Abstract
Even though the existence of phosphodiesterase (PDE) 7 in T cells has been proved, the lack of a selective PDE7 inhibitor has confounded an accurate assessment of PDE7 function in such cells. In order to elucidate the role of PDE7 in human T cell function, the effects of two PDE inhibitors on PDE7A activity, cytokine synthesis, proliferation and CD25 expression of human peripheral blood mononuclear cells (PBMC) were determined. Recombinant human PDE7A was obtained and subjected to cyclic AMP-hydrolysis assay. PBMC of Dermatophagoides farinae mite extract (Df)-sensitive donors were stimulated with the relevant antigen or an anti-CD3 monoclonal antibody (MoAb). PBMC produced IL-5 and proliferated in response to stimulation with Df, while stimulation with anti-CD3 MoAb induced CD25 expression and messenger RNA (mRNA) synthesis of IL-2, IL-4 and IL-5 in peripheral T cells. A PDE inhibitor, T-2585, which suppressed PDE4 isoenzyme with high potency (IC50 = 0·00013 μm) and PDE7A with low potency (IC50 = 1·7 μm) inhibited cytokine synthesis, proliferation and CD25 expression in the dose range at which the drug suppressed PDE7A activity. A potent selective inhibitor of PDE4 (IC50 = 0·00031 μm), RP 73401, which did not effectively suppress PDE7A (IC50 > 10 μm), inhibited the Df- and anti-CD3 MoAb-stimulated responses only weakly, even at 10 μm. PDE7 may play a critical role in the regulation of human T cell function, and thereby selective PDE7 inhibitors have the potential to be used to treat immunological and inflammatory disorders.
Keywords: cyclic AMP, cytokine, phosphodiesterase, T cell
INTRODUCTION
Cyclic AMP (cAMP) has been recognized as an important second messenger regulating immune and inflammatory responses. Agents with the ability to elevate intracellular cAMP levels have been demonstrated to possess immonosuppressive and anti-inflammatory properties [1,2]. These effects are caused in part by the inhibition of various T cell functions including proliferation [3], cytokine production [4–6] and expression of activation markers on the cell surface [7].
One mechanism by which cAMP may be elevated within cells is by inhibition of phosphodiesterase (PDE). PDE comprises a family of enzymes, currently known to exist in at least 11 different isoenzyme forms which are characterized by a variety of properties, including their sensitivity to different inhibitors [8–12]. With regard to T cell function, the existence of three distinct cAMP-PDE isoenzymes, PDE3, 4 and 7, has been implicated [13–16]. However, earlier studies have not clarified the comparative roles of these isoenzymes in the degradation of intracellular cAMP in human T cells. PDE4 is one of the most convincing candidates for this role, as inhibition of various T cell functions by ‘so-called’ selective PDE4 inhibitors has been documented [14,16–19]. Nevertheless, several earlier studies are in apparent contradiction with the idea of a principle role for PDE4, as the potency of PDE4 inhibitors to suppress cytokine production and proliferation of human peripheral T cells was not correlated with their ability to suppress PDE4 activity [19–23]. Regarding PDE3, few reports support the contribution of PDE3 in the regulation of T cell function, and a lack of effect of various PDE3 inhibitors on T cell activation has been documented [16–19,22,23]. The existence of a rolipram-insensitive cAMP-PDE type enzyme in T cells was described first by Ichimura and Kase [13] and thereafter it was identified as PDE7A by Bloom and Beavo [24]. However, the role of PDE7 in T cell function is still unclear, as a selective PDE7 inhibitor had not been established.
We have generated a new PDE inhibitor, T-2585 (2-{4-2,3-bis(hydroxymethyl)-6,7-diethoxy-1-naphthalenyl}-2-pyridinyl]-4-(3-pyridinyl)-1(2H)-phthalazinone) [25]. This agent displays potent inhibitory activity against PDE4 (IC50 = 0·00013 μm) and 14 000-fold greater selectivity compared with PDE3 [25]. Another PDE4 inhibitor, RP 73401 (3-cyclopentyloxy-N-(3,5-dichloro-4-pyridyl)-4-methoxybenzamide), has been evaluated to have similar potency of PDE4 suppression (IC50 = 0·00031 μm) to T-2585 [25,26]. RP 73401 failed to suppress the activity of other PDE isoenzymes even at 10 μm[25–27].
In this study, we obtained an interesting result in that T-2585 suppressed PDE7A activity with an IC50 of 1·7 μm, whereas RP 73401 suppressed it only weakly at 10 μm. This finding provides a useful insight into the significance of PDE7 in T cell function, since it is possible to predict the potential role of PDE7 by comparing the effects of the two PDE inhibitors. In this study, therefore, the effects of T-2585 and RP 73401 on various T cell functions were characterized using human peripheral blood mononuclear cells (PBMC).
MATERIALS AND METHODS
Materials
T-2585 and RP 73401 were synthesized by the Discovery Research Laboratory, Tanabe Seiyaku Co. (Osaka, Japan). Dermatophagoides farinae mite extract (Df; Torii Pharmaceutical Co., Tokyo, Japan), anti-CD3 monoclonal antibody (MoAb; Ortho, Raritan, NJ, USA), purified rat antimouse/human IL-5 monoclonal antibody (Pharmingen, San Diego, CA, USA), biotinylated rat antihuman IL-5 monoclonal antibody (Pharmingen), Cell Titer 96™ AQueous Non-Radioactive Cell Proliferation Assay kit (Promega, Madison, WI, USA), ISOGEN (Nippongene, Tokyo, Japan), murine Moloney leukaemia virus reverse transcriptase (Perkin-Elmer Cetus, Norwalk, CT, USA), GeneAmp® DNA polymerase (Perkin-Elmer Cetus), RT-PCR Amplimer Sets (Clontech, Palo Alto, CA, USA), fluorescein isocyanate (FITC)-labelled anti-CD25 antibody (Becton Dickinson, Franklin Lakes, NJ, USA), phycoerythrin (PE)-labelled anti-CD3 antibody (Becton Dickinson), Ficoll-Paque (Pharmacia, Uppsala, Sweden) and AIM-V medium (Gibco BRL, Gaithersburg, MD, USA) were used. All other reagents were obtained from Sigma (St Louis, MO, USA).
