Abstract
The aim of this study was to determine if the distribution in vivo of CD4+CD45RA+/CD45RO− (naive), CD4+CD45RA+/CD45RO+ (Ddull) and CD4+CD45RO+ (memory) lymphocytes differs in malnourished infected and well-nourished infected children. The expression of CD45RA (naive) and CD45RO (memory) antigens on CD4+ lymphocytes was analysed by flow cytometry in a prospectively followed cohort of 15 malnourished infected, 12 well-nourished infected and 10 well-nourished uninfected children. Malnourished infected children showed higher fractions of Ddull cells (11·4 ± 0·7%) and lower fractions of memory cells (20·3 ± 1·7%) than the well-nourished infected group (8·8 ± 0·8 and 28·1 ± 1·8%, respectively). Well-nourished infected children showed increased percentages of memory cells, an expected response to infection. Impairment of the transition switch to the CD45 isoforms in malnourished children may explain these findings, and may be one of the mechanisms involved in immunodeficiency in these children.
Keywords: CD4CD45RA, CD4CD45RA/CD45RO, CD4CD45RO, malnutrition, flow cytometry
Introduction
In Latin America, protein-energy malnutrition (PEM) associated infections are considered as directly or indirectly responsible for one third of the deaths in children under 6 years of age [1]. It is well known that malnourished individuals are more susceptible to infection, and are thus considered as immunodeficient [2–4]. The two major components of cause-specific mortality in malnourished children in developing countries are pneumonia and diarrhoea [5]. In a previous study, we found that all T lymphocyte subsets of malnourished children showed impaired activation capability in vitro, as CD3+CD69+, CD4+CD69+ and CD8+CD69+ lymphocyte percentages were significantly lower in these children than those observed in well-nourished infected children [6].
As T lymphocyte subsets of malnourished children show an impaired response to mitogens in vitro, we considered it important to characterize CD4+ T lymphocyte differentiation assessing the expression of different CD45RA and CD45RO antigen isoforms in vivo, in malnourished children. Hodge et al. in 1998 [7] reported that the CD45RA isoform is brightly expressed on newborn naive T cells and the CD45RO isoform marks memory T cells, which respond to recall antigenic stimulation by proliferation and provide helper activate for antigen-specific antibody synthesis. Naive cells acquire CD45RO and lose CD45RA expression, following in vitro antigenic stimulation [8–10]. Additionally these studies have shown that expression of dual positive CD45RA/CD45RO occurs after stimulation of adult T lymphocytes, before conversion to single CD45RO isoform expression. These cells have been shown to be important in the diagnostics and prognostics of several diseases [11–13].
The aim of this study was to determine if peripheral blood from malnourished infected children shows alterations to the in vivo distribution of CD4+CD45RA+/CD45RO−, CD4+ CD45RA+/CD45RO+ and CD4+CD45RA−/CD45RO+ lymphocytes as compared to well-nourished infected children.
Subjects and methods
Subjects
The study groups consisted of 12 well-nourished infected (WNI) and 15 malnourished infected children (MNI) of both sexes who were inpatients at the Xochimilco and Iztapalapa Paediatric Federal District Department (DDF) Hospitals in Mexico City, suffering from severe bacterial infection: respiratory and/or gastrointestinal. The WNI children had normal weight and height according to age, from 7 to 34 months. Four children showed respiratory infections, three gastrointestinal and five both respiratory and gastrointestinal infections (mixed). The MNI group who were primary admitted for severe infections aged 6–24 months. Six children with second degree malnutrition (weight/height deficit >25% and <40% according to age). Nine with severe (third degree) malnutrition, six with marasmus showing severe weight/height deficit (> 40% for age) and three with kwashiorkor, one showing only 2·3% weight/height deficit due to the presence of oedema. Three of these children had bacterial respiratory infection, seven gastrointestinal and five mixed infections. The severity of malnutrition was assessed on clinical signs and symptoms of malnutrition, as well as weight/height deficit according to the established values for Mexican children [14]. Bacterial infection was diagnosed rigorously on clinical data and laboratory routine tests. The children that were referred with viral infection or clinical suspicion of tuberculosis, cardiac diseases, or allergic diseases were excluded from the study. A group of 10 well-nourished uninfected children of both sexes who were outpatients at the same hospital were studied as controls. These children had normal weight/height ratios according to age. The study was approved by The Medical Ethics Committee of The General Direction of Medical Services (Federal District Department, Mexico).
