Abstract
Autoantibodies directed against human CD38 (an enzyme catalysing the interconversion of NAD+ and cyclic ADP-ribose) have been demonstrated recently in patients with type 2 diabetes. We tested 220 consecutive Caucasian patients with autoimmune chronic thyroiditis, 104 patients with Graves' disease, 220 subjects from the general population (control I) and 78 healthy control subjects not affected by thyroid autoimmune disorders (control II) for the presence of anti-CD38 autoimmunity. Using Western blot analysis and optical densitometry, a specific band corresponding to human recombinant CD38 was identified in the serum of several subjects. By defining anti-CD38 positivity as a standardized optical reading >3 s.d. higher than the mean value of control I, 10·4% of patients with thyroiditis and 7·7% of Graves' patients were anti-CD38 positive (P = 0·0009 versus 1·8% of control I). Similarly, 13·1% of patients with thyroiditis and 10·5% of Graves' patients had a standardized optical reading >3 s.d. higher than the mean value of the subjects not affected by thyroid autoimmune disorders (P = 0·002 versus 1·2% of control II). Anti-CD38 autoimmunity did not differ between euthyroid, hyperthyroid or hypothyroid patients or between patients with or without thyroid hypoechogenicity. Anti-CD38 autoantibodies were associated with higher levels of circulating antithyroid-peroxidase antibodies (P = 0·03) and they were more frequent in Graves' patients with ophthalmopathy (P < 0·05). Anti-CD38 autoantibodies are a new autoimmune marker in chronic autoimmune thyroiditis and Graves' disease. The specific role of CD38 and its autoantibodies in the modulation of thyroid cell function or growth remains to be investigated.
Keywords: anti-CD38 autoantibody, antithyroid-peroxidase autoantibody, chronic thyroiditis, Graves' disease, Graves' ophthalmopathy
Introduction
CD38 (adenosine 5′ diphosphate[ADP]-ribosyl cyclase/cyclic ADP-ribose hydrolase) is an enzyme catalysing the conversion of NAD+ to cyclic ADP-ribose (cADPR) as well as the reverse reaction [1,2]. CD38 is expressed on the surface of monocytes, platelets, NK cells, T and B lymphocytes, myeloid cells and vascular endothelial cells, and in tissues such as brain, cardiac and skeletal muscle, spleen, heart, liver, prostate and kidney [3]. Recent evidence has shown that CD38 is also expressed in both rat [4] and human [3] pancreatic islets. In immunocompetent cells, activation of CD38 by specific agonistic antibodies causes cell activation and proliferation, induces cytokine secretion and modulates apoptosis. According to a model proposed originally by Okamoto et al. [5], in pancreatic β-cells glucose-mediated ATP synthesis inhibits cADPR degradation by CD38, thereby leading to preponderance of the cyclase activity of the enzyme and accumulation of cADPR. This second messenger binds to ryanodine receptors, thereby triggering a cytoplasmic Ca2+ wave which depolarizes the membrane and favours further Ca2+ influx from the extracellular fluid. The resulting increase in cytosolic Ca2+ concentration ([Ca2+]i) leads to insulin release [6].
Autoantibodies reacting with CD38 have been detected by Western blot in 12% of Japanese patients with type 2 diabetes mellitus. The presence of anti-CD38 autoantibodies in these patients was not associated with antiglutamic acid decarboxylase (GAD) autoantibodies [7] and was independent of the co-existence of other autoimmune diseases such as lupus erythematosus systemicus or rheumathoid arthritis. We have reported recently on the presence of anti-CD38 autoantibodies in about 10% of Caucasian patients with type 2 or type 1 diabetes [8,9]. On these grounds, it has been postulated that anti-CD38 antibodies, in addition to being a new marker of autoimmune diabetes, may also participate in its pathogenesis via effects on insulin release [5].
CD38 is expressed in intraparenchymatous fibrous septa of the human thyroid [3]. Thus, the protein is a potential target for an immune reaction in autoimmune thyroid disease. We tested for the presence of anti-CD38 autoimmunity in two models of human autoimmune thyroid disease, namely chronic thyroiditis and Graves' disease.