Expression of recombinant PDE7A protein
Recombinant human PDE7A was prepared as described previously [12]. The cDNA encoding human PDE7A (GeneBank accession no. Q13946) [28], amplified by polymerase chain reaction (PCR), was cloned in pFLAG expression vector (Amersham Pharmacia Biotech, Uppsala, Sweden). The correct sequence of the resulting construct, pFLAG-PDE7A, was verified by sequencing. The plasmid was transfected into COS-7 cells by LipofectAMINE 2000 (Gibco BRL) according to the manufacturer’s instructions. Forty-eight hours after transfection, cells were washed with ice-cold phosphate-buffered saline and scraped in ice-cold homogenization buffer (20 mm Tris-HCl, pH 7·4, 2 mm magnesium acetate, 0·3 mm CaCl2, 1 mm dithiothreitol, 1·3 mm benzamidine and 1 mm NaN3). The cell suspension was disrupted by a sonicator (TOMY Seiko, Tokyo, Japan) for 15 s (twice at 1-min intervals), and homogenates were centrifuged at 100 000 ×g for 60 min The resultant supernatant was collected and stored at 4°C until use. The protein concentration of the supernatant was determined by a DC protein assay kit (Bio-Rad, CA, USA) using bovine serum albumin as a standard.
Assay of cAMP-PDE activity
PDE activity was determined by a modification of the method of Thompson et al.[29]. The assay buffer contained 50 mm Tris-HCl, pH 8·0, 5 mm MgCl2, 4 mm 2-mercaptoethanol, 0·33 mg/ml bovine serum albumin, 22 nm3H]cAMP and unlabelled cAMP. Reactions were started by adding 0·2–0·5 μl enzyme solution to 500 μl assay buffer, and incubated at 37°C for 30 min. After boiling for 90 s, the mixture was added to 100 μl of 1 mg/ml Crotalus atrox snake venom and incubated at 37°C for 30 min. Reactions were stopped by the addition of 500 μm methanol, and the resultant solutions were applied to Dowex (1 × 8–400) columns. Aqueous scintillation mixture was added to each eluate, and radioactivity was measured with a scintillation counter. In evaluation of the effects of PDE inhibitors, the agents examined were dissolved in dymethyl sulphoxide. Assays were performed in triplicate at three or four different concentrations, the mean of the determinations at each concentration was plotted, and IC50 values were determined graphically.
Preparation of human PBMC
Adult male volunteers who were employees of Tanabe Seiyaku Co. were enlisted. All subjects gave written informed consent to the protocol as approved by the Company’s ethics committee. As a result of preliminary examination, we decided to enroll nine donors whose PBMC produced IL-5 and proliferated in response to Df antigen in this study. No subjects were receiving medication. Heparinized venous blood was taken between 9 and 10 a.m. PBMC were prepared by Ficoll-Paque density gradient centrifugation as described previously [20]. Cells were washed and suspended in AIM-V medium.
Cell cultures
PBMC suspended in AIM-V medium (2 × 106/ml) were cultured in 24-well culture plates with or without Df for 6 days. In some experiments, PBMC were cultured with anti-CD3 MoAb (1 ng/ml) for the designated time periods. For cytokine assays, supernatants were harvested, and then frozen at – 70°C until used. Each test compound was added at the start of culture.
Quantification of IL-5 in culture supernatants
Concentration of IL-5 in the culture supernatant was measured by enzyme-immunoassay (EIA). Purified rat antimouse/human IL-5 MoAb and biotinylated rat antihuman IL-5 MoAb were used as the capture and detection antibodies, respectively. The range of detection of the assay system was 2 pg/ml to 10 ng/ml.
Cell proliferation assay
After PBMC (2 × 105/well) were cultured for 6 days with Df and test compound in 96-well flat-bottomed culture plates, proliferation was assessed by the bioreduction of tetrazolium salt into formazan as previously described [30] with Cell Titer 96™ AQueous Non-Radioactive Cell Proliferation Assay kit according to the manufacturer’s manual. Briefly, 20 μl tetrazolium assay solution was added to 100 μl cell culture in each well. After incubation for 4 h at 37°C, the absorbance of each well at 515 nm was measured. Results were expressed as stimulation index, which was calculated as the ratio of the absorbance in stimulated culture to that in control culture.
Cytokine messenger RNA (mRNA)expression
Gene expression of IL-2, IL-4 and IL-5 was analysed by the reverse transcription-polymerase chain reaction (RT-PCR) method, as reported previously [31]. Briefly, RNA was extracted from the pelleted cells essentially following the one-step acid guanidinium isothiocyanate/phenol chloroform extraction method [32] using ISOGEN. cDNA was synthesized from 1 μg cytoplasmic RNA using random primers and murine Moloney leukaemia virus reverse transcriptase. PCR was performed using the following RT-PCR amplimer sets.