Cell preparation and staining
Whole blood samples were collected using Vacutainer™ blood collection tubes (Becton Dickinson Vacutainer Systems, Franklin Lakes, NJ) with sodium heparin anticoagulant on the day of hospital admission and prior to treatment. The samples were processed on the day of collection. Cell viability was determined with double fluorescein diacetate and ethidium bromide staining [15]. More than 95% of the cells were viable. Lymphocyte subsets were determined using standard techniques for simultaneous, direct, 3-colour immunofluorescence staining [16] with minor modifications. Commercially conjugated antibodies to fluorescein isothiocyanate (FITC), phycoerythrin (PE) and peridin-chlorophyll protein (PerCP) dyes (Becton Dickinson Immunocytometry System, San Jose, CA) were used, including: (1) isotype control; (2) CD45 FITC/CD14 PE; (3) CD45RA FITC; (4) CD45RO PE; and (5) CD4 PerCP. Twenty microlitres of each monoclonal reagent pair were added to 100 μl of whole blood in 12 × 75 mm test tubes. Whole blood and monoclonal antibody mixture was gently stirred and incubated at room temperature for 20 min in the dark. Then, 2 ml of (1X) FACS brand lysing solution was added and incubated for 10 min at room temperature in the dark. The cell suspension was centrifuged for 5 min at 300 × g, the supernate was aspirated and the pellet resuspended in 2 ml PBS, centrifuged 5 min at 200 × g, the supernate was aspirated and the pellet resuspended in 1% paraformaldehyde for fixation.
Flow cytometry analysis
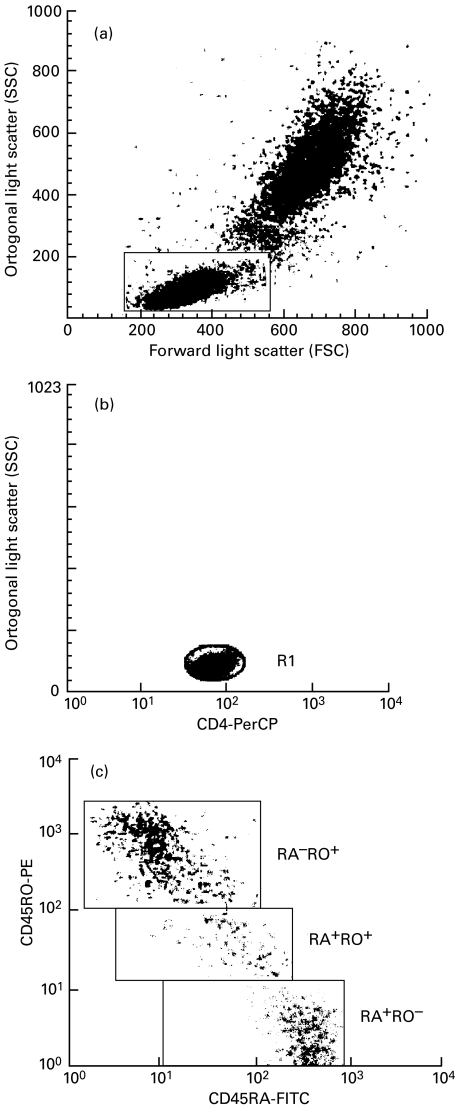
Data were acquired with a FACScan flow cytometer (Becton Dickinson Immunocytometry System, San Jose, CA). Instrument set-up was performed using CaliBRITE brand beads and AutoCOMP brand software. A minimum of 10 000 total events was acquired, using LYSYS II software and the LeucoGATE brand reagent (CD45/CD14) to establish an analysis gate including at least 95% of lymphocytes and no more than 5% of monocytes in the sample. Markers for determining positive and negative cells for reagents were set by LYSYS II software using conjugated antibodies of irrelevant specificity as negative controls. Figure 1 shows how gates were set on the FSC-SSC to obtain all lymphocytes, FL3-SSC distribution for gate on CD4+ cells, also a representative dot plot of the distribution of naive Ddull and memory cells. The absolute cells number (× 106/l) for each lymphocyte subset was calculated by multiplying the corrected relative value by the total lymphocyte count.
