Abstract
Variant alleles of the mannose binding lectin (MBL) gene are associated with increased susceptibility to infection and polymorphisms of tumour necrosis factor and lymphotoxin alpha genes (TNF, LTA) are associated with increased severity of infection. Studies have associated recurrent miscarriage with low serum mannose binding lectin concentrations and premature membrane rupture and preterm delivery with elevated maternal and fetal levels of TNF and the TNF (− 308) polymorphism. In this study the frequencies of variant MBL, TNF and LTA alleles in 76 Caucasian couples with idiopathic recurrent miscarriage were compared with those in 69 Caucasian control couples with no history of miscarriage and at least one previous live birth. A new assay based on hybridization to immobilized sequence-specific oligonucleotides (SSO) was used to rapidly detect nine MBL, two TNF and two LTA sequence variants. The assay genotyped all the structural and promoter MBL variants known to influence serum MBL concentrations. This assay was more reliable than restriction digestion or nested allele-specific PCR for the structural variants at codon 54 or 52, respectively. Reliability for codon 57 alleles was not assessed because of the low frequency in this population. The MBL haplotype frequencies in antenatal controls were similar to those reported in other control populations. The frequencies of structural variant MBL genes and of low, medium and high MBL level haplotypes were similar in the recurrent miscarriage and control couples. The TNF and LTA haplotype frequencies were similar in the recurrent miscarriage and control couples. In this carefully defined population no association has been found between recurrent miscarriage and variant alleles of the MBL, TNF or LTA genes.
Keywords: lectin, mannose, miscarriage, TNF
Introduction
Recurrent miscarriage is defined as the loss of three or more consecutive pregnancies and affects 1% of couples [1]. This prevalence is higher than expected by chance and suggests that some couples have an underlying systematic cause for repeated pregnancy loss. Recurrent miscarriage is a heterogeneous condition but comprehensive investigation identifies a cause in only 20% of couples [1]. Several infectious agents have been proposed as a cause for recurrent miscarriage [2] and preterm labour [3,4]. However, cohort studies have shown that individual organisms in the maternal genital tract or placenta have a poor positive predictive value in pregnancy [5,6]. This suggests that pregnancy outcome is determined by the maternal or fetal host immune response.The association of severe infantile infections with mannose binding lectin (MBL) deficiency raised the possibility that deficiency of this component of the immune response may lead to increased susceptibility to infection in utero and miscarriage. Indeed, several studies have reported an association between recurrent miscarriage and low serum mannose binding lectin concentrations (MBL) [7–9]. MBL, a calcium-dependent plasma lectin, is a component of the innate immune system [10]. MBL binds to sugars on microbial surfaces and activates two mannose-binding-lectin-associated serine proteases to cause complement activation [11]. This MBL pathway is the third complement activation pathway.
Human plasma levels of MBL are largely genetically determined. Three different alleles coding for structurally abnormal proteins have been identified in codons 52, 54 and 57 of exon 1 of the MBL gene [12–14]. These structural variants are common with frequencies ranging from 0·11 to 0·29 among different populations. Individuals who are homozygous or compound heterozygotes have plasma concentrations of less than 1% of the wild-type concentrations and heterozygotes have plasma concentrations of about 10% of the wild-type levels. Variant structural alleles of MBL have been associated with increased susceptibility to various infections in both children [12,15–17] and adults [18]. Variants in the 5′ flanking sequences of the MBL gene have also been identified. Some promoter haplotypes appear to influence plasma MBL concentrations, although this may reflect linkage between promoter polymorphisms and structural variants in exon 1 [19,20].Whereas MBL polymorphisms are associated with susceptibility to infection, TNF and LTA polymorphisms are associated with severity of infection. Elevated maternal and fetal levels of TNF and the TNF (− 308) polymorphism are associated with premature membrane rupture and preterm delivery [21,22]. These results suggest a possible association between TNF polymorphisms and recurrent late miscarriage due to genital tract infection. Individuals with the TNF (− 308) polymorphism are at increased risk of cerebral malaria [23], mucocutaneous leishmaniasis [24] and severe meningococcal disease [25]. Individuals heterozygous for the TNF (− 238) polymorphism may be at increased risk of severe malaria and tuberculosis [26]. Homozygotes for LTA codon 26 and LTA (+ 252) polymorphisms have been associated with mucocutaneous leishmaniasis [24] and a poor prognosis in severe sepsis [27]. However, TNF and LTA polymorphisms are in linkage disequilibrium and exist as gene haplotypes rather than as polymorphisms in isolation of each other.