Il-2 5′-CATGCACTAAGTCTTGCACTTGTCA-3′
5′-CGTTGATATTGCTGATTAAGTCCCTG-3′
Il-4 5′-ATGGGTCTCACCTCCCAACTGCT-3′
5′-CGAACACTTTGAATATTTCTCTCTCAT-3′
Il-5 5′-GCTTCTGCATTTGAGTTTGCTAGCT-3′
5′-TGGCCGTCAATGTATTTCTTTATTAAG-3′
β-actin 5′-ATGGATGATGATATCGCCGCG-3′
5′CTAGAAGCATTTGCGGTGGAC GATGGGGGCC-3′
To 50 μl (final volume) amplification solution (50 mm KCl, 10 mm Tris-HCl (pH 8·3), 2 mm MgCl2, 0·01% gelatin, 0·2 mm each deoxynucleotide triphosphate), 2 μl cDNA (corresponding to about 250 ng starting RNA material), 0·4 μm each primer, and 2 U GeneAmp® DNA polymerase were added. The mixture was heated at 95°C for 2 min, followed by 30 cycles, each consisting of incubation for 30 s at 95°C, 30 s at 60°C and 90 s at 73°C. The PCR products were analysed by 2% agarose gel electrophoresis in the presence of ethidium bromide. Expected sizes of PCR amplification products were 305, 456, 294, and 838 bp for IL-2, IL-4, IL-5 and β-actin, respectively.
Flow cytometric analysis of CD25 expression on cell surface of PBMC
After PBMC (2 × 106/well) were cultured for 3 days with anti-CD3 MoAb (1 ng/ml), cells were harvested, washed and resuspended in staining buffer (PBS supplemented with 0·25% BSA and 0·1% NaN3). After blocking with murine IgG for 1 h at 4°C, these cells were incubated with FITC-labelled anti-CD25 antibody or their control antibodies of appropriate isotype for 30 min at 4°C. In some experiments, cells were counter-stained with PE-labelled anti-CD3 antibody. After another two washes, cells were analysed using a FACScan® flow cytometer (Becton Dickinson, Mountain View, CA, USA). Dead cells were gated out by their forward and angle light scatter profile. Data were analysed using the CellQuest® program.
Statistics
All data are presented as mean or mean ± s.e. Statistical analysis was performed by paired or Student’s t-test for comparison between two groups and one-way anova with Bonferroni’s method for three groups or more. P < 0·05 was considered statistically significant.
RESULTS
Effects of PDE inhibitors on PDE7 activity
The first experiment was carried out to delineate the effects of T-2585 and RP 73401 on PDE7 activity, using purified recombinant human PDE7A. T-2585 inhibited PDE7A activity in a concentration-dependent manner (0·1–10 μm), and the IC50 value of PDE7A inhibition by T-2585 was estimated to be 1·7 μm (Table 1). On the contrary, relatively weak suppression of PDE7A activity was obtained with the other PDE inhibitor, RP 73401, with maximum inhibition at 10 μm being only 35%.
Table 1.
Effects of T-2585 and RP 73401 on PDE4 and 7 A activity
| IC50 (μm) | ||
|---|---|---|
| Isoenzyme | T-2585 | RP 73401 |
| PDE4* | 0·00013 | 0·00031 |
| PDE7A | 1·7 | >10 |
Recombinant human PDE7A was purified from pFLAG-PDE7A-transfected COS-7 cells and incubated in the presence of 3H]-cAMP with or without various concentrations of T-2585 or RP 73401. The radioactivity of hydrolysed 3H]-cAMP was then measured. IC50 values were determined graphically from a concentration–inhibition curve.Data are cited from our previous report [25].
Effects of PDE inhibitors on IL-5 production by PBMC
The effects of T-2585 and RP 73401 on Df-induced IL-5 production by PBMC were determined. PBMC were incubated with Df, and the resulting supernatants were assayed for IL-5. IL-5 was produced spontaneously by PBMC of several individuals, while there was a significant increase in IL-5 production upon stimulation with Df, and the antigen-specific IL-5 production reached a maximum on day 6 (Fig. 1a). The optimal concentration of antigen was 1–10 μg/ml in most subjects. Other cytokines, IL-2 and IL-4, were hardly detected in the culture supernatants of PBMC incubated with or without antigen. T-2585 suppressed antigen-induced IL-5 production in a concentration-dependent manner (0·1–10 μm) (Fig. 1b). However, RP 73401 caused little inhibition of IL-5 production by PBMC even at 10 μm (22%). No concentrations of T-2585 or RP-73401 used in this study displayed cytotoxicity to PBMC (data not shown). We have previously demonstrated that IL-5 produced by PBMC is derived exclusively from CD4+ T cells [31], suggesting that T-2585 suppresses IL-5 production by CD4+ T cells.
Fig.1.

Effects of T-2585 and RP 73401 on IL-5 production by human PBMC. PBMC (2 × 106/ml) were incubated with or without Df (10 μg/ml) for 6 days. Various concentrations of T-2585 (•) or RP 73401 (□) were included throughout the culture period. The quantity of IL-5 in the culture supernatants was measured by EIA. Data from each individual subject (a) and the percentage inhibition of Df-induced IL-5 production by PBMC (b) are shown (n = 4–9). **P < 0·01 (paired t-test). #P < 0·05, compared with control cultures (Bonferroni’s method). ††P < 0·01, compared with RP 73401 (Student’s t-test).
Effects of PDE inhibitors on proliferation of PBMC
The effects of T-2585 and RP 73401 on another antigen-specific response of PBMC, proliferation, were examined next. As an antigen-specific proliferative response of PBMC was detected on day 2 and reached a maximum on day 6, proliferation was assessed on day 6. The stimulation index was highest at an antigen concentration of 10 μg/ml (2·1 ± 0·22, n = 6). As shown in Fig. 2, antigen-specific proliferation of PBMC was inhibited by T-2585 in a concentration-dependent manner. On the other hand, the maximum inhibition of PBMC proliferation obtained with 0·1–10 μm RP 73401 was only 20%. Almost 90% of the living cells present after 6 days of culture with Df antigen were determined to be CD4+ T cells by flow cytometric analysis (data not shown), suggesting that T-2585 inhibited the proliferation of CD4+ T cells.