Patients and methods
Patients
From the out-patient clinic, we selected 220 consecutive Caucasian patients with chronic autoimmune thyroiditis and 104 subjects with Graves' disease (Table 1). The patients were referred to us by general practitioners or other hospitals because of the presence of circulating thyroid autoantibodies (n = 84), hypothyroidism (n = 26), hyperthyroidism (n = 55) or clinical suspicion of a thyroid disorder. The diagnosis of chronic autoimmune thyroiditis [10,11] was inferred from the clinical presentation (presence of a firm goitre, varying in size from small to very large, with a lobulated surface), and was confirmed subsequently by thyroid hormones and thyroid autoantibodies measurements and/or by thyroid ultrasonography (decreased, dyshomogeneous echogenicity). The majority of these patients had a normal thyroid volume (n = 138), while some showed goitre (n = 64) or atrophic thyroiditis (n = 18). A minority of patients (n = 51) were submitted to fine-needle aspiration to exclude the presence of thyroid cancer or lymphoma; in these cases, histology confirmed the presence of a lymphocytic infiltration. Graves' disease was diagnosed [10,12] on the basis of the presence or history of diffuse goitre, hyperthyroidism and detectable thyroid autoantibodies. Most (n = 51) of these patients had already been treated for hyperthyroidism, and had normal circulating free triiodotyronine (FT3) and free thyroxine (FT4) levels. Methimazole was used on 48 patients, thyroidectomy had been performed on eight patients and radioiodine had been administered to three patients. Among the patients with Graves' disease, 66 also had Graves' ophthalmopathy, with exophthalmus, lacrimation, photofobia, blurring of vision and/or diplopia.
Table 1.
Characteristics of the study subjects§
| Controls I | Thyroiditis | Graves' | P | |
|---|---|---|---|---|
| n | 220 | 220 | 104 | |
| Gender (F/M) | 186/34 | 186/34 | 83/21 | n.s. |
| Age (years) | 54 ± 16 | 48 ± 15* | 44 ± 11* | 0·0001 |
| CD38Ab (%) | 1·8 | 10·4* | 7·7* | 0·0009 |
Data are mean ± s.d.;
different from controls at P < 0·05 by anova or χ2 testing.
Controls
Two control groups were used. The first control group (controls I, n = 220) was extracted from a sample of 744 non-diabetic subjects of the general population from the same geographical area (north-western Tuscany) in a 1–1 sex match to the thyroiditis patients in order to estimate accurately the prevalence of anti-CD38 autoimmunity in this condition. A second control group (controls II, n = 78) consisted of a random sample of the general population from the same geographical area [13] in whom a complete thyroid work-up (history, physical examination, TSH, FT3, FT4, antithyroglobulin (AbTg) and antithyroid peroxidase (AbTPO) antibody measurements and ultrasonography) was available. None of the study subjects, patients or controls, had diabetes mellitus (fasting plasma glucose >7 mmol/l or known antidiabetic treatment). The study was approved by the local Ethical Commitee.
Ultrasonography of the neck and fine-needle aspiration (FNA)
Neck ultrasonography was performed by the same operator, who was unaware of the results of thyroid hormones and autoantibodies measurement, using a probe (Toshiba Tosbee) with a sectorial 7·5 MHz transducer, interposing a water bag [14]. Thyroid volume was calculated using the ellipsoid formula, as described [15]. The presence of hypoechoic and dyshomogeneous echogenicity was rated arbitrarily at three levels (0 = normal echogenicity; 1 = slight hypoechoic and dyshomogeneous; 2 = severely hypoechoic and dyshomogeneous) in order to evaluate structural abnormalities of thyroid tissue associated with thyroid autoimmunity [16]. The presence of thyroid nodules was recorded, and nodules with a diameter >10 mm were submitted to ultrasonography-guided FNA, which was performed by the same operator, using a freehand method as described previously [17].
Laboratory evaluation
Thyroid function and thyroid autoantibodies were measured as described previously [18]. Circulating FT3 and FT4 were measured by commercial RIA kits (Amerlex-MAB FT3/FT4 Kit; Amersham, UK). Serum TSH (DiaSorin, USA), AbTPO and AbTg (ICN Pharmaceuticals, USA) were evaluated by IRMA methods. Serum titres of antithyroid stimulating hormone (TSH)-receptor autoantibodies (AbTR) were measured with the use of a radioreceptor assay (Radim, Milano, Italy).