Fig. 1.
(a) The lymphocyte gate set in the FSC–SSC distribution. (b) The FL3–SSC distribution, the (R1) gate was set on to restrict the analysis to CD4 positive cells. (c) The FL1–FL2 distribution on the cells passed by the window of (a) for all lymphocytes or by the window of (b) to restrict the analysis on the RA/RO phenotype to CD4 positive cells. FL1 = CD45RA-FITC; FL2 = CD45RO-PE; FL3 = CD4-PerCP.
Statistical analysis
Results are expressed as mean ± standard error, and compared using the anova and nonparametric Wilcoxon/Kruskal–Wallis tests, with the JMP statistics program. Statistical significance was considered when P < 0·05.
Results
Clinical characteristics on hospital admission
The WNI and MNI children with gastrointestinal and mixed infections showed diarrhoea, fever and several degrees of dehydration. Respiratory infection was associated with fever, cough and respiratory distress. Mean haemoglobin concentration was 11·5 ± 0·7 g/l in WNI and 8·3 ± 1·0 g/l in MNI children. Mean total leucocyte counts were as expected for age (9·03 ± 1·6 × 106 and 10·47 ± 3·8 × 106 cells/l in WNI and MNI children, respectively). Circulating leucocyte counts showed 55·11 ± 10·6% lymphocytes and 43·78 ± 10·3% neutrophils in WNI children, and 46·14 ± 10·3% lymphocytes and 53·14 ± 10·3% neutrophils in the MNI group. These percentage values were slightly different to those expected for age [17].
Distribution of cells on RA/RO subsets
The mean percentages and absolutes values of naive, memory and Ddull lymphocytes for each group and the results of the statistical analyses are presented in Table 1. In general, the distribution of absolute values for lymphocyte subsets showed the same tendency as relative values. CD45RA+, CD45RA+/CD45RO+ and CD45RO+ surface antigen expression on nonactivated peripheral lymphocytes showed no significant differences among the three groups. The mean CD45RA+ (naive) cells were 75·3 ± 2·8% in WN, 77·3 ± 1·3% in WNI and 72·6 ± 2·3% in MNI children. The mean CD45RO+ (memory) cells were 13·6 ± 1·8%, 11·1 ± 1·2% and 11·5 ± 1·0% in WN, WNI and MNI children, respectively. and the mean CD45RA+/CD45RO+ (Ddull) cells were 7·5 ± 1·4%, 5·5 ± 0·5 and 7·0 ± 0·9% in the WN, WNI and MNI groups, respectively.
Table 1.
Percentage and absolute (per 106/l, in bold) numbers of peripheral blood naive, memory and Ddull lymphocyte subsets in well-nourished (WN), well-nourished infected (WNI) and malnourished infected (MNI) children
| Cell type | WN (n = 10) | WNI (n = 12) | MNI (n = 15) |
|---|---|---|---|
| CD45RA+ | 75·3 ± 2·8 | 77·3 ± 1·3 | 72·6 ± 2·3 |
| 3535 ± 444 | 3560 ± 280 | 2728 ± 211 | |
| CD45RO+ | 13·6 ± 1·8 | 11·1 ± 1·2 | 11·5 ± 1·0 |
| 577 ± 68 | 472 ± 45 | 430 ± 70 | |
| CD45RA+CD45RO+ | 7·5 ± 1·4 | 5·5 ± 0·5 | 7·0 ± 0·9 |
| 301 ± 45 | 242 ± 29 | 277 ± 54 | |
| CD4+CD45RA+ | 71·4 ± 1·9 | 62·3 ± 1·7* | 68·2 ± 2·2 |
| 3339 ± 401 | 2756 ± 246 | 2501 ± 129 | |
| CD4+CD45RO+ | 19·4 ± 1·5 | 28·1 ± 1·8* | 20·3 ± 1·7** |
| 778 ± 115 | 1297 ± 119* | 781 ± 119** | |
| CD4+CD45RA+CD45RO+ | 8·8 ± 1·0 | 8·8 ± 0·8 | 11·4 ± 0·7**# |
| 376 ± 37 | 434 ± 70 | 509 ± 52 |
Data are expressed as mean ± s.e.