Fig.2.
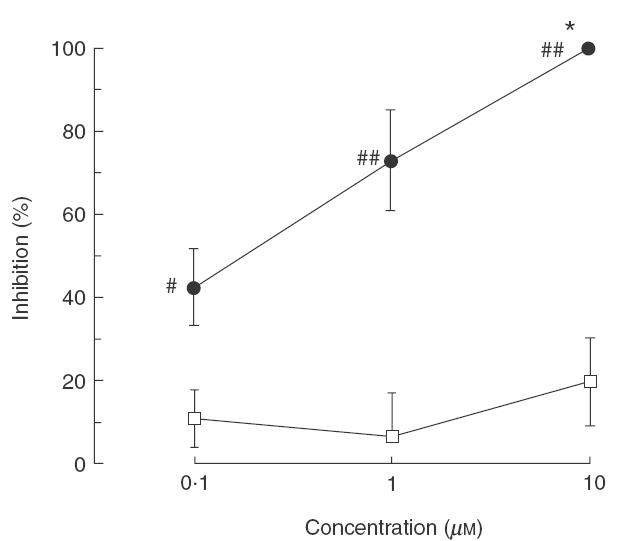
Effects of T-2585 and RP 73401 on the proliferation of human PBMC. PBMC (2 × 106/ml) were incubated with Df (10 μg/ml) in the presence or absence of T-2585 (•) or RP 73401 (□) for 6 days. The proliferation of PBMC was measured by a non-radioactive cell proliferation assay system. The percentage inhibition of Df-induced proliferation of PBMC is shown (n = 4). *P < 0·05, compared with control cultures (Bonferroni’s method). #P < 0·05, ##P < 0·01, compared with RP 73401 (Student’s t-test).
Effects of PDE inhibitors on cytokine mRNA expression in PBMC
The dose of T-2585 most effective at inhibiting proliferation (Fig. 2) was similar to that capable of inhibiting IL-5 production (Fig. 1), suggesting that the attenuation of IL-5 production by T-2585 was caused at least in part by the reduction in PBMC numbers. In order to evaluate whether T-2585 has a direct effect on cytokine synthesis, expression of cytokine mRNA in PBMC upon stimulation with anti-CD3 MoAb was examined. The CD3 molecule is associated with the T cell receptor, and the stimulation signal induced by anti-CD3 MoAb passes through this receptor. Anti-CD3 MoAb was therefore used to mimic the stimulus produced by a specific antigen. No obvious expression of any cytokine mRNA was obtained in unstimulated PBMC. Expression of IL-5 mRNA was induced in PBMC upon anti-CD3 MoAb (1 ng/ml) stimulation, and expression reached a maximum at 6 h (Fig. 3). Interestingly, mRNA for two other T cell cytokines, IL-2 and IL-4, was also expressed clearly upon anti-CD3 MoAb stimulation, even though neither cytokine was detected in the culture supernatant of PBMC stimulated with Df. IL-2 and IL-4 are T cell and/or B cell growth factors [33,34], and receptors for these two cytokines are expressed abundantly on the cell surface of PBMC, suggesting that both cytokines produced by peripheral T cells are quickly trapped by these receptors.
Fig. 3.

Effects of T-2585 and RP 73401 on cytokine mRNA expression in human PBMC. PBMC (4 × 106/ml) were incubated with anti-CD3 antibody (1 ng/ml) or PMA (20 nm) + ionomycin (1 μm) with or without T-2585 or RP 73401 for 6 h. Total RNA was then extracted, reverse transcribed and amplified by PCR. Lanes 1 and 5: no stimulation; 2–4: anti-CD3 antibody stimulation; 6–8: PMA + ionomycin stimulation; 3 and 7: + 10 μm T-2585; 4 and 8: + 10 μm RP 73401. This is one representative result from a total of three separate experiments.
As shown in Fig. 3, 10 μm T-2585 significantly diminished IL-2, IL-4 and IL-5 mRNA expression in anti-CD3 MoAb-stimulated PBMC. Suppression of mRNA expression of these cytokines by T-2585 was obtained even at 1 μm (data not shown). To confirm the specificity of the effect of T-2585, we next examined the possibility that T-2585 affects cytokine mRNA expression initiated by phorbol-ester and calcium ionophore. mRNA expression of IL-2, IL-4 and IL-5 was significantly up-regulated by stimulation with phorbol 12-myristate 13 acetate (PMA) + ionomycin. T-2585 (10 μm) did not, however, affect this PMA + ionomycin-induced cytokine mRNA expression at all (Fig. 3). Neither anti-CD3 MoAb- nor PMA + ionomycin-stimulated IL-2, IL-4 and IL-5 mRNA expression was affected by 10 μm RP 73401.
These findings suggest that T-2585 inhibits T cell receptor-mediated production of not only IL-5, but also IL-2 and IL-4 in human peripheral T cells at a point in the upstream signalling pathway leading to the regulation of gene transcription.