In this study we report the frequencies of MBL, TNF and LTA haplotypes in recurrent miscarriage women and their partners and women with normal obstetric histories and their partners. A new assay based upon hybridization to immobilized sequence-specific oligonucleotides (SSO) was used to rapidly detect nine MBL, two TNF and two LTA sequence variants. The frequencies of the MBL haplotypes known to influence serum MBL levels were similar in the recurrent miscarriage and control groups. Furthermore, TNF and LTA haplotypes were not associated with recurrent miscarriage.
Materials and methods
Recurrent miscarriage and control groups
Couples were recruited consecutively from the antenatal and recurrent miscarriage clinics at St Mary's Hospital, London and the antenatal clinic of the Royal Sussex County Hospital, Brighton, UK. Local ethics committees approved the study. Written informed consent was obtained from all subjects. The couples were interviewed about their gynaecological and obstetric history in current and previous relationships. Three hundred and fifty-one subjects were recruited, from whom age-matched recurrent miscarriage and control groups were selected. All were Caucasian subjects. A 5-ml venous blood sample was collected from each subject in EDTA and stored at −80°C until analysis. Couples attending the recurrent miscarriage clinic were investigated according to a standard protocol [28]. Seventy-six couples with a history of three consecutive miscarriages in that relationship were suitable for inclusion (mean maternal age 34 years, range 22–44). In brief, the exclusion criteria were the presence of a chromosomal abnormality in either partner, the primary antiphospholipid antibody syndrome in the female partner (two positive test results at least 6 weeks apart) or the presence of a uterine anatomical abnormality. The control group of 69 Caucasian couples with no history of miscarriage and at least one previous live birth were recruited from the antenatal clinics in London and Brighton (mean 32, range 22–45).
Mutation analysis
DNA was isolated from whole blood by standard methods including proteinase k digestion and phenol–chloroform extraction. Genotyping was performed by two methods without knowledge of the clinical data.
Standard assay
The details have been published elsewhere [17]. Briefly, a 330-bp fragment of exon 1 of the MBL gene was amplified by the polymerase chain reaction (PCR). Codon 54 mutations were detected by restriction digestion of the PCR product with Ban I. Codon 52 mutations were detected by nested allele-specific PCR.
Immobilized SSO assay
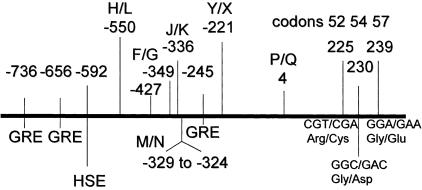
A total of 13 biallelic sites were genotyped using a multiplex PCR amplification followed by hybridization to immobilized oligonucleotide probes with colourimetric detection. Nine of these sites were within the MBL gene: three structural polymorphisms at codons 52, 54 and 57, the promoter polymorphisms at −550 (H/L), −221 (X/Y), −349 and −336, the deletion between −324 to −329; and the polymorphism at position +4 in the 5′ untranslated region of the gene [20] (Fig. 1). The assay does not distinguish between the MBL haplotypes LX/HY and LY/HX; however, HX is rare. The remaining four sites were within TNF and LTA genes, the (−308) and (−238) polymorphisms in the TNF [29,30] and the codon 26 and NcoI/252 polymorphisms in the LTA gene [31].
Fig. 1.
Map of 5′ flanking sequence and exon 1 of the MBL gene. The positions of the variable sites in the 5′ flanking sequences are shown as H/L, M/N, F/G, J/K, deletion -324 to -329, Y/X and P/Q. The positions of the structural variants in exon 1 at codons 52, 54 and 57 are shown. Consensus sequences for glucocorticoid-responsive elements (GRE) and a heat-shock promoter element (HSE) are present in the 5′ sequences.