†Significant difference from WN group, CD4+CD45RA+ (P < 0·02), CD4+CD45RO+ (P < 0·005 relative and P < 0·01 absolute values), CD4+CD45RA+/CD45RO+ MNI (P < 0·02).
Significant difference from WNI group, CD4+CD45RO+ (P < 0·005 relative and P < 0·01 absolute numbers), CD4+CD45RA+/CD45RO+ (P < 0·02).
The contrast of CD45RA and CD45RO isoform expression on CD4+ T lymphocytes in the WN and WNI groups showed that the mean CD4+CD45RA+ cells were significantly lower in WNI (62·3 ± 1·7%) than in WN children (71·4 ± 1·9%; P < 0·02), while the mean CD4+CD45RO+ cells were higher in WNI (28·1 ± 1·8%) than in WN children (19·4 ± 1·5%, P < 0·005). No significant difference between well-nourished groups in CD4+CD45RA+/CD45RO+ cells was detected (8·8 ± 0·8% versus 8·8 ± 1·0%, respectively).
The comparison of CD45RA+ antigen expression on CD4+ lymphocytes in WNI and MNI children showed no significant differences (62·3 ± 1·7% versus 68·2 ± 2·2%, respectively). However, WNI children showed significantly higher CD4+CD45RO+ cell fraction than in MNI children (28·1 ± 1·8% versus 20·3 ± 1·7%, P < 0·005). The mean CD4+CD45RA+/CD45RO+ cells were significantly lower in WNI than in MNI children (8·8 ± 0·8% versus 11·4 ± 0·7%, P < 0·02), similar data were observed in WN than in MNI children (8·8 ± 1·0% versus 11·4 ± 0·7%, P < 0·02).
The analysis of CD45 isoform expression in children with different types of infection revealed significant differences only in the CD4+CD45RO+ cells, which was higher in WNI children with mixed infections (31·0 ± 3·6%, n = 5) than in MNI children with mixed infections (19·0 ± 3·0%, P < 0·05, n = 5). Furthermore, isoform expression analysis of children with different types and degree of malnutrition showed differences only in the CD4+CD45RA+/CD45RO+ fraction, which was higher in children with second degree malnutrition (13·4 ± 1·0%, n = 6) as compared to marasmus children (8·9 ± 1·0%, P < 0·02, n = 6), whose CD4+CD45RA+/CD45RO+ cells were closer to that of WNI children.
Discussion
We analysed the expression of CD45RA, CD45RO and CD45RA/CD45RO antigens in all lymphocytes and CD4+ T lymphocytes in WN, WNI and MNI children in order to identify possible infection-related and malnutrition-related alterations. In general, malnourished children had severe infections, therefore the inclusion of a group of well-nourished infected children also hospitalized by severe infections was considered important. This group may indicate changes in CD45 isoforms related to infections.
The analysis of CD4+ T cells revealed that the presence of infection in WN children decreased the peripheral blood CD4+CD45RA+ cells and increased the CD4+CD45RO+, while the CD4+CD45RA+/CD45RO+ cells remained unaltered in relation to WN non-infected children. This sequence of events is expected, as it is known that CD45RA+ (naive) cells are transformed to CD45RO+ (memory) cells as a response to an antigenic stimulus by an infective organism. This has been observed in in vitro stimulation studies [9,10]. Specific infection diseases have shown alterations at the same level in vivo. Patients with tuberculosis showed higher CD45RO+ T cells in pleural fluid [18]; in patients with chronic hepatitis, within hepatic tissue the CD45RO expression was greater than that of the CD45RA isotype [19]. Cells from patients with severe pneumonia showed a higher proportion of CD45RO+ T cells than healthy volunteers [20]. We cannot make bacteriological determination in infected children, but in previous studies it has been established that enteropathogens most frequent in Mexican children are: Shigella spp., Salmonella spp., Escherichia coli (enteropathogenic) and Campylobacter spp. [21,22]. The most commonly isolated bacteria from invasive respiratory infections are Streptococcus pneumoniae, Haemophilus influenzae and Moraxela (Branhamella) catarrhalis in Mexico [23,24].