Effects of PDE inhibitors on CD25 expression of PBMC
We also examined the effects of T-2585 and RP 73401 on another T cell activation marker, CD25. CD25 is the IL-2 receptor α-chain and is expressed on the T cell surface membrane following cell activation. After PBMC had been stimulated with anti-CD3 MoAb, CD25 expression was determined by flow cytometry. CD25 was hardly expressed on the cell surface of unstimulated PBMC but its expression was clearly up-regulated by anti-CD3 MoAb (1 ng/ml) stimulation, reaching a maximum after 3 days of stimulation (21 ± 8·1% of PBMC, n = 3; Fig. 4a). As 60–70% of the CD25+ cells in PBMC were determined to be CD3+, (data not shown), the effect of the PDE inhibitors on CD25 expression was determined after the T cells in PBMC were gated by counter-staining with a PE-labelled CD3 antibody. As shown in Fig. 4b, T-2585 suppressed anti-CD3 MoAb-induced CD25 expression on human T cells in a concentration-dependent manner (0·1–10 μm). On the other hand, relatively weak inhibition of CD25 expression was obtained with RP 73401 (24–33%).
Fig.4.

Effects of T-2585 and RP 73401 on CD25 expression of human T cells. PBMC (2 × 106/ml) were incubated with anti-CD3 MoAb (1 ng/ml) with or without T-2585 (•) or RP 73401 (□) for 3 days. CD25 expression on PBMC was analysed by flow cytometry. (a) The histograms of PBMC correspond to representative experiments with (solid line) or without (bold line) stimulation. The dotted line indicates baseline fluorescence obtained with FITC-labelled control antibody. (b) Percentage inhibition of anti-CD3 MoAb-induced CD25 expression on CD3+ cells is shown (n = 3). *P < 0·05, compared with control cultures (Bonferroni’s method). #P < 0·05, compared with RP 73401 (paired t-test).
DISCUSSION
Our findings demonstrate that the PDE inhibitor, T-2585, acts on human peripheral T cells to suppress: IL-5 protein production; IL-2, IL-4 and IL-5 mRNA expression; cell proliferation; and CD25 expression in the dose range at which the drug inhibits PDE7A activity. However, another potent PDE inhibitor RP 73401, which does not inhibit PDE7A activity effectively (IC50 = > 10 μm), suppressed only those T cell responses weakly at 10 μm. Since the IC50 of RP73401 for PDE4 suppression is 0·00031 μm, this suggests that PDE7, but not PDE4, plays a substantial role in regulating human T cell function.
cAMP-elevating agents affect T cell function [1,4,35,36] and the inhibition of human cytokine production by such agents has been documented [5,6,20]. Prostaglandin E2, forskolin and a cAMP-analogue, dibutyryl cAMP, have been shown to suppress IL-2, IL-4 and IL-5 production by T cell receptor-stimulated PBMC from atopic asthmatics [20]. The suppression of both CD25 expression and proliferation of human T cells by prostaglandin E2 and other cAMP-affecting agents has also been reported [7,35,37,38]. In addition, several selective and nonselective PDE inhibitors suppress human T cell responses such as proliferation, mRNA expression and protein synthesis of cytokines [14,16–23]. We have reported previously that a PDE inhibitor that is structurally related to T-2585 inhibited cAMP-PDE activity in human PBMC and subsequently increased the level of intracellular cAMP [20]. Several groups have reported that cAMP suppresses Ca2+ release from intracellular stores [39,40]. Such suppression is mediated by the cAMP-activated protein kinase PKA acting on the signalling pathway downstream of the T cell receptor [40,41]. We demonstrated recently that prostaglandin E2 suppresses both intracellular Ca2+ elevation and IL-5 production of human T cells stimulated by anti-CD3 MoAb [42]. This suggests that the suppression of T cell function by cAMP is caused, at least in part, by down-modulation of intracellular Ca2+ influx. This is consistent with the result that cytokine mRNA expression in PBMC induced by anti-CD3 MoAb, but not PMA + ionomycin, is suppressed by T-2585. Taken together, it is reasonable to propose that the inhibition of PDE7 activity by T-2585 results in the suppression of T cell function as a consequence of the increase in the level of intracellular cAMP.
Recently, Li et al. reported that PDE7 is induced in T cells upon stimulation with anti-CD3 and anti-CD28 antibody [43]. The significance of PDE7 for T cell function was implicated by the fact that a selective reduction in PDE7 expression by antisense oligonucleotide inhibits T cell proliferation. Our present findings support these findings and further demonstrate that PDE7 may modulate those T cell functions induced by stimulation with a specific antigen.
In apparent contradiction to our present findings, a number of earlier studies have demonstrated that PDE4 plays a role in the regulation of multiple T cell functions by using the selective PDE4 inhibitor, rolipram, which does not inhibit PDE7 activity [13,14,16–19]. However, in some reports, the effective dose of rolipram required to inhibit T cell activation was higher than that required to suppress PDE4 activity [21–23]. Interestingly, in our experiments, RP 73401 displayed very little suppression (10–30%) of most of the T cell functions examined (Figs 1–4) in the dose range at which the drug inhibits the activity of PDE4, but not PDE7A, completely (0·1–1 μm). Therefore, PDE4 may have a supplemental role in the regulation of the intracellular cAMP level in human T cells, and consequently in T cell function. It is possible that T-2585 inhibits the variety of T cell receptor initiated T cell functions via the combined effects on PDE4 and PDE7.
From our findings, a possible contribution of PDE3 inhibition to T-2585-mediated suppression of T cell function has not been completely ruled out, since the inhibitory potency of T-2585 on PDE3 activity isolated from guinea pig heart was similar to that on PDE7A activity [25]. Nevertheless, previous reports from at least three separate groups demonstrating that PDE3 inhibitors failed to affect cytokine mRNA expression and proliferation of human PBMC [16–19,22,23] rule against a critical role for PDE3 in T cell function. We also confirmed by preliminary experiments that IL-5 production was not inhibited by CI-930, a PDE3 inhibitor, in our experimental system (data not shown). In order to elucidate additional details of the comparative roles of PDE7 and other PDE isoenzymes in T cell function, it will be necessary to delineate the effects of currently existing PDE inhibitors on PDE7 activity in human T cells. Most importantly, the generation of a selective PDE7 inhibitor will be eagerly awaited.