The multiplex PCR pool consisted of six pairs of biotinylated primers. Three primer pairs amplified fragments of between 130 and 305 bp from the promoter and exon 1 regions of the MBL gene. One primer pair amplified a 220-bp fragment of the TNF promoter region and two primers amplified 140- and 160-bp fragments from the LTA gene. Primer concentrations were adjusted for generally comparable yields of all targets and ranged from 0·2 to 0·425 µm.
Approximately 25 ng of total genomic DNA was used for each assay. In addition to the primer pool, each 50-μl reaction contained 15 mm Tris-HCl (0.15 m stock, pH 8.0 at 2–5°C), 50 mm KCl, 3.0% DMSO (v/v), 0.1 mm dATP, 0.1 mm dCTP, 0.1 mm dGTP, 0.2 mm dUTP, 1.7 mm MgCl2, and 6 units of AmpliTaq Gold™ DNA polymerase (Applied Biosystems, Foster City, CA, USA). Samples were amplified in a GeneAmp® PCR System 9600 (Applied Biosystems) using a 1·6-h thermal cycling profile: initial hold of 94°C for 12·5 min, then 33 cycles of 95°C for 10 s, 60°C for 30 s, 70°C for 20 s and a final extension step of 68°C for 5 min.
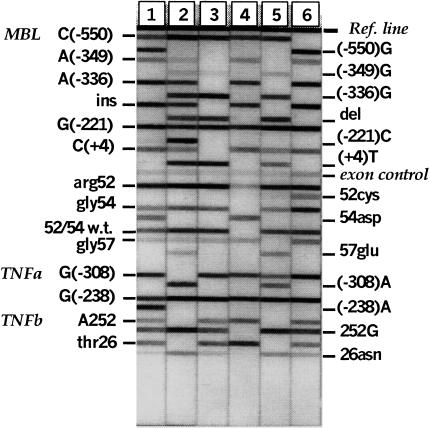
In general, two probes were designed for each biallelic site, to detect and distinguish between the variant sequences. Specificity and sensitivity were optimized using genomic DNA of known genotype. For codon 52, non-specific signals from the 52cys probe were observed for some samples carrying either the 54asp or 57glu sequence variation. Consequently, pooled pairs of probes were used to detect each allele at codon 52. In addition, one probe was designed specifically for the arg52–gly54 sequence. Concentrations of all probes were chosen to achieve signal balance between alleles at each variable site and for generally comparable intensities among all of the loci. A probe complementary to the MBL exon 1 amplicon region upstream of codon 52 was used at a very low concentration to serve as a signal intensity control. Probes were conjugated at their 5′-ends to bovine serum albumin (BSA) by methods similar to [32] and then applied in a linear array to sheets of backed nylon membrane using a Linear Striper and Multispense2000™ controller (IVEK, N. Springfield, VT, USA). Each sheet was cut into probe strips approximately 7·5 cm long and 4–5 mm in width. Amplified alleles were detected essentially as described in [33], except at 45°C and with a stringent wash buffer of 1× SSPE and 0·3% SDS. Briefly, PCR product pools were denatured then hybridized to the immobilized probe strips. Detection of bound PCR product was based upon streptavidin–horseradish peroxidase conjugate and substrates 3,3′,5,5′-tetramethylbenzidine and H2O2. Typical results are shown in Fig. 2.
Fig. 2.
Immobilized sequence-specific oligonucleotide assay which rapidly genotypes nine MBL, two TNF and two LTA sequence variants. Typical results for six strips are shown. The strips are aligned to the Ref.line on a reference grid and the genotypes of the DNA sample are read off.
Sequencing
For samples with discordant results between the ‘standard’ and ‘immobilized SSO’assays, the exon 1 region of MBL was amplified and sequenced using Big Dye Terminator chemistry on an ABI310 (Applied Biosystems, Foster City, CA, USA).
MBL serum assay
In 146 subjects serum MBL concentrations were measured. Serum MBL levels were determined using an enzyme linked immunosorbent assay (ELISA) with a mouse monoclonal antibody against MBL (Statens Serum Institut, Denmark). The details have been published elsewhere [13]. Purified recombinant human MBL [34] was used as the standard. This standard was later calibrated against a serum sample of known MBL concentration kindly provided by Prof. M. Turner (London).