Detection of anti-CD38 autoantibodies
To screen for the presence of anti-CD38 autoantibodies, immunoblotting was performed using a recombinant CD38-maltose binding protein (MBP) fusion protein of 68 kDa obtained, as described previously [19], from a human CD38 cDNA encoding amino acids 45–300. Twenty µg of recombinant CD38-MBP were separated by electrophoresis on 10% SDS polyacrylamide gel, and electrotransferred to a polyvinylidene fluoride membrane (PVDF, Immobilon P, Millipore, Bedford, MA, USA). The membrane was incubated subsequently with a blocking solution of phosphate buffered saline (PBS) containing 5% non-fat dry milk and 0·15% Tween 20 for 60 min. Sera from patients and controls were diluted 1 : 1000 in the same buffer and incubated with the membrane for 60 min using a Screener Blotter Mini 56 (Samplatec, Osaka, Japan). After washing with PBS containing 0·15% Tween 20, the membrane was incubated for 60 min with a rabbit antihuman antibody labelled with horseradish peroxidase (American Quialex, La Mirada, CA, USA) diluted 1 : 1600. The signals were revealed by using an enhanced chemiluminescence detection system (ECL, Amersham Corp., Arlington, IL, USA), according to the manufacturer's instructions. The autoradiographic signals were quantified by densitometry; images were acquired using a Hamamatsu CCD C3077/-01 video camera (Hamamatsu Photonics KK, Japan) connected to an IQ Base software (Hamamatsu Photonics KK) and analysed using the Image 1·28 software (W. Rasband, National Institute of Health, Research Service Branch, NIMH, Bethesda, MD, USA). Each sample was measured twice and the mean value was used as the anti-CD38 titre.
The specificity of the signals was tested as follows. (1) In preabsorption experiments, three thyroiditis sera-positive for anti-CD38 antibodies were incubated overnight at 4°C with or without 100 µg/ml of human recombinant CD38-MBP or LacZ-MBP in blocking solution [7], and used in immunoblotting as described above. (2) Three anti-CD38 positive and three anti-CD38 negative thyroiditis sera diluted 1 : 1000 were immunoblotted against the extracellular domain of human CD38, with the putative glycosylation sites eliminated by site-directed mutagenesis, obtained by recombinant expression technique from the yeast Pichia pastoris (10 µg/per gel) [20]. (3) In preabsorption experiments, five thyroiditis sera positive for anti-CD38 antibodies were incubated overnight at 4°C with or without 30 µg/ml of CD38 obtained by P. pastoris in blocking solution, and used in the immunoblotting as described above. (4) Three thyroiditis sera positive for anti-CD38 antibodies were preincubated with 50 µg/ml protein A for 1 h at 37°C, and used in the immunoblotting. (5) Signals obtained from thyroiditis or normal sera were compared with those produced by an antihuman CD38 monoclonal antibody (T16, Cosmo Bio Co. Ltd, Tokyo, Japan) tested on the same PVDF membrane at the concentration of 5 µg/ml. (6) Serum samples positive for the presence of anti-CD38 antibodies were immunoblotted with a recombinant LacZ-MBP fusion protein to exclude the presence of antibodies directed against MBP.
Data analysis
CD38 optical density readings were corrected for background and standardized against an internal control sample; the ratio of the unknown to the control was multiplied by 100. Positivity for anti-CD38 autoimmunity was defined as an optical density value >3 s.d. above the mean value of the control population. For AbTg, AbTPO and AbTR titres, positivity was set at >50, > 10 and >10 U/ml, respectively.