It is significant that T-2585 but not RP 73401 effectively suppressed IL-5 production by PBMC because accumulating evidence suggests that IL-5 is the key cytokine involved in allergic diseases, such as asthma and atopic dermatitis, associated with eosinophilic inflammation. IL-5 is a lymphokine produced primarily by activated T cells and it enhances the proliferation, differentiation and survival of eosinophils [44,45]. Activated T cells expressing IL-5 mRNA were found in increased numbers in the bronchial mucosa of asthmatic patients [46] and the numbers were further increased upon antigen challenge [47]. Administration of an anti-IL-5 neutralizing antibody abrogated allergic eosinophilic inflammation in experimental allergic models [48–50]. Our present findings suggest that selective inhibitors for PDE7, but not PDE4, are promising drugs for the management of chronic allergic disorders. Furthermore, PDE7 inhibitors able to down-regulate total T cell function have the potential for treating a wide range of diseases associated with an abnormal T cell response.
In conclusion, PDE7 has the potential to regulate human T cell functions including cytokine production, proliferation and expression of activation markers. This suggests the possible management of various immunological diseases by treatment with selective PDE7 inhibitors.
Acknowledgments
The authors thank Ms Rieko Kameda, Sumie Kikkawa and Kaoru Akiyama for technical assistance and Dr Wendy A. Gray for reviewing this manuscript.
REFERENCES
- 1.Goodwin JS, Ceuppens J. Regulation of immune response by prostaglandins. J Clin Immunol. 1983;3:295–315. doi: 10.1007/BF00915791. [DOI] [PubMed] [Google Scholar]
- 2.Moore AR, Willoughby DA. The role of cAMP regulation in controlling inflammation. Clin Exp Immunol. 1995;101:387–9. doi: 10.1111/j.1365-2249.1995.tb03123.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lingk DS, Chan MA, Gelfand EW. Increased cyclic adenosine monophosphate levels block progression but not initiation of human T cell proliferation. J Immunol. 1990;145:449–55. [PubMed] [Google Scholar]
- 4.Chouaib S, Welte K, Mertelsmann R, Dupont B. Prostaglandin E2 acts at two distinct pathways of T lymphocyte activation: inhibition of interleukin 2 production and down-regulation of transferrin receptor expression. J Immunol. 1985;135:1172–9. [PubMed] [Google Scholar]
- 5.Bastin B, Payet MD, Dupuis G. Effects of modulators of adenylyl cyclase on interleukin-2 production, cytosolic Ca2+ elevation, and K+ channel activity in Jurkat cells. Cell Immunol. 1990;128:385–99. doi: 10.1016/0008-8749(90)90035-p. [DOI] [PubMed] [Google Scholar]
- 6.Mary D, Aussel C, Ferrua B, Fehlmann M. Regulation of interleukin 2 synthesis by cAMP in human T cells. J Immunol. 1987;139:1179–84. [PubMed] [Google Scholar]
- 7.Krause DS, Deutsch C. Cyclic AMP directly inhibits IL-2 receptor expression in human T cells. Expression of both p55 and p75 subunits is affected. J Immunol. 1991;146:2285–96. [PubMed] [Google Scholar]
- 8.Soderling SH, Beavo JA. Regulation of cAMP and cGMP signaling: new phosphodiesterases and new functions. Curr Opin Cell Biol. 2000;12:174–9. doi: 10.1016/s0955-0674(99)00073-3. [DOI] [PubMed] [Google Scholar]
- 9.Beavo JA. Cyclic nucleotide phosphodiesterases: functional implications of multiple isoforms. Physiol Rev. 1995;75:725–48. doi: 10.1152/physrev.1995.75.4.725. [DOI] [PubMed] [Google Scholar]
- 10.Nicholson CD, Shahid M. Inhibitors of cyclic nucleotide phosphodiesterase isoenzymes – their potential utility in the therapy of asthma. Pulm Pharmacol. 1994;7:1–17. doi: 10.1006/pulp.1994.1001. [DOI] [PubMed] [Google Scholar]
- 11.Fawcett L, Baxendale R, Stacey P, et al. Molecular cloning and characterization of distinct human phophodiesterase gene family: Pde11a. Proc Natl Acad Sci USA. 2000;97:3702–7. doi: 10.1073/pnas.050585197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fujishige K, Kotera J, Michibata H, et al. Cloning and characterization of a novel human phosphodiesterase that hydrolyzes bothe cAMP and cGMP (PDE10A) J Biol Chem. 1999;274:18438–45. doi: 10.1074/jbc.274.26.18438. [DOI] [PubMed] [Google Scholar]
- 13.Ichimura M, Kase H. A new cyclic nucleotide phosphodiesterase isozyme expressed in the T-lymphocyte cell lines. Biochem Biophys Res Commun. 1993;193:985–90. doi: 10.1006/bbrc.1993.1722. 10.1006/bbrc.1993.1722. [DOI] [PubMed] [Google Scholar]
- 14.Robicsek SA, Blanchard DK, Djeu JY, Krzanowski JJ, Szentivanyi A, Polson JB. Multiple high-affinity cAMP-phosphodiesterases in human T-lymphocytes. Biochem Pharmacol. 1991;42:869–77. doi: 10.1016/0006-2952(91)90047-9. [DOI] [PubMed] [Google Scholar]
