Abstract
Cancer-associated retinopathy (CAR) is a paraneoplastic syndrome that is characterized by degeneration of the retina as a remote effect of cancer outside the eye. The detection of autoantibodies associated with the retinopathy may precede the diagnosis of the underlying cancer. We have examined the sera of two patients with CAR by Western blot analysis. Autoantibodies to a 40kD antigen doublet and a 35 kD antigen were detected. Tissue specificity of the autoantigens was determined by testing several different tissues. The 40 kD antigen doublet was most abundant in retinal extract but was also present in lung and spleen extracts. The 35 kD antigen showed little tissue specificity and was present in all tissues tested. Fractionation of retinal proteins into water-soluble and-insoluble proteins revealed that the 40 kD antigen doublet was highly insoluble and probably represented membrane-associated proteins. Immunohistochemical analysis of the retina showed that the 40 kD antigens locate to the photoreceptors while the 35 kD antigen is located in the outer plexiform layer.
Keywords: cancer-associated retinopathy, CAR, autoantibodies, paraneoplasm, autoimmune
INTRODUCTION
Patients with the paraneoplastic disorder cancer-associated retinopathy (CAR) suffer from a progressive visual dysfunction as a remote effect of cancer in a distant part of the body. The general finding of circulating autoantibodies in these patients directed to specific retinal structures suggests that a humoral autoimmune response is involved in the retinal degeneration. The most frequently described retinal autoantigen in CAR is the 23 kD antigen, recoverin, a photoreceptor and bipolar cell-specific calcium-binding protein [1–3]. In addition to this retinal component, other autoantigens have been described in CAR, including enolase [4], a heat-shock cognate protein [5, 6], a tubby-like protein 1 [7] and several unidentified antigens of various molecular weights [8–13]. This suggests that an immune response against various retinal antigens may lead to visual impairment as observed in CAR. The molecular pathophysiology of CAR has not been fully elucidated and two possible mechanisms have been proposed. One hypothesis for the induction of anti-retinal antibodies in CAR is that aberrant expression by cancer cells of retinal proteins, normally present in the immune-privileged environment of the eye, induces an immune response. Alternatively, antibodies generated against cancer cell antigens might cross-react with retinal epitopes and cause autoimmune retinal degeneration. All CAR antigens described so far are intracellular proteins, suggesting that these antigens are sequestered and seem unavailable for the autoantibodies. However, several studies have shown that autoantibodies, including anti-recoverin and anti-enolase, are able to penetrate living cells and trigger cell death via apoptosis [5, 14, 15, 16]. In this study, the autoantibody repertoire of two CAR patients with similar visual problems was analysed. Autoantibodies from both patients recognized an antigen doublet of 40 kD preferentially expressed in the retina and localized to the membranes of the photoreceptor outer segments. In addition, a protein of 35 kD present in the retinal outer plexiform layer was recognized by serum antibodies of one patient.
PATIENTS AND METHODS
Patients’ history
Patient 1 was a 73-year-old woman. Her visual acuity of the right eye had gradually decreased to 12/20. The acuity of the left eye was 20/20. She complained of decreased vision of both eyes, especially in the dark. Visual field analysis (Hymphrey central 24–2) showed parafoveal decrease sensitivity confirmed in the pattern deviation. Clinical examination showed clear media and minimal retinal pigment epithelial mottling of both eyes. Colour vision showed a red-green deficit in the right eye (6 faults Ishihara test).
Six months earlier, a colon resection was performed, showing a moderate differentiated adenocarcinoma which was totally excised. At follow-up one year later, her visual complaints were similar, but no deterioration was measured. The tumour was in complete remission.
Patient 2 was a 69-year-old man. He complained of difficulties with reading. His visual acuity was 20/20 of the right eye and 8/20 of the left eye. There was a moderate nuclear lens sclerosis in the left eye. Ocular tension was 27/27 with aplanation tonometry. The discs of the optic nerves showed an increased cupping of the left eye, with a cup disc ratio of 0·7 (0·2 for the right eye); repeated visual field analysis showed decreased pericentral sensitivity in both eyes. Because of the suspicion of glaucoma, he was treated locally with betaxolol, resulting in a diminution of the ocular pressure to 17/17. A colour vision test showed a severe red-green deficiency of both eyes. His dark adaptation was also severely diminished. Six years earlier, a prostate carcinoma was detected which was treated with radiotherapy. Six months after his first eye examination, bone metastasis was detected and this was treated with busereline, a testosterone-depressing gonadoreline analogue.