Statistics
A Caucasian population has a 25% prevalence of heterozygotes for MBL structural alleles. We assumed a similar prevalence in a normal antenatal population. A prestudy calculation indicated that to detect a significant difference in the recurrent miscarriage group with an 80% level of confidence required a sample size of 150. Comparisons between the groups were made with the chi-squared test except in the case of small numbers, where Fisher's exact test was used. The haplotypes represented by the TNF (− 238) and (− 308) sites together with LTA codon 26 and nucleotide 252 were estimated using Arlequin (version 2·0,http://anthro.unige.ch/arlequin) [35]. Arlequin was also used to confirm Hardy–Weinberg equilibrium. The distribution of the maximum-likelihood haplotype frequencies between cases and controls (males and females compared separately) and between the controls and a UK population reported by Fanning et al. [36] were compared using the log likelihood ratio or G statistic in a row by column test for heterogeneity [37].
Results and discussion
Until this study the association between recurrent miscarriage and MBL has been based on comparing serum MBL concentrations in recurrent miscarriage couples and blood donor controls [7–9]. There are conflicting data on the role of paternal plasma MBL concentrations in recurrent miscarriage [7,8]. MBL is an acute phase protein [38] and there is a marked overlap of serum concentrations in wild-type subjects and subjects heterozygous for structural variant alleles. The overlap and the effects of promoter haplotypes make it impossible to reliably predict genotype from serum concentrations or vice versa. Furthermore, MBL structural variants are inherited as autosomal co-dominant characteristics so that genotyping the parents allows a statistical prediction of fetal genotype. This study was restricted to Caucasians because the frequency of variant MBL alleles differs in other ethnic populations.
Comparison between genotyping assays
The 351 Caucasian individuals were initially genotyped by our standard methods of restriction digestion and nested allele-specific PCR. The samples were then reanalysed using the immobilized SSO assay. The assays gave concordant results in 308 samples (88%). Of the 43 samples (12%) that gave discordant results, 40 were available for sequencing that showed that the immobilized SSO assay was correct in 34 (85%). In six samples (2%) where the immobilized SSO assay was wrong, review showed the misreading to be due to faint bands. The three samples not available for sequencing were excluded from subsequent analysis. The results indicate that this non-radioactive immobilized SSO assay is more reliable than the standard methods of restriction digestion and nested allele-specific PCR. The linear array format also permits a high throughput of samples.
MBL haplotype frequencies in Caucasian antenatal control group
The haplotype frequencies in 138 normal Caucasians recruited from antenatal clinics are shown in Table 1. LX, LY and HY are promoter haplotypes that are associated with low, medium and high serum MBL levels, respectively. G, K and N are recently-described promoter variants [20] that usually form a haplotype with LYQ (Fig. 1). Variants at codon 52, 54 and 57 are structural variants that have a profound lowering effect on serum MBL levels. The haplotype frequencies in antenatal controls were similar to those reported in a UK control population [39]. It is noteworthy that these normal antenatal patients and their partners had frequencies of variable MBL sites similar to blood donors who are subject to the criticism that they are positively selected for good health.
Table 1.
MBL gene haplotypes in 138 controls
| MBL producer phenootype | Haplotype | Female | Male | Total | % |
|---|---|---|---|---|---|
| High | HYA/HYA | 6 | 8 | 14 | 10 |
| HYA/LYA | 15 | 10 | 25 | 18 | |
| HYA/LXA | 5 | 10 | 15 | 11 | |
| LYA/LYA | 7 | 1 | 8 | 6 | |
| LYA/LXA | 6 | 8 | 14 | 10 | |
| Medium | LXA/LXA | 3 | 1 | 4 | 3 |
| HYA-54asp | 10 | 8 | 18 | 13 | |
| LYA-54asp | 2 | 9 | 11 | 8 | |
| HYA-57gluq1 | 2 | 3 | 2 | ||
| LYA-57glu | 1 | 1 | 1 | ||
| HYA-52cys | 1 | 2 | 3 | 2 | |
| LYA-52cys | 3 | 1 | 4 | 3 | |
| Low | LXA-54asp | 3 | 6 | 9 | 7 |
| LXA-57glu | 1 | 1 | 1 | ||
| LXA-52cys | 3 | 3 | 2 | ||
| Homozygotes/ compoundheterozygotes | 2 | 3 | 5 | 4 |
A, wild-type. See Fig. 1 and text for details.