The comparative analysis of MNI and WN children showed differences only in the proportion of CD4+ Ddull cells, which were higher in MNI children. However, the comparison between WNI and MNI groups revealed differences that may be related to malnutrition: increase of peripheral CD4+CD45RA+/CD45RO+ cells and decrease of CD4+CD45RO+ cells, as compared to the response observed in WNI children.
The results obtained in this study may indicate that the fraction of Ddull lymphocytes was raised in peripheral blood because they are unable to transform to memory cells. It is known that Ddull cells are transient. After 48 h of in vitro activation, Dd cells increase. After 72 h of culture this cell population was transformed to memory cells [25]. However, CD4+CD45RA+/CD45RO+ cells kept in culture for prolonged periods (14 days) did not switch to CD45RO expression in absence of stimulation of the T-cell receptor. These cells maintained stable expression of both CD45RA and CD45RO isoforms but a small subset reexpress the CD45RA isoforms, and a fraction of the CD45RO cells may gain a Ddull phenotype in the absences of T-cell receptor crosslinking [26]. Moreover, there is evidence that Ddull cells have the capacity to provide help for B cells containing antigen-specific precursor cells, although the concentration of secreted immunoglobulins reached only about half the level of that in the presence of CD45RO cells [26].
The increased proportion of CD4+ Ddull cells and the decrease of CD4+CD45RO+ cells in MN children may be due to an alteration in the switch from the Ddull to memory cells and/or that the CD45RO cells may reexpress a Ddull phenotype. There are several possible factors that may contribute to alter this process in MN children: incapability of specific antigen recognition at the T-cell receptor (TCR); lack of nutrients which would alter the production of new proteins needed to synthesize surface receptor to trigger cell differentiation; or a combination of both mechanisms may explain the immunosupression in malnourished children.
Several reports have indicated that the immune system impairment in severe malnourished children may be related mainly to the altered physiology of lymphocytes, rather than to a fall in the number of peripheral blood T lymphocyte subpopulations. It has been suggested that these are unable to secrete normal quantities of lymphokines which mediate and regulate differentiation, activation and proliferation of lymphocytes to achieve an adequate immunological function [27]. In addition, an impaired early activation of CD4 and CD8 T lymphocytes of malnourished children has been detected [6]. Moreover, in relation to the proliferation kinetics of lymphocytes from malnourished children a faster response was found at 48 h of culture. Nevertheless, this initial response did not persist at 72 h of culture in these children [28,29].
According to the degree of malnutrition the analysis showed a higher percentage of CD4+CD45RA+/CD45RO+ cells in children with second degree malnutrition as compared to marasmus children. The proportion of Ddull cells was similar between marasmus and WNI children. This may be related to the fact that marasmus children have suffered from chronic malnutrition allowing the organism to adapt to this condition. Moreover a previous report concluded that most malnutrition-related deaths are attributable to second degree malnutrition rather than to severe malnutrition [30]. Differences observed between degrees of malnutrition are important findings that require further studies including a greater number of patients.
We found no studies on Ddull and memory cells in malnourished children. In mice, malnutrition may change the ratio of naïve to memory cells by increasing naïve T lymphocytes population in splenic [31], as well as in mesenteric lymph nodes and whole blood [32]. In the same way, increased naive CD45RA+ cells with a parallel decrease in memory CD45RO+ T cells have been observed in elderly humans with severe malnutrition compared to healthy elderly subjects [33]. In this study, we did not find differences in total lymphocyte CD45RA isoforms when comparing WN and MNI children, although the latter group showed a nonsignificant decrease of the CD45RO+ cells.
In other immunodeficiency diseases it has been demonstrated that the CD4+CD45RO+ fraction was lower than in healthy individuals [34] or higher levels of CD4+ Ddull cells [35]. In addition, the role of these cell subsets in the development of bone marrow transplantation has been studied [36].
Naive and memory on CD4+ and CD8+ T lymphocytes have been analysed in different infectious diseases, and has proved to be of prognostic value in certain infections such as HIV [37,38]. CD4+CD45RO+ and CD4+CD45RA+/CD45RO+ isoforms expression is a test which can be used in vivo for screening and monitoring of infection in the newborn [8,39].