- 15.Tenor H, Staniciu L, Schudt C, et al. Cyclic nucleotide phosphodiesterase from purified human CD4+ and CD8+ T lymphocytes. Clin Exp Allergy. 1995;25:616–24. doi: 10.1111/j.1365-2222.1995.tb01109.x. [DOI] [PubMed] [Google Scholar]
- 16.Giembycz MA, Corrigan CJ, Seybold J, Newton R, Barnes PJ. Identification of cyclic AMP phosphodiesterases 3, 4 and 7 in human CD4+ and CD8+ T-lymphocytes: role in regulating proliferation and biosynthesis of interleukin-2. Br J Pharmacol. 1996;118:1945–58. doi: 10.1111/j.1476-5381.1996.tb15629.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Essayan DM, Huang SK, Undem BJ, Kagey-Sobotka A, Lichtenstein LM. Modulation of antigen- and mitogen-induced proliferative responses of peripheral blood mononuclear cells by nonselective and isozyme selective cyclic nucleotide phosphodiesterase inhibitors. J Immunol. 1994;153:3408–16. [PubMed] [Google Scholar]
- 18.Essayan DM, Huang S, Kagey-Sobotka A, Lichtenstein LM. Effects of nonselective and isozyme selective cyclic nucleotide phosphodiesterase inhibitors on antigen-induced cytokine gene expression in peripheral blood mononuclear cells. Am J Respir Cell Mol Biol. 1995;13:692–702. doi: 10.1165/ajrcmb.13.6.7576707. [DOI] [PubMed] [Google Scholar]
- 19.Banner KH, Roberts NM, Page CP. Differential effect of phosphodiesterase 4 inhibitors on the proliferation of human peripheral blood mononuclear cells from normals and subjects with atopic dermatitis. Br J Pharmacol. 1995;116:3169–74. doi: 10.1111/j.1476-5381.1995.tb15120.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kaminuma O, Mori A, Suko M, Kikkawa H, Ikezawa K, Okudaira H. Interleukin-5 production by peripheral blood mononuclear cells of asthmatic patients is suppressed by T-440: relation to phosphodiesterase inhibition. J Pharmacol Exp Ther. 1996;279:240–6. [PubMed] [Google Scholar]
- 21.van Wauwe J, Aerts F, Walter H, de Boer M. Cytokine production by phytohemagglutinin-stimulated human blood cells. Effects of corticosteroids, T cell immunosuppressants and phosphodiesterase IV inhibitors. Inflamm Res. 1995;44:400–5. doi: 10.1007/BF01797868. [DOI] [PubMed] [Google Scholar]
- 22.Essayan DM, Kagey-Sobotka A, Lichtenstein LM, Huang SK. Regulation of interleukin-13 by type 4 cyclic nucleotide phosphodiesterase (PDE) inhibitors in allergen-specific human T lymphocyte clones. Biochem Pharmacol. 1997;53:1055–60. doi: 10.1016/s0006-2952(97)00102-0. [DOI] [PubMed] [Google Scholar]
- 23.Essayan DM, Kagey-Sobotka A, Lichtenstein LM, Huang SK. Differential regulation of human antigen-specific Th1 and Th2 lymphocyte responses by isozyme selective cyclic nucleotide phosphodiesterase inhibitors. J Pharmacol Exp Ther. 1997;282:505–12. [PubMed] [Google Scholar]
- 24.Bloom TJ, Beavo JA. Identification and tissue-specific expression of PDE7 phosphodiesterase splice variants. Proc Natl Acad Sci USA. 1996;93:14188–92. doi: 10.1073/pnas.93.24.14188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ukita T, Sugahara M, Terakawa Y, et al. Novel, potent and selective phosphodiesterase-4 inhibitors as antiasthmatic agents: synthesis and biological activities of a series of 1-phridylnaphthalene derivatives. J Med Chem. 1999;42:1088–99. doi: 10.1021/jm980314l. [DOI] [PubMed] [Google Scholar]
- 26.Ashton MJ, Cook DC, Fenton G, et al. Selective type IV phosphodiesterase inhibitors as antiasthmatic agents. The syntheses and biological activities of 3-(cyclopentyloxy) -4-methoxybenzamides and analogues. J Med Chem. 1994;37:1696–703. doi: 10.1021/jm00037a021. [DOI] [PubMed] [Google Scholar]
- 27.Souness JE, Maslen C, Webber S, et al. Suppression of eosinophil function by RP 73401, a potent and selective inhibitor of cyclic AMP-specific phosphodiesterase: comparison with rolipram. Br J Pharmacol. 1995;115:39–46. doi: 10.1111/j.1476-5381.1995.tb16317.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Michaeli T, Bloom TJ, Martins T, et al. Isolation and characterization of a previously undetected human cAMP phosphodiesterase by complementation of cAMP-phosphodiesterase-deficient Saccharomyces cerevisiae. J Biol Chem. 1993;268:12925–32. [PubMed] [Google Scholar]
- 29.Thompson WJ, Terasaki WL, Epstein PM, Strada SJ. Assay of cyclic nucleotide phosphodiesterase and resolution of multiple molecular forms of the enzyme. Adv Cyclic Nucleotide Res. 1979;10:69–92. [PubMed] [Google Scholar]
- 30.Roehm NW, Rodgers GH, Hatfield SM, Glasebrook AL. An improved colorimetric assay for cell proliferation and viability utilizing the tetrazolium salt XTT. J Immunol Meth. 1991;142:257–65. doi: 10.1016/0022-1759(91)90114-u. [DOI] [PubMed] [Google Scholar]
- 31.Mori A, Suko M, Nishizaki Y, et al. IL-5 production by CD4+ T cells of asthmatic patients is suppressed by glucocorticoids and the immunosuppressants FK506 and cyclosporin A. Int Immunol. 1995;7:449–57. doi: 10.1093/intimm/7.3.449. [DOI] [PubMed] [Google Scholar]