Values are given as the mean ±s.d. Mean group values were compared by using Student's t-test or one-way analysis of variance (anova) for normally distributed variables (age and body mass index), otherwise by the Mann–Whitney U or Kruskal–Wallis. Proportions were compared by the χ2 test. Post-hoc comparisons on normally distributed variables were carried out using the Bonferroni–Dunn test. Univariate and multivariate analysis were performed by standard methods.
Results
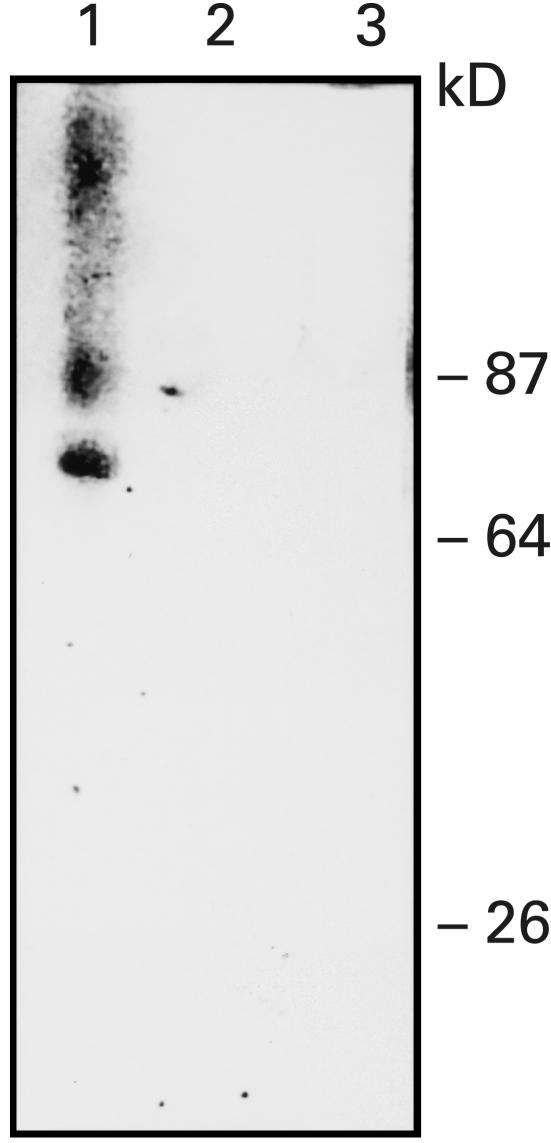
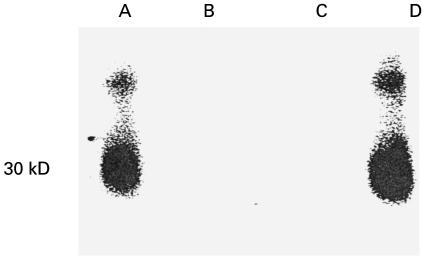
A band of ∼68 kDa was observed with the sera of some patients with chronic thyroiditis or Graves' disease as well as normal subjects (Fig. 1, lane 1). The autoradiographic signals were abolished by preincubating anti-CD38 positive sera with recombinant CD38 from Escherichia coli (Fig. 1, lane 2), whereas they were still detectable when the sera were preincubated with LacZ-MBP. Preincubation of sera with anti-CD38 activity with Staphylococcus aureus Protein A covalently linked to Sepharose CL-4B abolished the autoradiographic signals, indicating that the observed anti-CD38 activity was due to IgG (Fig. 1, lane 3). T16, an antihuman CD38 monoclonal antibody, yielded an autoradiographic pattern similar to that obtained with anti-CD38-positive human sera. Upon immunoblotting sera containing putative anti-CD38 autoantibodies against rCD38 from P. pastoris, a band of apparent molecular mass of ∼ 30 kDa (corresponding to the soluble CD38) was revealed; also evident was a second band of higher molecular weight, as reported previously (Fig. 2, lanes A and D) [8,9]. No signals were produced by sera from subjects lacking anti-CD38 activity. The autoradiographic signals were abolished by preincubating sera with anti-CD38 activity with rCD38 from P. pastoris (Fig. 2, lane B). Also with the use of rCD38 from P. pastoris, preincubation of anti-CD38 positive sera with S. aureus Protein A covalently linked to Sepharose CL-4B abolished the autoradiographic signals, suggesting that the observed anti-CD38 activity was due to IgG (Fig. 2, lane C). When serum samples positive for the presence of anti-CD38 antibodies were immunoblotted with a recombinant LacZ-MBP fusion protein, the autoradiographic signals were abolished.
Fig. 1.
Western blot of a human serum from a patient with chronic thyroiditis with activity against rCD38 from Escherichia coli (lane 1). After preincubation with rCD38 from E. coli (lane 2) or with preincubation with Staphylococcus aureus Protein A (lane 3) the signal is abolished.
Fig. 2.
Western blot of a human serum from a patient with chronic thyroiditis with activity against rCD38 from Pichia pastoris (lanes A and D). After preincubation with rCD38 from P. pastoris (lane B) or with preincubation with Staphylococcus aureus Protein A (lane C) the signal is abolished.
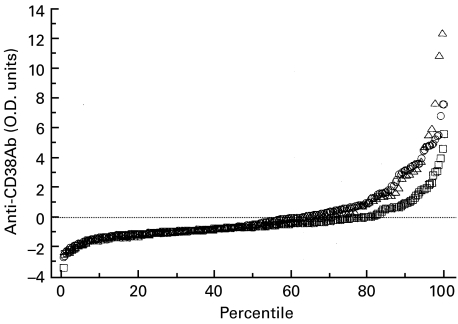
The distribution of anti-CD38 values for the chronic thyroiditis and Graves' patients clearly diverged from that of the control subjects (controls I) above the 80th percentile (Fig. 3). By defining anti-CD38 positivity as a standardized optical density value at least 3 s.d. above the mean value of the control group, 23 of 220 patients with thyroiditis and eight of 104 patients with Graves' disease were positive for anti-CD38 antibodies versus four of 220 sex-matched control subjects (Table 1).
Fig. 3.
Distribution of anti-CD38 antibodies (CD38Ab) (by optical densitometry) in 220 patients with chronic autoimmune thyroiditis, 104 patients with Graves' disease and 220 healthy control subjects (controls I). □, Controls; ○, thyroiditis; ▵, Graves'.