The data obtained in our work indicate that the analysis of CD45RO and CD45RA/CD45RO expression on CD4+ lymphocytes is a valuable immunosupression test in malnutrition. This sort of analysis in malnourished infected children may help to understand the mechanisms of immunodeficiency in malnutrition. Further studies using in vitro activation and other surface antigens may be necessary to analyse this finding.
In conclusion, the results obtained in this study show that CD4+ lymphocytes of well-nourished infected children show adequate differentiation capability, as the CD4+CD45RO+ (memory) cell fraction increased and the CD4+CD45RA+ (naive) cell decreased in peripheral blood. In contrast, CD4+ lymphocytes of malnourished children are unable to achieve adequate memory cell fractions to provide protection against external antigens and to provide helper activity for antigen-specific antibody synthesis. These alterations contribute to understanding the mechanisms of immunodeficiency observed in malnourished children.
Acknowledgments
This work was supported in part by CONACyT, México grants 3005P-M9606, F282-M-9208 and 114128/114816, and FOMES, México grant 98–35–28.
REFERENCES
- 1.Cravioto MJ, Ortega ER, Arrieta MR. La nutrición y la salud de las madres y niños mexicanos. México: SSA, Fondo de Cultura Económica; 1990. Desnutrición en la infancia; pp. 251–6. Chapter XXV. [Google Scholar]
- 2.Chandra RK. Nutrition and immunity: lesson from the past and new insights into the future. Am J Clin Nutr. 1991;53:1087–101. doi: 10.1093/ajcn/53.5.1087. [DOI] [PubMed] [Google Scholar]
- 3.Bhaskaram P. Nutritional modulation of immunity to infection. Indian J Pathol Microbiol. 1992;35:392–400. [PubMed] [Google Scholar]
- 4.Chevalier P, Sevilla R, Zalles L, Sejas E, Belmonte G, Parent G, Jambon B. Immuno-nutritional recovery of children with severe malnutrition. Santé. 1996;6:201–8. [PubMed] [Google Scholar]
- 5.Yoon PW, Black RE, Moulton LH, Becker S. y of age in Cebu, Philippines. Am J Clin Nutr. 1997;65:1070–7. doi: 10.1093/ajcn/65.4.1070. The effect of malnutrition on the risk of diarrheal and respiratory mortality in children < 2. [DOI] [PubMed] [Google Scholar]
- 6.Nájera O, González C, Toledo G, Cortés E, López L, Betancourt M, Ortiz R. Early activation of T, B and NK lymphocytes in infected malnourished and infected well-nourished children. J Nutr Immunol. 2001;5:43–51. doi: 10.1046/j.1365-2249.2001.01694.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hodge S, Hodge R, Flower R, Han P. Surface activation markers of T lymphocytes: role in the detection of infection in neonates. Clin Exp Immunol. 1998;113:33–8. doi: 10.1046/j.1365-2249.1998.00626.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Osugi Y, Hara J, Kurahashi H, et al. Age-related changes in surface antigens on peripheral lymphocytes of healthy children. Clin Exp Immunol. 1995;100:543–8. doi: 10.1111/j.1365-2249.1995.tb03735.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fahey JL, Schnelle JF, Boscardin J, Thomas JK, Gorre ME, Aziz N, Sadeghi H, Nishanian P. Distinct categories of immunologic changes in frail elderly. Mech Ageing Dev. 2000;115:1–20. doi: 10.1016/s0047-6374(00)00094-4. [DOI] [PubMed] [Google Scholar]
- 10.Amolot PL, Tahami F, Chinn D, Rawlings E. Activation antigen expression on human T cells. I. Analysis by two-colour flow cytometry of umbilical cord blood, adult blood and lymphoid tissue. Clin Exp Immunol. 1996;105:176–82. doi: 10.1046/j.1365-2249.1996.d01-722.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Plaeger-Marshall S, Hultin P, Bertolli J, et al. Activation and differentiation antigens on T cells of healthy, at-risk and HIV-infected children. J AIDS. 1993;6:984–93. [PubMed] [Google Scholar]