Local ethical approval and informed consent was obtained from all blood donors.
Preparation of protein extracts
Human retinal tissue was obtained from donor eyes that were processed at the Eye Bank of the Netherlands Ophthalmic Research Institute. Human and rat tissues were isolated, immediately followed by snap-freezing in liquid nitrogen. A total protein extract was obtained by adding approximately 100 mg of tissue to 500 μl sodium dodecyl sulphate (SDS)-polyacrylamide gel electrophoresis (PAGE) sample buffer (125 mm Tris HCl pH 6·8, 2% sodium dodecyl sulphate, 5% beta-mercapto-ethanol, 10% glycerol, 0·005% bromophenol blue), followed by vigorous stirring to shear the chromosomal DNA, boiling for 5 min and centriguging for 5 min at 10 000 g. To separate rat retinas into water-soluble and a water-insoluble fractions, four retinas were homogenized in 300 μl phosphate-buffered saline (PBS), pH 7·4, using a mortar. The homogenate was centrifuged at 10 000 g for 5 min, and to the supernatant fluid containing the water-soluble fraction, an equal volume of 2× concentrated SDS sample buffer was added. To obtain the water-insoluble fraction, the remaining pellet was washed twice with 1 ml PBS and resuspended in 200 μl SDS sample buffer, followed by vigorous stirring and boiling for 5 min. The remaining insoluble material, consisting mainly of lipids, was removed from the extract by centrifugation for 5 min at 10 000 g. A retinal membrane protein fraction was obtained essentially as previously described [17]. Briefly, a water-insoluble fraction of rat retina was dissolved in a volume of 10 mm Tris HCl (pH 7·5), 2% Triton X-100 and 12 μm phenylmethylsulphonyl fluoride (PMSF), and stirred for 1·5 h. After centrifugation, the pellet was re-extracted under the same conditions. SDS sample buffer was added to these two fractions and the remaining pellet before size fractionation by PAGE.
Western blot analysis
Protein extracts (15–20 μg per lane) were size-fractionated by 10% SDS-PAGE and electroblotted onto nitrocellulose filters (Schleicher and Schuell, Dassel, Germany). Blots were incubated with diluted serum from CAR patients or pooled normal human serum, washed, and incubated with a 1:1000 dilution of a goat anti-human Ig horseradish peroxidase-conjugated secondary antibody (DAKO, Glostrup, Denmark). Bound antibodies were visualized using diaminobenzidine tetrahydrochloride substrate according to the manufacturer’s instructions (ICN Biomedicals, Aurora, OH, USA).
Immunohistochemical analysis
Cryosections (10 μm) of adult Wistar rat eyes were acetone-fixed and incubated with diluted patient or control serum. Sections were washed and incubated with a peroxidase-conjugated mouse monoclonal antibody to human IgG (CLB, Amsterdam, The Netherlands), followed by washing and staining with diaminobenzidine.
RESULTS
The sera of two patients with different types of cancer (colon carcinoma and prostate carcinoma) and clinical symptoms of cancer-associated retinopathy (CAR) were analysed for the presence of anti-retinal autoantibodies by Western blot analysis using total rat retinal extracts. The serum of these patients appeared to contain IgG antibodies to several proteins, of approximately 40 kD, in both human and rat retinal extracts (Fig. 1). As negative controls, pooled normal human serum and the sera of 25 uveitis patients were tested. Although some of the control sera gave weak bands of various molecular weight, none reacted with these particular antigens (not shown). To determine the tissue specificity of these autoantigens, Western blots containing an equivalent amount of total protein from rat retina and various other rat tissues were incubated with diluted sera from patient 1 and 2, and normal human control serum. As shown for patient 1, the protein doublet around 40 kD is highly expressed in the retina, but could also be detected in the spleen and the lung (Fig. 2, closed arrowheads). The serum of patient 2 labelled the same doublet (although the signal in the lane containing lung extract was very weak) but in addition, contained autoantibodies directed to an antigen which was expressed in all tissues tested (open arrowhead). This suggests that the expression of the 40 kD doublet antigen shows tissue specificity, with high expression in the retina and moderate-to-weak expression in spleen and lung tissue.