Patients with thyroid disease were younger and more often female than controls II (Table 2), and body mass index (BMI) was significantly lower in Graves' patients. In univariate analysis the echogenicity score was lower (P = 0·0004), whereas thyroid volume was higher (P = 0·003) in men compared to women. Echogenicity was significantly increased in both chronic thyroiditis and Graves' patients, whereas thyroid volume was increased in Graves' patients only; serum FT4 and FT3 were higher, and TSH was lower, in Graves' than in chronic thyroiditis patients. The distribution of anti-CD38 values for the chronic thyroiditis and Graves' patients clearly diverged from that of the control subjects above the 90th percentile. By defining anti-CD38 positivity as a standardized optical density value at least 3 s.d. above the mean value of this control group (controls II), 29 of 220 patients with thyroiditis (13·1%) and 11 of 104 (10·5%) patients with Graves' disease were positive for anti-CD38 antibodies versus one of 78 (1·2%) control subjects (Table 3). No significant difference in anti-CD38 antibody titre was observed between patients with euthyroid versus hypothyroid chronic thyroditis, or between Graves' patients who were hyperthyroid versus those with normal serum FT3 and FT4. Interestingly, among Graves' patients only one of 38 subjects without ophthalmopathy (2·6%) was positive for anti-CD38 antibodies, while 10 of 66 patients with ophthalmopathy (15·1%) were positive for anti-CD38 antibodies (P < 0·05). No statistically significant difference in sex, age or BMI was found between anti-CD38-positive and -negative patients with chronic thyroiditis or Graves' disease. Positivity for AbTg was higher in chronic thyroiditis patients, whereas positivity for AbTR was higher in Graves' patients, positivity for AbTPO being similar in the two patient groups (Table 3).
Table 2.
Characteristics of the study subjects with chronic thyroiditis (thyroiditis), Graves'disease (Graves') and controls‡
| Controls II | Thyroiditis | Graves' | P† | |
|---|---|---|---|---|
| n | 78 | 220 | 104 | |
| Gender (F/M) | 36/42 | 186/34* | 83/21* | 0·0001 |
| Age (years) | 55 ± 18 | 48 ± 15* | 44 ± 11* | 0·0001 |
| Body mass index (kg/m2) | 26·6 ± 5·1 | 25·6 ± 4·8 | 24·3 ± 4·4* | 0·02 |
| Echogenicity (units) | 0·43 ± 0·74 | 0·91 ± 0·86* | 1·29 ± 0·80* | 0·0001 |
| Thyroid volume (ml) | 19·3 ± 16·0 | 19·2 ± 18·3 | 28·7 ± 25·0* | 0·0001 |
| fT3 (ng/ml) | 3·70 ± 0·46 | 3·42 ± 1·2 | 4·34 ± 2·78* | 0·0001 |
| fT4 (nl/l) | 9·21 ± 1·73 | 9·92 ± 3·56 | 12·56 ± 6·86* | 0·01 |
| TSH (mUI/ml) | 1·34 ± 0·82 | 2·6 ± 7·2 | 1·00 ± 1·39* | 0·0001 |
Data are mean ± s.d.;
by anova, Kruskall–Wallis or χ2 testing;
different from controls at the P < 0·05 level or less by Mann–Whitney or Bonferroni–Dunn test.
Table 3.
Prevalence of autoantibodies against CD38 (CD38Ab), thyroglobulin (AbTg), thyroid peroxidase (AbTPO) and thyroid stimulating hormone [TSH]-receptor (AbTR)
| Controls II | Thyroiditis | Graves' | P* | |
|---|---|---|---|---|
| CD38Ab (%) | 1·2 | 13·1 | 10·5 | 0·002 |
| AbTg (%) | 0 | 52·9 | 32·9 | 0·0001 |
| AbTPO (%) | 0 | 73·0 | 67·4 | 0·0001 |
| AbTR (%) | 0 | 5·3 | 43·4 | 0·0001 |
χ2 test.
The association between serum autoantibody titres and clinical variables (serum thyroid hormone levels, thyroid echogenicity and thyroid volume) is reported in Table 4. After adjusting for gender, age, BMI and clinical condition, AbTPO titre was significantly related to high levels of AbTg and TSH. Hypoechoic, dyshomogeneous thyroid glands were found in association with high titres of AbTPO and AbTR. High circulating AbTR titres were also associated independently with an increased thyroid volume and high FT3 and FT4 levels (Table 4). Anti-CD38 antibodies were correlated significantly with high levels of AbTPO.
Table 4.