- 12.Sing G, Butterworth L, Chen X, Bryant A, Cooksley G. Composition of peripheral blood lymphocyte populations during different stages of chronic infection with hepatitis B virus. J Viral Hepatitis. 1998;5:83–93. doi: 10.1046/j.1365-2893.1998.00088.x. [DOI] [PubMed] [Google Scholar]
- 13.Pawlik I, Mackiewicz U, Lacki JK, Wirtorowicz K, Konys J. The differences in the expression of CD45 isoforms on peripheral blood lymphocytes derived from patients with seasonal or perennial atopic allergy. Tohoku J Exp Med. 1997;182:1–8. doi: 10.1620/tjem.182.1. [DOI] [PubMed] [Google Scholar]
- 14.Ramos-Galván R. Somatometría Pediátrica. Arch Inv Med (México) 1976;6:5. [Google Scholar]
- 15.Strauss GHS. Non-random cell killing in criopreservation: Implications for performance of the battery of leukocyte tests (BLT) I. Toxic and immunotoxic effects. Mutation Res. 1991;252:1–5. doi: 10.1016/0165-1161(91)90247-6. [DOI] [PubMed] [Google Scholar]
- 16.Kotylo PK, Fineberg NS, Freeman KS, Redmond NI, Charland C. Reference ranges for lymphocyte subsets in paediatric patients. Am J Clin Pathol. 1993;100:111–5. doi: 10.1093/ajcp/100.2.111. [DOI] [PubMed] [Google Scholar]
- 17.Games EJ, Palacios TJL. Introducción a pediatría. México: Mendez Editores; 1995. pp. 557–8. [Google Scholar]
- 18.Montes J, Gambón-Deza F, Pacheco M, Cerdá T. Linfocitos T de memoria durante la infección y enfermedad tuberculosa. Arch Bronconeumología. 1998;34:384–7. doi: 10.1016/s0300-2896(15)30383-5. [DOI] [PubMed] [Google Scholar]
- 19.Fiore G, Galetta V, Piazzolla G, Angarano I, Jirillo E, Schiraldi O, Antonaci S. CD45RA and CD45RO isoforms expression on intrahepatic T-lymphocytes in chronic hepatitis C. Microbios. 1997;92:73–82. [PubMed] [Google Scholar]
- 20.Kodera M, Iwagaki H, Miromoto Y, Kobashi K, Hizuta A, Tanaka N. Involvement of apoptosis in activation-induced cell death of bacterial-reactive human CD45RO+ T cells. Res Commun Mol Pathol Pharmacol. 1999;104:205–18. [PubMed] [Google Scholar]
- 21.Olarte J. El laboratorio de bacteriología intestinal del Hospital Infantil de México contribuciones acerca de la etiología de la diarrea aguda. Bol Med Hospital Infant Mex. 1997;54:591–5. [Google Scholar]
- 22.Pérez MA, Mejía AE. Evolución temporal del patrón de aislamiento de enteropatógenos en diarrea aguda. Bioquimia. 1998;23:873–7. [Google Scholar]
- 23.López-Antuñano FJ, Silva-Sanchez J. Bacterial resistance to antibiotics in acute respiratory infections (ARIs) Arch Med Res. 1997;28:195–203. [PubMed] [Google Scholar]
- 24.Spiritus EM. Diagnóstico y tratamiento de infecciones agudas en vías respiratorias inferiores. Infectología. 1994;14:517–24. [Google Scholar]
- 25.Johannisson A, Festin R. Phenotype transition of CD4+ T cells from CD45RA to CD45RO is accompanied by cell activation and proliferation. Cytometry. 1995;19:343–52. doi: 10.1002/cyto.990190409. [DOI] [PubMed] [Google Scholar]
- 26.Hamann D, Baars PA, Hooibrink B, van Lier RAW. Heterogeneity of the human CD4+ T-cell population: two distinct CD4+ T-cell subsets characterised by coexpression of CD45RA and CD45RO isoforms. Blood. 1996;88:3513–21. [PubMed] [Google Scholar]
- 27.González C, Rodriguez L, Bonilla E, Betancourt M, Siller N, Zumano E, Ortiz R. Electrophoretic analysis of plasmatic and lymphocyte secreted proteins in malnourished children. Med Sci Res. 1997;25:643–6. [Google Scholar]