Fig.1.

Sera of CAR patients were tested by Western blot analysis of total protein extracts of rat and human retina. Note that the signal from the serum of patient 2 on human retinal extract is weak. The molecular weight standards for 31 kD and 45 kD are indicated.
Fig.2.
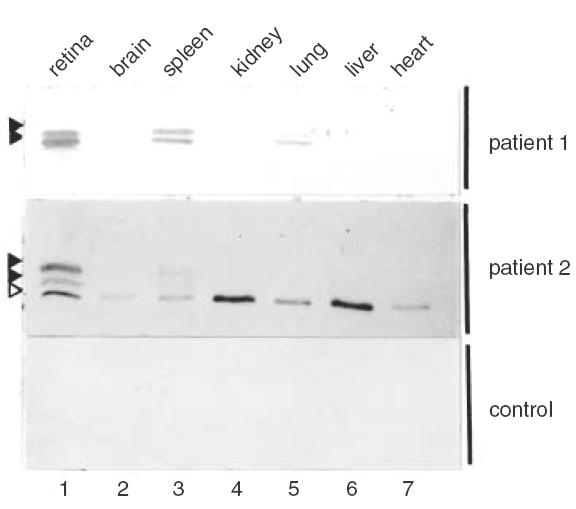
Tissue specificity of CAR antigens. Western blots containing total protein extracts of several rat tissues were incubated with a 1:250 dilution of patient or pooled normal human serum. The position of the 40 kD doublet is indicated by closed arrowheads, and the non-tissue-specific autoantigen running at 35 kD is indicated by an open arrowhead.
To determine the location of the antigens in the retina in more detail, cryosections of whole adult Wistar rat eyes were incubated with diluted patient and control sera. Control sera gave no specific staining above background, while the serum of patient 1 gave weak staining of the outer segments of the photoreceptors (not shown). Serum of patient 2 gave strong staining of the outer segments of the photoreceptors and in addition, a sharp line of intense staining at the position of the outer plexiform layer (OPL; Fig. 3, left panel). Serum of both patients contained antibodies against the 40 kD doublet antigen and gave staining of the photoreceptor outer segments in immunohistochemistry. This suggests that the 40 kD doublet antigen constitutes part of the photoreceptor outer segments, while the additional antigen recognized by patient 2 corresponds to the autoantigen in the OPL. To confirm these results, diluted serum of patient 2 was pre-absorbed with rat retinal extract. Although the absorption procedure led to some reduction in overall staining, the autoantibodies directed to the 40 kD autoantigen doublet were efficiently removed, while the intensity of the 35 kD antigen remained similar to the mock-absorbed diluted serum (Fig. 4).
Fig.3.
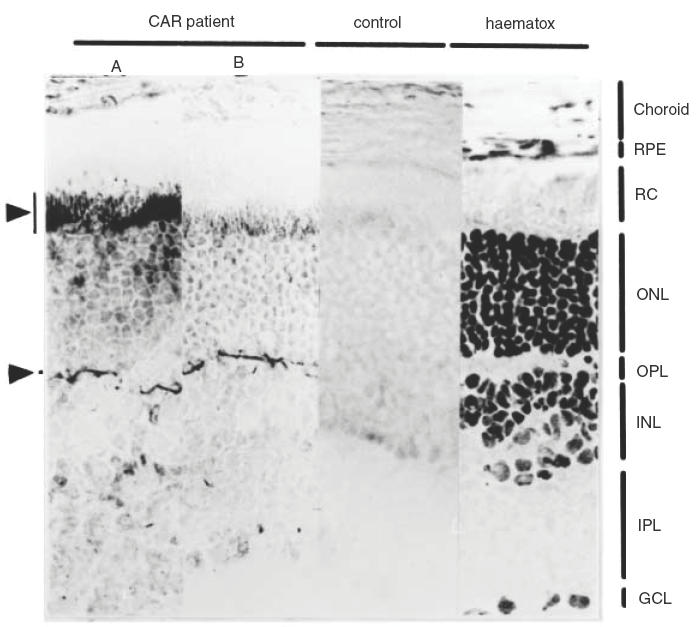
Acetone-fixed cryosections of rat retina were incubated with a 1:200 dilution of patient 2 serum without prior absorbtion (A), or pre-absorbed with total rat retinal extract (B), and a 1:200 dilution of pooled normal human serum (control). A haematoxylin-stained section is also shown (haematox). The retinal layers are indicated as follows: RPE, retinal pigment epithelium; RC, layer of rods and cones; ONL, outer nuclear layer; OPL, outer plexiform layer; INL, inner nuclear layer; IPL, inner plexiform layer; GCL, ganglion cell layer.