Clinical correlates of autoantibody positivity*
| Anti-CD38Ab | AbTg | AbTPO | AbTR | |
|---|---|---|---|---|
| Anti-CD38Ab (titre) | – | – | – | – |
| AbTg (titre) | n.s. | – | – | – |
| AbTPO (titre) | 0·03 (+) | 0·0001 (+) | – | – |
| AbTR (titre) | n.s. | n.s. | n.s. | – |
| Echogenicity (units) | n.s. | n.s. | 0·01 (+) | 0·03 (+) |
| Thyroid volume (ml) | n.s. | n.s. | n.s. | 0·008 (+) |
| TSH (mUI/ml) | n.s. | n.s. | 0·005 (+) | n.s. |
| fT3 (ng/ml) | n.s. | n.s. | n.s. | 0·0001(+) |
| fT4 (ng/l) | n.s. | n.s. | n.s. | 0·001(+) |
Entries are P-values for the association of antibody titres (columns) and clinical variables (rows), adjusted by gender, age, BMI and clinical condition; the sign of the association is given in parenthesis, antibody titres were log-transformed.
Discussion
The thyroid patients screened in the present study were typical with respect to clinical phenotype and autoimmune markers. In general, their thyroid glands were hypoechoic and dyshomogeneous, the more so the higher their autoantibody titres. Thyroid volume was significantly larger in the patients with Graves' disease because of the presence of high levels of circulating AbTR. In contrast, thyroid volume was not significantly different between chronic thyroiditis patients and controls, probably because of a relatively large thyroid volume in the control population (due to the presence of iodine deficiency in the past decades) [21]. Serum FT3 and FT4 concentrations were higher and TSH levels were lower in the Graves' patients, whereas the slightly raised TSH levels in the thyroiditis group was due to the inclusion of some hypothyroid patients. A high proportion of patients with chronic thyroiditis or Graves' had high titres of AbTg and AbTPO. As expected, the prevalence of an elevated AbTR titre, which is pathogenetically involved in Graves' disease, was higher in these patients than in those with thyroiditis. AbTR positivity in patients with Graves' disease was somewhat less than that reported in other studies [22] because a high proportion of our patients had been treated by methimazole at the time of study.
Positivity for anti-CD38 autoantibodies was significantly higher in patients with thyroiditis or Graves' disease than in either group of control subjects. The slightly higher estimated prevalence of anti-CD38 positivity when using controls II versus controls I is due probably to the larger number among controls I of women, who are more prone to thyroid autoimmunity and/or circulating antithyroid autoantibodies. With regard to the prevalence of anti-CD38 autoimmunity, it should also be noted that a recent study in diabetic patients [23] has confirmed the presence of anti-CD38 autoantibodies with the use of an enzyme immunoassay, with a frequency similar to that reported by our laboratory [8,9].
The observed prevalence of anti-CD38 antibodies in the present series of thyroid patients was similar to that reported in Japanese patients with type 2 diabetes [7], and to that found previously in Caucasian patients with type 2 diabetes [8,9]. No significant difference in anti-CD38 positivity was evident between chronic thyroiditis and Graves' patients, between euthyroid and hypothyroid subjects or between euthyroid and hyperthyroid Graves' patients. It was of interest to find that the prevalence of anti-CD38 antibodies was significantly higher among Graves' patients with ophthalmopathy than in those without ophthalmopathy. In the whole dataset, no relationship was detected between serum anti-CD38 titre and sex, age or BMI. Some cases of anti-CD38 positivity were observed in thyroiditis or Graves' patients who were negative for AbTg or AbTPO or AbTR.
Thus, anti-CD38 antibodies are a new marker of autoimmunity in both chronic autoimmune thyroiditis and Graves' disease; this marker has a lower frequency than, and is largely independent of, the classical markers of thyroid autoimmunity. The weak association between anti-CD38 autoantibodies and AbTPO may be due to more aggressive thyroid autoimmunity (Table 4). In support of this interpretation is the finding of similar associations between AbTPO and AbTg or AbTR, which are definitely distinct molecules. Except for more frequent ophtalmopathy, the presence of anti-CD38 autoimmunity does not appear to identify a clinical phenotype with respect to primary diagnosis or thyroid function indices.
Abnormal thyroid ultrasound patterns, characterized by diffuse low echogenicity, have been reported in patients with chronic thyroiditis and Graves' disease [24,25]. In the current study, when evaluating associations between markers of thyroid autoimmunity and clinical parameters (Table 4), both AbTPO and AbTR titres were associated with more pronounced structural abnormalities of thyroid tissues (hypoechogenicity), as noted by other authors [16]. In contrast, positivity for anti-CD38 antibodies or AbTg was not associated with significant changes of thyroid echogenicity
Although the precise significance of anti-CD38 antibodies in the natural history of autoimmune thyroid disease remains to be established, the current results indicate that, in patients with thyroid autoimmune disorders, the presence of anti-CD38 antibodies marks for more aggressive autoimmunity, both at the level of circulating antibodies (AbTPO) and clinically (ophthalmopathy in Graves' patients). CD38 tissue expression is almost ubiquitous [3,26–28], and low-prevalence anti-CD38 autoimmunity has also been found in type 2 and type 1 diabetes. Therefore, the precise role of anti-CD38 autoimmunity remains to be established in each of these conditions. In pancreatic β-cells CD38 is involved in insulin release [5], and human anti-CD38 autoantibodies have been shown to exert [Ca2+]i-mobilizing activity and to stimulate insulin release in isolated human islets [9]. Studies are under way to investigate the role of CD38 and its autoantibodies in the modulation of thyroid cell function or growth.