We then examined the effects of the variable MBL sites on serum MBL levels. The data (not shown) confirmed previous reports [13,20] that the structural variants had the most profound lowering effects on serum MBL levels. LX, LY and HY haplotypes were associated with low, medium and high serum levels although this may reflect linkage between promoter polymorphisms and structural variants in exon 1; the GKN haplotype did not influence MBL levels.
Association of MBL and TNF-LTA haplotypes with recurrent miscarriage
Comparison of recurrent miscarriage couples and control couples showed no differences in the number of structural variant MBL genes (data not shown) or the frequency of low, medium or high MBL level haplotypes in both partners (Table 2). The frequency of very serum low MBL haplotypes (structural variant homozygotes/compound heterozygotes and LX/structural heterozygotes) was identical in the two groups (13%). There was no significant difference between recurrent miscarriage couples and control couples for any combination of mutations using Fisher's exact test. The frequencies of codon 52 and 54 mutations did not differ significantly between males and females or for females between the two groups. The proportions of patients homozygous for structural variant alleles were similar in the recurrent miscarriage (4·5%) and control groups (3·5%). The proportions of couples with ≥2 structural variant alleles were similar in both groups (21% versus 23%). Thirty women in the recurrent miscarriage group had a history of late miscarriage (between 13 and 24 weeks of gestation) or stillbirths. The proportion of MBL variant alleles in this group was not significantly different from the controls.Arlequin predicted the same four major TNF and LTA haplotypes reported by Fanning et al. [36].
Table 2.
MBL gene haplotypes in recurrent miscarriage and controls
| Female | Male | UK controls | |||||||
|---|---|---|---|---|---|---|---|---|---|
| RM | Controls | RM | Controls | Ref. [39] | |||||
| Haplotypes | n | % | n | % | n | % | n | % | % |
| High MBL producing | 36 | 47 | 39 | 56 | 47 | 62 | 37 | 54 | 53 |
| Medium MBL producing | 28 | 37 | 21 | 30 | 20 | 26 | 23 | 33 | 32 |
| Low MBL producing | 12 | 16 | 9 | 13 | 9 | 12 | 9 | 13 | 11 |
RM, recurrent miscarriage (n = 76 couples); controls n = 69 couples. Haplotypes: high MBL producing = wild-type (A) + any promoter haplotype except LXA/LXA. Medium MBL producing =LYP-54asp or HYP-52cys or LYQ=57glu + HYA or LYA and LXA/LXA. Low MBL producing = homozygous or compound heterozygous for exon mutations or heterozygous for exon mutation + LXA.
Arlequin also postulated the existence of three other haplotypes, GAasnG, AGthrA and AAasnG; however, the accuracy of predicting very low frequency haplotypes is not high and these other haplotypes were estimated at less than 0·02. No statistically significant differences were observed either between the control couples and the previously described UK control population [36] or between recurrent miscarriage couples and control couples (Table 3). Although the sample size was small there is no evidence indicating that specific TNF-LTA haplotypes contribute to the risk of recurrent miscarriage.
Table 3.
TNF and LTA haplotype frequencies in recurrent miscarriage and controls
| Female | Male | UK controls | |||
|---|---|---|---|---|---|
| Haplotypes | RM | Controls | RM | Controls | Ref. [36] |
| G G thr A | 0·60 | 0·62 | 0·57 | 0·60 | 0·59 |
| G G asn G | 0·20 | 0·18 | 0·17 | 0·16 | 0·17 |
| G A asn G | 0·18 | 0·16 | 0·20 | 0·19 | 0·18 |
| A G thr Av | 0·02 | 0·03 | 0·04 | 0·05 | 0·06 |
RM, recurrent miscarriage (n = 76 couples); controls n = 69 couples. Four major haplotypes were predicted by Arlequin. The order was chosen to match Fanning et al. [36]: TNF(− 238) – TNF(− 308) – LTA codon 26 – LTA (+ 252). See text for details.