Fig.4.
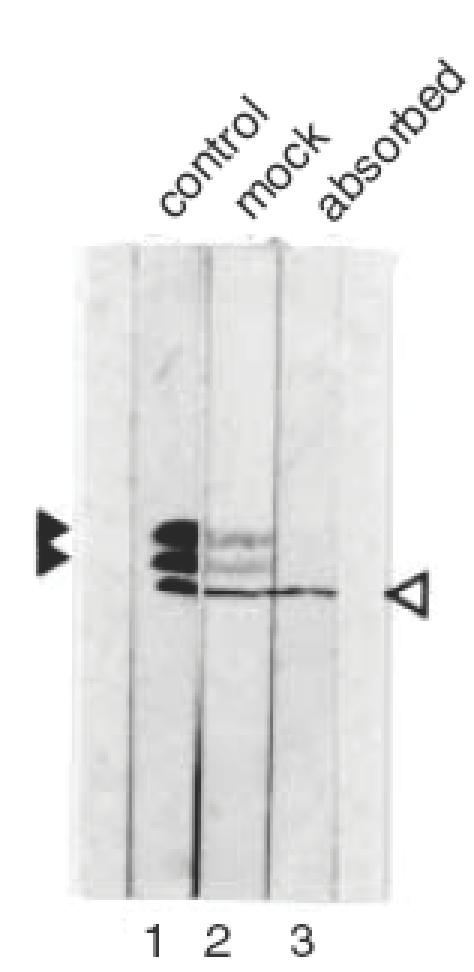
Absorbtion of CAR serum with retinal extract. A Western blot containing rat total retinal protein was incubated with a 1:200 dilution of patient serum 2 (lane 1), pre-absorbed with total rat retinal proteins (lane 3) or pre-absorbed with BSA only (lane 2). The position of the 40 kD doublet is indicated by closed arrowheads, and the position of the 35 kD antigen is indicated by an open arrowhead.
The absorption efficiency was monitored by Western blot analysis (Fig. 4). This showed that this procedure efficiently removed the autoantibodies directed to the 40 kD autoantigen doublet while the intensity of the 35 kD antigen (see also Fig. 2) remained similar to the mock-absorbed diluted serum. When this pre-absorbed serum was subsequently used for immunohistochemical analysis, the strong staining of the photoreceptors was reduced to background level, while the intense staining of the line of the OPL was not affected (Fig. 3, panel B).
The 40 kD autoantigen doublet always appeared as relatively broad bands on standard SDS-PAGE followed by Western blot analysis (see Figs 1, 3, 4). Water-insoluble proteins are known to display such aberrant electrophoretic behaviour. To test whether these photoreceptor-associated autoantigens were indeed water-insoluble, a retinal extract was fractionated into water-soluble and water-insoluble proteins. Both fractions were analysed by Western blot using diluted serum of the CAR patients and several negative controls. Patient 1 recognized the 40 kD doublet autoantigen in the water-insoluble fraction only (not shown). However, the blot incubated with serum from patient 2 showed complete separation of the photoreceptor 40 kD doublet, which was in the water-insoluble fraction, and the OPL-specific 35 kD antigen, which was completely in the water-soluble fraction (Fig. 5). These same fractions were analysed by Coomassie staining and showed that whereas the 40 kD antigen doublet appeared as prominent bands in the total extract and the water-insoluble extract, it was absent from the water-soluble fraction (Fig. 6). These results indicate that the 40 kD doublet antigen constitutes a significant part of the total photoreceptor cell protein. To test whether these water-insoluble antigens were membrane-associated proteins, a Triton X-100-soluble protein fraction was prepared from a pellet of water-insoluble retinal proteins. As shown in Fig. 7, the first extraction with Triton X-100 yielded only residual water-soluble protein, while the second extraction resulted in partial recovery of the 40 kD autoantigen doublet from the pellet. These results suggest that the autoantigens are membrane-bound proteins.