Acknowledgments
This work was supported through a grant from the Yamanouchi European Foundation.
REFERENCES
- 1.Howard M, Grimaldi JC, Bazan JF, et al. Formation and hydrolysis of cyclic ADP-ribose catalyzed by lymphocyte antigen CD38. Science. 1993;262:1056–9. doi: 10.1126/science.8235624. [DOI] [PubMed] [Google Scholar]
- 2.Takasawa S, Tohgo A, Noguchi N, et al. Synthesis and hydrolysis of cyclic ADP-ribose by human leukocyte antigen CD38 and inhibition of the hydrolysis by ATP. J Biol Chem. 1993;268:26052–4. [PubMed] [Google Scholar]
- 3.Fernàndez JE, Deaglio S, Donati D, et al. Analysis of the distribution of human CD38 and of its ligand CD31 in normal tissues. J Biol Regul Homeost Agents. 1998;12:81–91. [PubMed] [Google Scholar]
- 4.Deaglio S, Dianzani U, Horenstein AL, et al. A human CD38 ligand: a 120 kDa protein predominantly expressed by endothelial cells. J Immunol. 1996;156:727–34. [PubMed] [Google Scholar]
- 5.Okamoto H, Takasawa S, Nata K. The CD38-cyclic ADP-ribose signalling system in insulin secretion: molecular basis and clinical implications. Diabetologia. 1997;40:1485–91. doi: 10.1007/s001250050854. 10.1007/s001250050854. [DOI] [PubMed] [Google Scholar]
- 6.Takasawa S, Akiyama T, Nata K, et al. Cyclic ADP-ribose and inositol 1,4,5-trisphosphate as alternate second messangers for intracellular Ca2+ mobilization in normal and diabetic β-cells. J Biol Chem. 1998;273:2497–500. doi: 10.1074/jbc.273.5.2497. [DOI] [PubMed] [Google Scholar]
- 7.Ikehata F, Sato J, Nata K, et al. Autoantibodies against CD38 (ADP-ribosyl-cyclase/cyclic ADP-ribose hydrolase) that impare glucose induced insulin secretion in noninsulin-dependent diabetes patients. J Clin Invest. 1998;102:395–401. doi: 10.1172/JCI1656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pupilli C, Giannini S, Marchetti P, et al. Autoantibodies to CD38 (ADP-ribosyl-cyclase/cyclic ADP-ribose hydrolase) in Caucasian patients with diabetes. Diabetes. 1999;48:2309–15. doi: 10.2337/diabetes.48.12.2309. [DOI] [PubMed] [Google Scholar]
- 9.Antonelli A, Baj G, Marchetti P, et al. Human anti-CD38 autoantibodies raise intracellular calcium and stimulate insulin release in human pancreatic islets. Diabetes. 2001;50:985–91. doi: 10.2337/diabetes.50.5.985. [DOI] [PubMed] [Google Scholar]
- 10.Antonelli A, Saracino A, Alberti, et al. High-dose intravenous immunoglobulin treatment in Graves' Ophthalmopathy. Acta Endocrinol. 1992;126:13–23. doi: 10.1530/acta.0.1260013. [DOI] [PubMed] [Google Scholar]
- 11.Antonelli A, Palla R, Casarosa L, et al. In vitro solubilization of deposits of IgG immune complexes by gamma-globulins in patients with Graves' disease, Graves' ophthalmopathy, pretibial myxoedema and Hashimoto's thyroiditis. Pharmacol Res. 1992;26(Suppl. 2):170–1. doi: 10.1016/1043-6618(92)90649-v. [DOI] [PubMed] [Google Scholar]
- 12.Antonelli A, Navarranne A, Palla R, et al. Pretibial myxoedema and high-dose intravenous immunoglobulin treatment. Thyroid. 1994;4:30–5. doi: 10.1089/thy.1994.4.399. [DOI] [PubMed] [Google Scholar]
- 13.Antonelli A, Ferri C, Fallahi P. Thyroid cancer in patients with hepatitis C infection. JAMA. 1999;281:1588. doi: 10.1001/jama.281.17.1588. [DOI] [PubMed] [Google Scholar]
- 14.Antonelli A, Miccoli P, Derzhitski VE, et al. Epidemiologic and clinical evaluation of thyroid cancer in children coming from the Gomel region (Belarus) World J Surg. 1996;20:867–1. doi: 10.1007/s002689900132. [DOI] [PubMed] [Google Scholar]