We cannot explain the discrepancy between the earlier reports based upon serum MBL data [7–9] and the genotyping reported here. By assaying both structural and promoter MBL variants all the genetic factors known to influence serum MBL concentrations were determined in this study. Furthermore, since variant allele frequencies were similar in the women and their partners in both groups neither maternal nor fetal MBL deficiency can be invoked as a risk factor for recurrent miscarriage. In conclusion, this study has been unable to demonstrate associations between recurrent miscarriage and genetic polymorphisms of MBL.
Acknowledgments
We thank all the couples that took part in the study and the midwives at St Mary's Hospital for help with sample collection. We thank Miss J. Montgomery, Mr R. Bradley and Mr R. Beard for permission to study patients under their care and the antenatal clinic staff at the Royal Sussex County Hospital, Brighton. Mr Philip Bates provided technical and scientific support and advice. Dr Richard Bellamy and Prof. Adrian Hill (Wellcome Trust Centre for Human Genetics, Oxford) provided control DNA bearing the codon 57 variation. We thank Dr William Klitz (School of Public Health, University of California at Berkeley) for help with the haplotype analysis. This work was supported by Wellbeing and the Save the Baby Charity.
REFERENCES
- 1.Regan L. Sporadic and recurrent miscarriage. In: Grudzinskas JG, O'Brien PMS, editors. Problems in early pregnancy: advances in diagnosis and management. London: RCOG Press; pp. 31–52. [Google Scholar]
- 2.Llahi-Camp JM, Rai R, Ison C, Regan L, Taylor-Robinson D. Association of bacterial vaginosis with a history of second trimester miscarriage. Hum Reprod. 1996;11:1575–8. doi: 10.1093/oxfordjournals.humrep.a019440. [DOI] [PubMed] [Google Scholar]
- 3.Kurki T, Sivonen A, Renkonen O, Savia E, Ylikorkala O. Bacterial vaginosis in early pregnancy and pregnancy outcome. Obstet Gynecol. 1992;80:173–7. [PubMed] [Google Scholar]
- 4.Hay PE, Lamont RF, Taylor-Robinson D, Morgan DJ, Ison C, Pearson J. Abnormal bacterial colonisation of the genital tract and subsequent preterm delivery and late miscarriage. Br Med J. 1994;308:295–9. doi: 10.1136/bmj.308.6924.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McDonald HM, O'Loughlin JA, Vigneswaran R, et al. Impact of metronidazole therapy on preterm birth in women with bacterial vaginosis flora (Gardnerella vaginalis): a randomised, placebo controlled trial. Br J Obstet Gynaecol. 1997;104:1391–7. doi: 10.1111/j.1471-0528.1997.tb11009.x. [DOI] [PubMed] [Google Scholar]
- 6.Carey JC, Klebanoff MA, Hauth JC, et al. Metronidazole to prevent preterm delivery in pregnant women with asymptomatic bacterial vaginosis. National Institute of Child Health and Human Development Network of Maternal-Fetal Medicine Units. N Engl J Med. 2000;342:534–40. doi: 10.1056/NEJM200002243420802. [DOI] [PubMed] [Google Scholar]
- 7.Kilpatrick DC, Bevan BH, Liston WA. Association between mannan binding protein deficiency and recurrent miscarriage. Hum Reprod. 1995;10:2501–5. doi: 10.1093/oxfordjournals.humrep.a136330. [DOI] [PubMed] [Google Scholar]
- 8.Christiansen OB, Kilpatrick DC, Souter V, Varming K, Thiel S, Jensenius JC. Mannan-binding lectin deficiency is associated with unexplained recurrent miscarriage. Scand J Immunol. 1999;49:193–6. doi: 10.1046/j.1365-3083.1999.00473.x. 10.1046/j.1365-3083.1999.00473.x. [DOI] [PubMed] [Google Scholar]
- 9.Kilpatrick DC, Starrs L, Moore S, Souter V, Liston WA. Mannan binding lectin concentration and risk of miscarriage. Human Reprod. 1999;9:2379–80. doi: 10.1093/humrep/14.9.2379. [DOI] [PubMed] [Google Scholar]
