F, Lenig D, Mackay CR, et al. Flexible programs of chemokine receptor expression on human polarized T helper 1 and 2 lymphocytes. J Exp Med. 1998;187:875–83. doi: 10.1084/jem.187.6.875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Annunziato F, Cosmi L, Galli G, et al. Assessment of chemokine receptor expression by human Th1 and Th2 cells in vitro and in vivo. J Leukoc Biol. 1999;65:691–9. doi: 10.1002/jlb.65.5.691. [DOI] [PubMed] [Google Scholar]
- 12.Mattapallil JJ, Dandekar S, Canfield DR, et al. A predominant Th1 type of immune response is induced early during acute Helicobacter pylori infection in rhesus macaques. Gastroenterology. 2000;118:307–15. doi: 10.1016/s0016-5085(00)70213-7. [DOI] [PubMed] [Google Scholar]
- 13.Bamford KB, Fan X, Crowe SE, et al. Lymphocytes in the human gastric mucosa during Helicobacter pylori have a T helper cell 1 phenotype. Gastroenterology. 1998;114:482–92. doi: 10.1016/s0016-5085(98)70531-1. [DOI] [PubMed] [Google Scholar]
- 14.Mach F, Sauty A, Iarossi AS, et al. Differential expression of three T lymphocyte-activating CXC chemokines by human atheroma-associated cells. J Clin Invest. 1999;104:1041–50. doi: 10.1172/JCI6993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gasperini S, Marchi M, Calzetti F, et al. Gene expression and production of the monokine induced by IFN-gamma (MIG), IFN-inducible T cell alpha chemoattractant (I-TAC), and IFN-gamma-inducible protein-10 (IP-10) chemokines by human neutrophils. J Immunol. 1999;162:4928–37. [PubMed] [Google Scholar]
- 16.Uguccioni M, Gionchetti P, Robbiani DF, et al. Increased expression of IP-10, IL-8, MCP-1, and MCP-3 in ulcerative colitis. Am J Pathol. 1999;155:331–6. doi: 10.1016/S0002-9440(10)65128-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dwinell MB, Lugering N, Eckmann L, et al. Regulated production of interferon-inducible T-cell chemoattractants by human intestinal epithelial cells. Gastroenterology. 2001;120:49–59. doi: 10.1053/gast.2001.20914. [DOI] [PubMed] [Google Scholar]
- 18.Simpson JE, Newcombe J, Cuzner ML, et al. Expression of the interferon-gamma-inducible chemokines IP-10 and Mig and their receptor, CXCR3, in multiple sclerosis lesions. Neuropathol Appl Neurobiol. 2000;26:133–42. doi: 10.1046/j.1365-2990.2000.026002133.x. 10.1046/j.1365-2990.2000.026002133.x. [DOI] [PubMed] [Google Scholar]
- 19.Sauty A, Dziejman M, Taha RA, et al. The T cell-specific CXC chemokines IP-10, Mig, and I-TAC are expressed by activated human bronchial epithelial cells. J Immunol. 1999;162:3549–58. [PubMed] [Google Scholar]
- 20.Knipp U, Birkholz S, Kaup W, et al. Partial characterization of a cell proliferation-inhibiting protein produced by Helicobacter pylori. Infect Immun. 1996;64:3491–6. doi: 10.1128/iai.64.9.3491-3496.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hiroshe M, Watanabe S, Wange XE. A water extract of Heliobacter pylori inhibited gastric epithelial restoration in vitro. Gastroenterology. 1995;108:A115. [Google Scholar]
- 22.Haeberle HA, Kubin M, Bamford KB, et al. Differential stimulation of Interleukin-12 (IL-12) and IL-10 by live and killed Helicobacter pylori in vitro and association of IL-12 production with gamma Interferon-producing T cells in the human gastric mucosa. Infect Immun. 1997;65:4229–35. doi: 10.1128/iai.65.10.4229-4235.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rieder G, Hatz RA, Moran AP, et al. Role of adherence interleukin-8 induction in Helicobacter pylori-associated gastritis. Infect Immun. 1997;65:3622–30. doi: 10.1128/iai.65.9.3622-3630.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lohoff M, Rollinghoff M, Sommer F. Helicobacter pylori gastritis: a Th1 mediated disease. J Biotechnol. 2000;29:33–6. doi: 10.1016/s0168-1656(00)00295-9. [DOI] [PubMed] [Google Scholar]