Fig. 5.
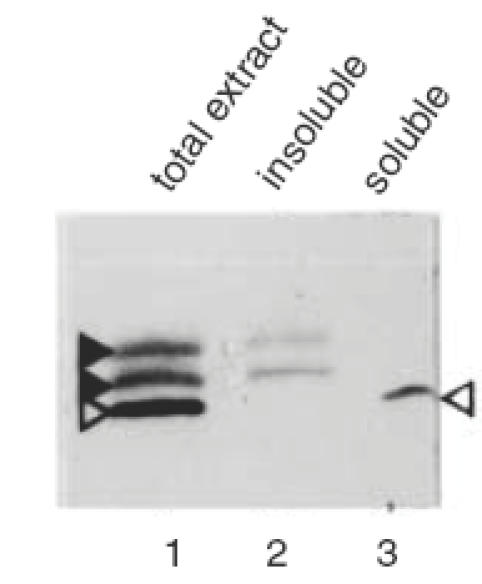
Western blot analysis of rat total retinal protein (lane 1), the water-insoluble fraction (lane 2) and the water-soluble fraction (lane 3). The applied serum (1:200) was from CAR patient 2. The position of the antigens is indicated by arrowheads as in Fig. 4
Fig. 6.
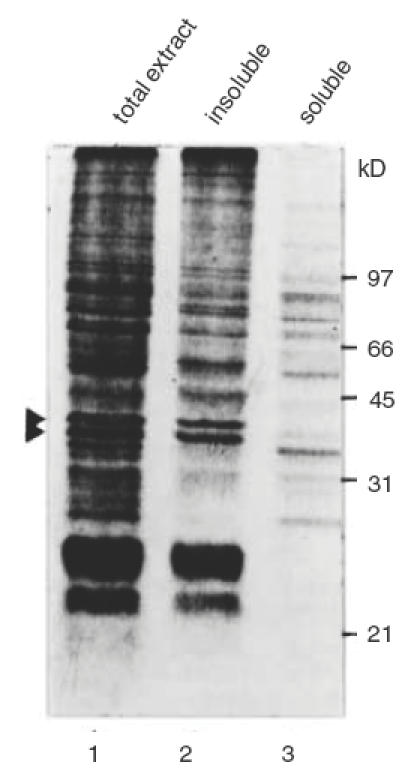
Coomassie-stained gel of total rat retinal proteins (lane 1), the water-insoluble fraction (lane 2) and the water-soluble fraction (lane 3). The position of the 40kD doublet is indicated by arrowheads. Molecular weight marker values are indicated at the right.
Fig. 7.