- 10.Sumiya M, Summerfield JA. The role of collectins in host defense. Semin Liver Dis. 1997;17:311–8. doi: 10.1055/s-2007-1007207. [DOI] [PubMed] [Google Scholar]
- 11.Thiel S, Vorup Jensen T, Stover CM, et al. A second serine protease associated with mannan-binding lectin that activates complement. Nature. 1997;386:506–10. doi: 10.1038/386506a0. [DOI] [PubMed] [Google Scholar]
- 12.Sumiya M, Super M, Tabona P, et al. Molecular basis of opsonic defect in immunodeficient children. Lancet. 1991;337:1569–70. doi: 10.1016/0140-6736(91)93263-9. [DOI] [PubMed] [Google Scholar]
- 13.Lipscombe RJ, Sumiya M, Hill AV, et al. High frequencies in African and non-African populations of independent mutations in the mannose binding protein gene. Hum Mol Genet. 1992;1:709–15. doi: 10.1093/hmg/1.9.709. [DOI] [PubMed] [Google Scholar]
- 14.Madsen HO, Garred P, Kurtzhals JA, et al. A new frequent allele is the missing link in the structural polymorphism of the human mannan-binding protein. Immunogenetics. 1994;40:37–44. doi: 10.1007/BF00163962. [DOI] [PubMed] [Google Scholar]
- 15.Garred P, Madsen HO, Hofmann B, Svejgaard A. Increased frequency of homozygosity of abnormal mannan-binding-protein alleles in patients with suspected immunodeficiency. Lancet. 1995;346:941–3. doi: 10.1016/s0140-6736(95)91559-1. [DOI] [PubMed] [Google Scholar]
- 16.Summerfield JA, Sumiya M, Levin M, Turner MW. Association of mutations in mannose binding protein gene with childhood infection in consecutive hospital series. Br Med J. 1997;314:1229–32. doi: 10.1136/bmj.314.7089.1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hibberd ML, Sumiya M, Summerfield JA, Booy R, Levin M. Association of variants of the gene for mannose binding lectin with susceptibility to meningococcal disease. Lancet. 1999;353:1049–53. doi: 10.1016/s0140-6736(98)08350-0. 10.1016/s0140-6736(98)08350-0. [DOI] [PubMed] [Google Scholar]
- 18.Summerfield JA, Ryder S, Sumiya M, et al. Mannose binding protein gene mutations associated with unusual and severe infections in adults. Lancet. 1995;345:886–9. doi: 10.1016/s0140-6736(95)90009-8. [DOI] [PubMed] [Google Scholar]
- 19.Madsen HO, Garred P, Thiel S, et al. Interplay between promoter and structural gene variants control basal serum level of mannan-binding protein. J Immunol. 1995;155:3013–20. [PubMed] [Google Scholar]
- 20.Madsen HO, Satz ML, Hogh B, Svejgaard A, Garred P. Different molecular events result in low protein levels of mannan-binding lectin in populations from Southeast Africa and South America. J Immunol. 1998;161:3169–75. [PubMed] [Google Scholar]
- 21.Ferriman EL, Simpson NAB, Reid JG, et al. Fetal carriage of TNF*2 is associated with premature membrane rupture and subsequent preterm delivery. J Soc Gynecol Invest. 2000;7(Suppl.):127A. [Google Scholar]
- 22.Roberts AK, Monson-Bordonoba F, Van Deerlin PG, et al. Association of polymorphism within the promoter of the tumor necrosis factor alpha gene with increased risk of preterm premature rupture of the fetal membranes. Am J Obstet Gynecol. 1999;180:1297–302. doi: 10.1016/s0002-9378(99)70632-0. [DOI] [PubMed] [Google Scholar]
- 23.McGuire W, Hill AVS, Allsopp CEM, Greenwood BM, Kwiatkowski D. Variations in the TNF-α promoter region associated with susceptibility to cerebral malaria. Nature. 1994;371:508–11. doi: 10.1038/371508a0. [DOI] [PubMed] [Google Scholar]
- 24.Cabrera M, Shaw M, Sharples C, et al. Polymorphism in tumor necrosis factor genes associated with mucocutaneous leishmaniasis. J Exp Med. 1995;182:1259–64. doi: 10.1084/jem.182.5.1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nadel S, Newport MJ, Booy R, Levin M. Variation in the TNF-α gene promoter region may be associated with death from meningococcal disease. J Infect Dis. 1996;174:878–80. doi: 10.1093/infdis/174.4.878. [DOI] [PubMed] [Google Scholar]