- 28.Ortiz R, Campos C, Gómez JL, Espinoza M, Ramos-Motilla M, Betancourt M. Sister chromatic exchange and cell proliferation in lymphocytes from infected and non-infected children with severe protein calorie malnutrition. Mutation Res. 1994;312:33–7. doi: 10.1016/0165-1161(94)90006-x. [DOI] [PubMed] [Google Scholar]
- 29.Ortiz R, Campos C, Gómez JL, Espinoza M, Ramos-Motilla M, Betancourt M. Effect of renutrition on the proliferation kinetics of PHA stimulated lymphocytes from malnourished children. Mutation Res. 1995;334:235–41. doi: 10.1016/0165-1161(95)90016-0. [DOI] [PubMed] [Google Scholar]
- 30.Pelletier DI, Frongill EA, Schroeder DG, Habicht J-P. Efectos de la malnutrición en la mortalidad de menores de 5 años en países en desarrollo. Bol Oficina Sanit Panam. 1996;120:425–32. [Google Scholar]
- 31.Woodward BD, Bezanson KD, Hillyer LM, Lee WH. The CD45 RA+ (quiescent) cellular phenotype is overabundant: relative: relative to the CD4RA– phenotype within the involuted splenic T-cell population of weanling mice subjected to wasting protein-energy malnutrition. J Nutr. 1995;125:2471–82. doi: 10.1093/jn/125.10.2471. [DOI] [PubMed] [Google Scholar]
- 32.Woodward B, Hillyer L, Hunt K. T cells with a quiescent phenotype (CD45RA+) are overabundant in the blood and involuted lymphoid tissues in wasting protein and energy deficiencies. Immunology. 1999;96:246–53. doi: 10.1046/J.1365-2567.1999.00694.x. 10.1046/j.1365-2567.1999.00694.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lesourd BM, Mazari L. Immune response during recovery from protein-energy malnutrition. Clin Nutr. 1997;16:37–46. doi: 10.1016/s0261-5614(97)80047-7. [DOI] [PubMed] [Google Scholar]
- 34.Crockard AD, Boyd NAM, McNell TA, McCluskey DR. CD4 lymphocyte subset abnormalities associated with impaired delayed cutaneous hypersensitivity reactions in patients with X-linked agammaglobulinaemia. Clin Exp Immunol. 1992;88:29–34. doi: 10.1111/j.1365-2249.1992.tb03034.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kretowski A, Mysliwiec J, Turowski D, Wysocka J, Kinalska I. Analysis of recently activated, memory and naive lymphocyte T subsets in the peripheral blood of patients with Graves'disease and insulin-dependent diabetes mellitus. Med Bialymst. 1999;44:226–34. [PubMed] [Google Scholar]
- 36.de Vries E, van Tol MJ, van de Bergh RE, Waaijer JL, ten Dam MM, Hermans J, Vossen JM. Reconstitution of lymphocytes subpopulations after paediatric bone marrow transplantation. Bone Marrow Transplant. 2000;25:267–75. doi: 10.1038/sj.bmt.1702141. [DOI] [PubMed] [Google Scholar]
- 37.Benito JM, Zabay JM, Gil J, Bermejo M, Escudero A, Sánchez E. Fernández-Cruz. Quantitative alterations of the functionally distinct subsets of CD4 and CD8 T lymphocytes in asymtomatic HIV infection: changes in the expression of CD45RO, CD45RA, CD11b, CD38, HLA-DR and CD25 antigens. J AIDS Human Retrovirol. 1997;14:128–35. doi: 10.1097/00042560-199702010-00005. [DOI] [PubMed] [Google Scholar]
- 38.Ullum H, Cozzi LA, Victor J, Skinhoj P, Philips AN, Klarlund PB. Increased losses of CD4CD45 cells in late stages of HIV infection is related to increased risk of death: evidence from a cohort of 347 HIV-infected individuals. AIDS. 1997;11:1479–89. doi: 10.1097/00002030-199712000-00012. [DOI] [PubMed] [Google Scholar]
- 39.Bruning A, Daiminger A, Enders G. Diagnostic value of CD45RO expression on circulating T lymphocytes of fetuses and newborn infants with pre, peri or early post-natal infections. Clin Exp Immunol. 1997;107:306–11. doi: 10.1111/j.1365-2249.1997.268-ce1165.x. [DOI] [PMC free article] [PubMed] [Google Scholar]