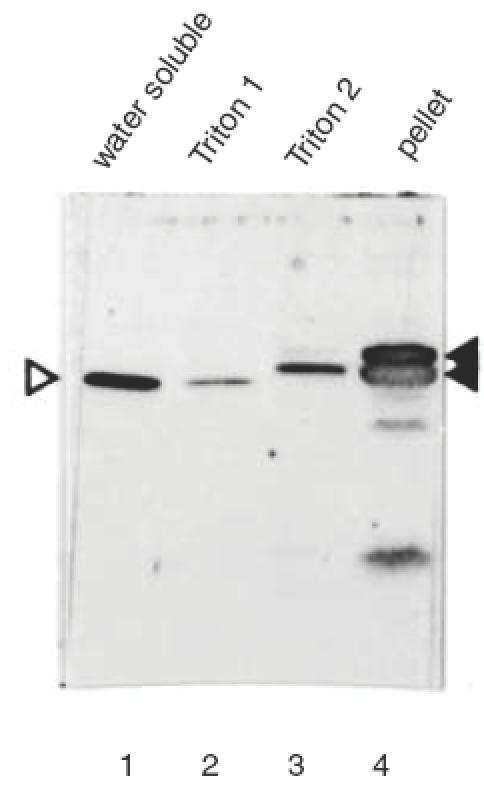
Triton X-100 extraction of rat retinal water-insoluble protein. Rat retinas were fractionated into a water-soluble (lane 1) and a water-insoluble fraction. The water-insoluble fraction was extracted twice with Triton-X100 to isolate membrane-associated proteins (lanes 2 and 3). The remaining pellet was also analysed (lane 4). The applied patient serum (1:200) was from CAR patient 2. The position of the antigens is indicated by arrowheads as in Fig. 4
DISCUSSION
In this report, we describe two patients with similar ophthalmologic complaints, including reduced colour vision and disturbed dark adaptation, in association with prostate or colon carcinoma. Both patients had circulating autoantibodies directed against an antigen doublet of around 40 kD, while one patient revealed an additional strong antibody reaction with a 35kD antigen. These antigens are different from known autoreactive proteins in CAR patients but similar to the major 23 kD CAR-associated autoantigen, recoverin; the 40 kD doublet showed predominant expression in the retina. Tissue specificity of autoantigens is, however, not a prerequisite for organ-specific immunological damage. Autoantibodies to proteins showing little tissue specificity, such as the 46 kD enolase antigen [4], neurofilaments of 58–62 kD, 145 kD and 205 kD [10], and the 70 kD heat-shock cognate protein [5, 6], have also been implicated in the aetiology of CAR. Therefore the autoantibodies to the 35 kD antigen, located as a sharp line in the OPL, might well have contributed to the visual deterioration. Labelling of the OPL was also reported in cutaneous melanoma-associated retinopathy but the antigen was not further characterized [18]. In contrast, the retinopathy in the patients described by Mizener et al.[19] had circulating antibodies against structures present only in the IPL. Both the OPL and the IPL contain synaptic connections between the various retinal neurones, and a high level of autoantibody to components of these delicate structures is likely to contribute to the visual problems of these patients. Remarkably, the 35 kD autoantigen appeared to be present in all tissues tested, with the highest expression in kidney and liver. Possibly, this protein is part of a transmembrane channel, which are abundant in these two organs and retinal synapses.
The exact mechanisms by which autoantibodies directed to intracellular retinal antigens are generated and lead to visual impairment are poorly understood, and have only been studied in detail for recoverin and enolase [14, 15]. Cancer cells of CAR patients have been shown to express the retina-specific recoverin, which may lead to a humoral autoimmune response [20–22]. These anti-recoverin antibodies were able to penetrate living cells and to induce apoptosis [23]. Although recoverin-specific T cells have been described in a patient with CAR, the presence of these cells was not accompanied by overt disease. Whether the autoantibodies identified in this study have cytotoxic potential remains to be investigated, but the fact that these antibodies are directed against abundant membrane-bound proteins of photoreceptors and the OPL indicates that probably, the photoreceptors themselves are involved rather than the bipolar, horizontal or Müller cells (35 kD) which are the target of autoantibodies in other studies [12, 24]. The abundance of the 40 kD autoantigen doublet in the insoluble fraction of the retinal extract indicates that these proteins are major constituents of retinal tissue. Although highly expressed in the retina, the antigens were also detected in the spleen and at a low level in the lung. This indicates that these proteins are unlikely to be abundant photoreceptor membrane-specific proteins like rhodopsin, peripherin/RDS or rod outer segment membrane protein, since they have not been reported to be expressed outside the retina. Although several attempts were made to identify the nature of the 40 kD autoantigen doublet by protein sequence analysis, the N-terminal block and the insolubility of these proteins have precluded such an analysis.