- 26.Hill AVS. Genetic susceptibility to malaria and other infectious diseases: from the MHC to the whole genome. Parasitology. 1996;112:S75–S84. doi: 10.1017/s003118200007668x. [DOI] [PubMed] [Google Scholar]
- 27.Stuber F, Petersen M, Bokelmann F, Schade U. A genomic polymorphism within the tumor necrosis factor locus influences plasma tumor necrosis factor alpha concentrations and outcome of patients with severe sepsis. Crit Care Med. 1996;24:381–4. doi: 10.1097/00003246-199603000-00004. [DOI] [PubMed] [Google Scholar]
- 28.Clifford K, Rai RS, Watson H, Regan L. An informative protocol for the investigation of recurrent miscarriage: preliminary experience of 500 consecutive cases. Hum Reprod. 1994;9:1328–32. doi: 10.1093/oxfordjournals.humrep.a138703. [DOI] [PubMed] [Google Scholar]
- 29.D'Alfonso S, Richiardi PM. A polymorphic variation in a putative regulation box of the TNFA promoter region. Immunogenetics. 1994;39:150–4. doi: 10.1007/BF00188619. [DOI] [PubMed] [Google Scholar]
- 30.Wilson AG, di Giovine FS, Blakemore AIF, Duff GW. Single base polymorphism in the human tumour necrosis factor alpha (TNF alpha) gene detectable by NcoI restriction of PCR product. Hum Molec Genet. 1992;1:353. doi: 10.1093/hmg/1.5.353. [DOI] [PubMed] [Google Scholar]
- 31.Messer G, Spengler U, Jung MC, et al. Polymorphic structure of the tumor necrosis factor (TNF) locus: an NcoI polymorphism in the first intron of the human TNF-beta gene correlates with a variant amino acid in position 26 and a reduced level of TNF-beta production. J Exp Med. 1991;173:209–19. doi: 10.1084/jem.173.1.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tung CH, Rudolph MJ, Stein S. Preparation of oligonucleotide-peptide conjugates. Bioconjug Chem. 1991;2:464–5. doi: 10.1021/bc00012a015. [DOI] [PubMed] [Google Scholar]
- 33.Cheng S, Grow MA, Pallaud C, et al. A multilocus genotyping assay of candidate markers of cardiovascular disease risk. Genome Res. 1999;9:936–49. doi: 10.1101/gr.9.10.936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tabona P, Mellor A, Summerfield JA. Mannose binding protein is involved in first-line host defence: evidence from transgenic mice. Immunology. 1995;85:153–9. [PMC free article] [PubMed] [Google Scholar]
- 35.Excoffier L, Slatkin M. Maximum-likelihood estimation of molecular haplotype frequencies in a diploid population. Mol Biol Evol. 1995;12:921–7. doi: 10.1093/oxfordjournals.molbev.a040269. [DOI] [PubMed] [Google Scholar]
- 36.Fanning GC, Bunce M, Black CM, Welsh KI. Polymerase chain reaction haplotyping using 3′ mismatches in the forward and reverse primers: application to the biallelic polymorphisms of tumour necrosis factor and lymphotoxin a. Tissue Antigens. 1997;50:23–31. doi: 10.1111/j.1399-0039.1997.tb02829.x. [DOI] [PubMed] [Google Scholar]
- 37.Sokal RR, Rohlf FJ. Biometry. 3. New York: WH Freeman; 1995. [Google Scholar]
- 38.Thiel S, Holmskov U, Hviid L, Laursen SB, Jensenius JC. The concentration of the C-type lectin, mannan-binding protein, in human plasma increases during an acute phase response. Clin Exp Immunol. 1992;90:31–5. doi: 10.1111/j.1365-2249.1992.tb05827.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mullighan CG, Marshall SE, Welsh KI. Mannose binding lectin polymorphisms are associated with early age of disease onset and autoimmunity in common variable immunodeficiency. Scand J Immunol. 2000;51:111–22. doi: 10.1046/j.1365-3083.2000.00697.x. 10.1046/j.1365-3083.2000.00697.x. [DOI] [PubMed] [Google Scholar]