- 25.Moser B, Loetscher P. Lymphocyte traffic control by chemokines. Nature Immunol. 2001;2:123–8. doi: 10.1038/84219. [DOI] [PubMed] [Google Scholar]
- 26.Bodger K, Crabtree JE. Helicobacter pylori and gastric inflammation. Br Med Bull. 1998;54:139–50. doi: 10.1093/oxfordjournals.bmb.a011664. [DOI] [PubMed] [Google Scholar]
- 27.Ohmori Y, Hamilton TA. The interferon-stimulated response element and a kappa B site mediate synergistic induction of murine IP-10 gene transcription by IFN-gamma and TNF-alpha. J Immunol. 1995;154:5235–44. [PubMed] [Google Scholar]
- 28.Ohmori Y, Schreiber RD, Hamilton TA. Synergy between interferon-gamma and tumor necrosis factor-alpha in transcriptional activation is mediated by cooperation between signal transducer and activator of transcription 1 and nuclear factor kappaB. J Biol Chem. 1997;272:14899–907. doi: 10.1074/jbc.272.23.14899. 10.1074/jbc.272.23.14899. [DOI] [PubMed] [Google Scholar]
- 29.Eck M, Schmausser B, Scheller K, et al. CXC chemokines Gro(alpha)/IL-8 and IP-10/MIG in Helicobacter pylori gastritis. Clin Exp Immunol. 2000;122:192–9. doi: 10.1046/j.1365-2249.2000.01374.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kolios G, Wright KL, Jordan NJ, et al. C-X-C and C-C chemokine expression and secretion by the human colonic epithelial cell line, HT-29: differential effect of T lymphocyte-derived cytokines. Eur J Immunol. 1999;29:530–6. doi: 10.1002/(SICI)1521-4141(199902)29:02<530::AID-IMMU530>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 31.Yang SK, Eckmann L, Panja A, et al. Differential and regulated expression of C-X-C, C-C, and C-chemokines by human colon epithelial cells. Gastroenterology. 1997;113:1214–23. doi: 10.1053/gast.1997.v113.pm9322516. [DOI] [PubMed] [Google Scholar]
- 32.Izzo RS, Witkon K, Chen AI, et al. Neutrophil-activating peptide (interleukin-8) in colonic mucosa from patients with Crohn's disease. Scand J Gastroenterol. 1993;28:296–300. doi: 10.3109/00365529309090244. [DOI] [PubMed] [Google Scholar]
- 33.Mazzucchelli L, Hauser C, Zgraggen K, et al. Expression of interleukin-8 gene in inflammatory bowel disease is related to the histological grade of active inflammation. Am J Pathol. 1994;144:997–1007. [PMC free article] [PubMed] [Google Scholar]
- 34.Grimm MC, Elsbury SK, Pavli B, et al. Interleukin 8: cells of origin in inflammatory bowel disease. Gut. 1996;38:90–8. doi: 10.1136/gut.38.1.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lee CK, Soike K, Hill J, et al. Immunization with recombinant Helicobacter pylori urease decreases colonization levels following experimental infection of rhesus monkeys. Vaccine. 1999;17:1493–505. doi: 10.1016/s0264-410x(98)00365-x. [DOI] [PubMed] [Google Scholar]
- 36.Meyer F, Wilson KT, James SP. Modulation of innate cytokine responses by products of Helicobacter pylori. Infect Immun. 2000;68:6265–72. doi: 10.1128/iai.68.11.6265-6272.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Klinmann DM, Yi AK, Beaucage SL, et al. CpG motifs present in bacterial DNA rapidly induce lymphocytes to secrete interleukin 6, interleukin 12, and interferon-γ. Proc Natl Acad Sci. 1996;93:2879–83. doi: 10.1073/pnas.93.7.2879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Knipp U, Birkholz S, Kaup W, et al. Immune suppressive effects of Helicobacter pylori on human peripheral blood mononuclear cells. Med Microbiol Immunol. 1993;182:63–76. doi: 10.1007/BF00189374. [DOI] [PubMed] [Google Scholar]