The autoimmune reaction against recoverin has been most extensively investigated, and determination of antibodies against this protein is considered to be a valid test for the diagnosis of CAR, although in patients with retinitis pigmentosa, anti-recoverin immunoreactivity was found without systemic malignancy [25]. In some cases of CAR, the occurrence of autoantibodies precedes the diagnosis of cancer, and this has led to a search for additional autoantigens in cancer patients with unexplained retinopathies. In this study, we have identified three more autoantigens; two of these are abundant water-insoluble proteins of approximately 40 kD and located in membranes of the photoreceptor outer segment, and one of approximately 35 kD is located in the OPL. Although this is the first report to describe circulating autoantibodies to the OPL of the retina in CAR, the autoantibodies directed to the water-insoluble (photoreceptor) proteins could have been present in CAR patients more often; in studies using Western blot with water-soluble retinal proteins for the analysis of patients’ sera, these autoantbodies would not have been detected. The use of total retinal extracts for these analyses is likely to add more retinal proteins to the still growing list of autoantigens involved in cancer-associated retinopathy.
References
- 1.Adamus G, Guy J, Schmied JL, Arendt A, Hargrave PA. Role of anti-recoverin autoantibodies in cancer-associated retinopathy. Invest Ophthalmol Vis Sci. 1993;34:2626–33. [PubMed] [Google Scholar]
- 2.Polans AS, Bucczlko J, Crabb J, Palczewski K. A photoreceptor calcium binding protein is recognized by autoantibodies obtained from patients with cancer-associated retinopathy. J Cell Biol. 1991;112:981–9. doi: 10.1083/jcb.112.5.981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Thirkill CE, Tait RC, Tyler NK, Roth AM, Keltner JN. The cancer-associated retinopathy antigen is a recoverin-like protein. Invest Ophthalmol Vis Sci. 1992;3:2768–72. [PubMed] [Google Scholar]
- 4.Adamus G, Aptsiauri N, Guy J, Heckenlively J, Flannery J, Hargrave PA. The occurrence of serum autoantibodies against enolase in cancer-associated retinopathy. Clin Immunol Immunopathol. 1996;78:120–9. doi: 10.1006/clin.1996.0021. 10.1006/clin.1996.0021. [DOI] [PubMed] [Google Scholar]
- 5.Ohguro H, Ogawa K, Maeda T, Maeda A, Maruyama I. Cancer-associated retinopathy induced by both anti-recoverin and anti-hsc70 antibodies in vivo. Invest Ophthalmol Vis Sci. 1999;40:3160–7. [PubMed] [Google Scholar]
- 6.Ohguro H, Ogawa K, Nakagawa T. Recoverin and Hsc 70 are found as autoantigens in patients with cancer-associated retinopathy. Invest Ophthalmol Vis Sci. 1999;40:82–9. [PubMed] [Google Scholar]
- 7.Kikuchi T, Arai J, Shibuki H, Kawashima H, Yoshimura N. Tubby-like protein 1 as an autoantigen in cancer-associated retinopathy. J Neuroimmunol. 2000;103:26–33. doi: 10.1016/s0165-5728(99)00163-0. [DOI] [PubMed] [Google Scholar]
- 8.Crofts JW, Bachynski BN, Odel JG. Visual paraneoplastic syndrome associated with undifferentiated endometrial carcinoma. Can J Ophthalmol. 1988;23:128–32. [PubMed] [Google Scholar]
- 9.Jacobson DM, Thirkill CE, Tipping SJ. A clinical triad to diagnose paraneoplastic retinopathy. Ann Neurol. 1990;28:162–7. doi: 10.1002/ana.410280208. [DOI] [PubMed] [Google Scholar]
- 10.Korngruth SE, Kalinke T, Grunwald GB, Schutta H, Dahl D. Anti-neurofilament antibodies in the sera of patients with small cell carcinoma of the lung and with visual paraneoplastic syndrome. Cancer Res. 1986;46:2588–95. [PubMed] [Google Scholar]