- 32.Chomczynski P, Sacchi N. Single step method of RNA isolation by acid guanidinium thiocyanate-phenol chloroform extraction. Anal Biochem. 1987;162:156–60. doi: 10.1006/abio.1987.9999. 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- 33.Banchereau J, Briere F, Galizzi JP, Miosseac P, Rousset F. Human interleukin 4. J Lipid Med. 1994;9:43–53. [PubMed] [Google Scholar]
- 34.Minami Y, Kono T, Miyazaki T, Taniguchi T. The IL-2 receptor complex. Its structure, function, and target genes. Annu Rev Immunol. 1993;11:245–67. doi: 10.1146/annurev.iy.11.040193.001333. [DOI] [PubMed] [Google Scholar]
- 35.Minakuchi R, Wacholtz MC, Davis LS, Lipsky PE. Delineation of the mechanism of inhibition of human T cell activation by PG E2. J Immunol. 1990;145:2616–25. [PubMed] [Google Scholar]
- 36.Oppenheimer-Marks N, Kavanaugh AF, Lipsky PE. Inhibition of the transendothelial migration of human T lymphocytes by prostaglandin E2. J Immunol. 1994;152:5703–13. [PubMed] [Google Scholar]
- 37.Anastassiou ED, Paliogianni F, Balow JP, Yamada H, Boumpas DT. Prostaglandin E2 and other cyclic AMP-elevating agents modulate IL-2 and IL-2R alpha gene expression at multiple levels. J Immunol. 1992;148:2845–52. [PubMed] [Google Scholar]
- 38.Rincon M, Tugores A, Lopez Rivas A, et al. Prostaglandin E2 and the increase of intracellular cAMP inhibit the expression of interleukin 2 receptors in human T cells. Eur J Immunol. 1988;18:1791–6. doi: 10.1002/eji.1830181121. [DOI] [PubMed] [Google Scholar]
- 39.Quinton TM, Dean WL. Cyclic AMP-dependent phosphorylation of the inositol-1,4,5-triphosphate receptor inhibits Ca2+ release from platelet membranes. Biochem Biophys Res Commun. 1992;184:893–9. doi: 10.1016/0006-291x(92)90675-b. [DOI] [PubMed] [Google Scholar]
- 40.Supattapone S, Danoff SK, Theibert A, Joseph SK, Steiner J, Snyder SH. Cyclic AMP-dependent phospholyration of a brain inositol triphosphate receptor decreases its release of calcium. Proc Natl Acad Sci USA. 1988;85:8747–50. doi: 10.1073/pnas.85.22.8747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ferris CD, Cameron AM, Bredt DS, Huganir RL, Snyder SH. Inositol 1,4,5-triphosphate receptor is phosphorylated by cyclic AMP-dependent protein kinase at serines 1755 and 1589. Biochem Biophys Res Commun. 1991;175:192–8. doi: 10.1016/s0006-291x(05)81219-7. [DOI] [PubMed] [Google Scholar]
- 42.Kaminuma O, Mori A, Ogawa K, et al. Cyclic AMP suppresses interleukin-5 synthesis by human helper T cells via the downregulation of the calcium mobilization pathway. Br J Pharmacol. 1999;127:521–9. doi: 10.1038/sj.bjp.0702558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Li L, Yee C, Beavo JA. CD3- and CD28-dependent induction of PDE7 required for T cell activation. Science. 1999;283:848–51. doi: 10.1126/science.283.5403.848. [DOI] [PubMed] [Google Scholar]
- 44.Sanderson CJ, Campbell HD, Young IG. Molecular and cellular biology of eosinophil differentiation factor (interleukin-5) and its effects on human and mouse B cells. Immunol Rev. 1988;102:29–50. doi: 10.1111/j.1600-065x.1988.tb00740.x. [DOI] [PubMed] [Google Scholar]
- 45.Sanderson CJ. Interleukin-5, eosinophils, and disease. Blood. 1992;79:3101–9. [PubMed] [Google Scholar]
- 46.Hamid Q, Azzawi M, Ying S, et al. Expression of mRNA for interleukin-5 in mucosal bronchial biopsies from asthma. J Clin Invest. 1991;87:1541–6. doi: 10.1172/JCI115166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Robinson DS, Hamid Q, Bentley A, Ying S, Kay AB, Durham SR. Activation of CD4+ T cells, increased TH2-type cytokine mRNA expression, and eosinophil recruitment in bronchoalveolar lavage after allergen inhalation challenge in patients with atopic asthma. J Allergy Clin Immunol. 1993;92:313–24. doi: 10.1016/0091-6749(93)90175-f. [DOI] [PubMed] [Google Scholar]
- 48.Chand N, Harrison JE, Rooney S, et al. Anti-IL-5 monoclonal antibody inhibits allergic late phase bronchial eosinophilia in guinea pigs: a therapeutic approach. Eur J Pharmacol. 1992;211:121–3. doi: 10.1016/0014-2999(92)90273-7. [DOI] [PubMed] [Google Scholar]
- 49.Kaminuma O, Mori A, Ogawa K, et al. Successful transfer of late phase eosinophil infiltration in the lung by infusion of helper T cell clones. Am J Respir Cell Mol Biol. 1997;16:448–54. doi: 10.1165/ajrcmb.16.4.9115756. [DOI] [PubMed] [Google Scholar]
- 50.van Oosterhout AJ, Ladenius AR, Savelkoul HF, van Ark I, Delsman KC, Nijkamp FP. Effect of anti-IL-5 and IL-5 on airway hyperreactivity and eosinophils in guinea pigs. Am Rev Respir Dis. 1993;147:548–52. doi: 10.1164/ajrccm/147.3.548. [DOI] [PubMed] [Google Scholar]