- 15.Wesche MF, Tiel-van Buul MM, Smits NJ, et al. Ultrasonographic versus scintigraphic measurements of thyroid volume in patients referred for 131I therapy. Nucl Med Commun. 1998;19:341–6. doi: 10.1097/00006231-199804000-00008. [DOI] [PubMed] [Google Scholar]
- 16.Vitti P. Grey scale thyroid ultrasonography in the evaluation of patients with Graves' disease. Eur J Endocr. 2000;142:22–4. doi: 10.1530/eje.0.1420022. [DOI] [PubMed] [Google Scholar]
- 17.Antonelli A, Miccoli P, Ferdeghini M, et al. Role of neck ultrasonography in the follow-up of patients operated on for thyroid cancer. Thyroid. 1995;5:25–9. doi: 10.1089/thy.1995.5.25. [DOI] [PubMed] [Google Scholar]
- 18.Baschieri L, Antonelli A, Nardi S, et al. Intravenous immunoglobulin versus corticosteroid in treatment of Graves' ophthalmopathy. Thyroid. 1997;4:579–85. doi: 10.1089/thy.1997.7.579. [DOI] [PubMed] [Google Scholar]
- 19.Tohgo A, Munakata H, Takasawa S, et al. Lysine 129 of CD38 (ADP-ribosyl cyclase/cyclic ADP-ribose hydrolase) partecipates in the binding of ATP to inhibit the cyclic ADP-ribose hydrolase. J Biol Chem. 1997;272:3879–82. doi: 10.1074/jbc.272.7.3879. [DOI] [PubMed] [Google Scholar]
- 20.Munshi CB, Fryxell KB, Lee HC, et al. Large-scale production of human CD38 in yeast by fermentation. Meth Enzymol. 1997;280:318–30. doi: 10.1016/s0076-6879(97)80123-1. [DOI] [PubMed] [Google Scholar]
- 21.Donati L, Antonelli A, Bertoni F, et al. Clinical picture of endemic cretinism in central Appennines (Montefeltro) Thyroid. 1992;2:283–90. doi: 10.1089/thy.1992.2.283. [DOI] [PubMed] [Google Scholar]
- 22.Vitti P, Rago T, Mancusi F, et al. Thyroid hypoechogenic pattern at ultrasonography as a tool for predicting recurrence of hyperthyroidism after medical treatment in patients with Graves' disease. Acta Endocrinol (Copenh) 1992;126:128–31. doi: 10.1530/acta.0.1260128. [DOI] [PubMed] [Google Scholar]
- 23.Mallone R, Ortolan E, Baj G, et al. Autoantibody response to CD38 in caucasian patients with type 1 and 2 diabets. Diabetes. 2001;50:752–62. doi: 10.2337/diabetes.50.4.752. [DOI] [PubMed] [Google Scholar]
- 24.Espinasse P. L'echographie thyroidienne dans les thyroidites lymphocytaires chroniques autoimmunes. J Radiol. 1983;64:537–44. [PubMed] [Google Scholar]
- 25.Gutenkust R, Hafermann W, Mansky T, et al. Ultrasonography related to clinical and laboratory findings in lymphocytic thyroiditis. Acta Endocrinol (Copenh) 1989;121:129–35. doi: 10.1530/acta.0.1210129. [DOI] [PubMed] [Google Scholar]
- 26.Takasawa S, Nata S, Yonekura H, et al. Cyclic ADP-ribose in insulin secretion from pancreatic β cells. Science. 1993;259:370–3. doi: 10.1126/science.8420005. [DOI] [PubMed] [Google Scholar]
- 27.Koguma T, Takasawa S, Tohgo A, et al. Cloning and characterization of cDNA encoding rat ADP-ribosyl cyclase/cyclic ADP-ribose hydrolase (homologue to human CD38) from islets of Langerhans. Biochem Biophys Acta. 1994;1223:160–2. doi: 10.1016/0167-4889(94)90087-6. [DOI] [PubMed] [Google Scholar]
- 28.Mizuguchi M, Otsuka N, Sato M, et al. Neuronal localization of CD38 antigen in the human brain. Brain Res. 1995;697:235–40. doi: 10.1016/0006-8993(95)00885-t. 10.1016/0006-8993(95)00885-t. [DOI] [PubMed] [Google Scholar]