- 11.Ohkawa T, Kawashima H, Makino S, et al. Cancer-associated retinopathy in a patient with endometrial cancer. Am J Ophthalmol. 1996;122:740–2. doi: 10.1016/s0002-9394(14)70501-x. [DOI] [PubMed] [Google Scholar]
- 12.Peek R, Verbraak F, Coevoet HM, Kijlstra A. Müller cell-specific autoantibodies in a patient with progressive loss of vision. Invest Ophthalmol Vis Sci. 1998;39:1976–9. [PubMed] [Google Scholar]
- 13.Thirkill CE, Roth AM, Keltner JL. Cancer-associated retinopathy. Arch Ophthalmol. 1987;105:372–5. doi: 10.1001/archopht.1987.01060030092033. [DOI] [PubMed] [Google Scholar]
- 14.Adamus G, Amundson D, Seigel GM, Machnicki M. Anti-enolase-alpha autoantibodies in cancer-associated retinopathy: epitope mapping and cytotoxicity on retinal cells. J Autoimmun. 1998;11:671–7. doi: 10.1006/jaut.1998.0239. 10.1006/jaut.1998.0239. [DOI] [PubMed] [Google Scholar]
- 15.Adamus G, Machnicki M, Elerding H, Sugden B, Blocker YS, Fox DA. Antibodies to recoverin induce apoptosis of photoreceptor and bipolar cells in vivo. J Autoimmun. 1998;11:523–33. doi: 10.1006/jaut.1998.0221. 10.1006/jaut.1998.0221. [DOI] [PubMed] [Google Scholar]
- 16.Chen W, Elias RV, Cae W, Lerious V, McGinnis JF. Anti-recoverin antibodies cause the apoptotic death of mammalian photoreceptor cells in vitro. J Neurosci Res. 1999;57:706–18. 10.1002/(sici)1097-4547(19990901)57:5<706::aid-jnr12>3.3.co;2-7. [PubMed] [Google Scholar]
- 17.Broekhuyse RM, Kuhlmann E. Uveitogenic 28/30kD and 43kD polypeptides in pigment epithelial membranes of the retina. Ocular Immunol Inflamm. 1997;5:19–26. doi: 10.3109/09273949709085046. [DOI] [PubMed] [Google Scholar]
- 18.Kiratli H, Thirkill CE, Bilgic S, Eldem B, Kececi A. Paraneoplastic retinopathy associated with metastatic cutaneous melanoma of unknown primary site. Eye. 1997;11:889–92. doi: 10.1038/eye.1997.227. [DOI] [PubMed] [Google Scholar]
- 19.Mizener JB, Kimura AE, Adamus G, et al. Autoimmune retinopathy in the absence of cancer. Am J Ophthalmol. 1997;123:607–18. doi: 10.1016/s0002-9394(14)71073-6. [DOI] [PubMed] [Google Scholar]
- 20.Polans AS, Witkowski D, Haley TL, Amundson D, Baizer L, Adamus G. Recoverin, a photoreceptor specific calcium-binding protein, is expressed by the tumor of a patient with cancer-associated retinopathy. Proc Natl Acad Sci USA. 1995;92:9176–80. doi: 10.1073/pnas.92.20.9176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Maeda A, Ohguro H, Maeda T, et al. Aberrant expression of photoreceptor-specific calcium-binding protein (recoverin) in cancer cell lines. Cancer Res. 2000;60:1914–20. [PubMed] [Google Scholar]
- 22.Yamaji Y, Matsubara S, Yamadori I, et al. Characterization of a small-cell-lung-carcinoma cell line from a patient with cancer-associated retinopathy. Int J Cancer. 1996;65:671–6. doi: 10.1002/(SICI)1097-0215(19960301)65:5<671::AID-IJC18>3.0.CO;2-A. 10.1002/(sici)1097-0215(19960301)65:5<671::aid-ijc18>3.3.co;2-4. [DOI] [PubMed] [Google Scholar]
- 23.Adamus G, Machnicki M, Seigel GM. Apoptotic retinal cell death induced by antirecoverin autoantibodies of cancer-associated retinopathy. Invest Opthalmol Vis Sci. 1997;38:283–91. [PubMed] [Google Scholar]
- 24.Thirkill CE. Lung cancer-induced blindness. Lung Cancer. 1996;14:253–64. doi: 10.1016/0169-5002(96)00551-x. 10.1016/0169-5002(96)00551-x. [DOI] [PubMed] [Google Scholar]
- 25.Heckenlively JR, Fawzi AA, Oversier J, Jordan BL, Aptsiauri N. Autoimmune retinopathy: patients with antirecoverin immunoreactivity and panretinal degeneration. Arch Ophthalmol. 2000;118:1525–33. doi: 10.1001/archopht.118.11.1525. [DOI] [PubMed] [Google Scholar]


