Abstract
An increase in mRNA levels for TNF and Tnfrsf1 in the bile ducts of Tnfsf5–/–(CD40 ligand or CD154 knockout) mice developing cholangitis following infection by Cryptosporidium parvum (CP) is accompanied by staining for TNFα in areas of inflammation. To determine whether TNF contributed to the bile duct damage seen in chronically-infected animals, we bred B6 mice with disrupted genes for Tnfrsf1a, Tnfrsf1b and Tnfsf5. Following CP infection, the Tnfsf5–/– Tnfrsf1a & 1b–/– mice were spared from cholangitis, even though their intestinal and bile duct infection by CP persisted. Mice with disruptions of Tnfsf5, and either Tnfrsf1a or Tnfrsf1b, developed bile duct sclerosis similar to that seen in CD40 and Tnfsf5 knockouts. Our data indicate that signalling through either TNF receptor is sufficient for the bile duct damage that follows chronic CP infection in mice, with disruption of the Tnfsf5 molecule.
Keywords: immunodeficiency, apicomplexans, cholangitis, Tnfrsf, CD40 ligand
INTRODUCTION
Signalling through CD40 and CD154 (now re-named Tnfsf5) is required for mice to clear a Cryptosporidium parvum (CP) infection from the gut [1]. Mice with disruptions of CD40 or Tnfsf5 become chronically infected with CP, and the parasites spread to the bile ducts and gall bladder to cause cholangitis [2]. The consequent sclerosis is severe enough to cause jaundice in up to one third of mice on the C57BL/6 background. This mouse model is important because it reproduces the course of CP infection that is seen in boys with mutations of their Tnfsf5 (also known as CD40 ligand and CD154) who have X-linked immunodeficiency with hyperIgM. In about a third of these patients, a chronic bile duct inflammation progresses to sclerosing cholangitis that is severe enough to necessitate liver transplantation [3]. The mechanisms of bile duct injury in these instances are unknown, but they are clearly important if therapeutic interventions are to be developed. TNF is known to be made in animal models of sclerosing cholangitis [4,5], with both inflammatory cells [6] and the bile duct cells participating in the production [7]. Inflammatory responses to CP in explants of human intestine are also associated with the production of TNF [8]. To determine whether TNF might contribute to bile duct sclerosis in chronic CP infection, we first established that mRNA transcripts for TNF and TNF receptors were present in infected bile duct, and then showed that TNFα was detectable by immunostaining. We then bred B6 mice for disruption of both TNF receptors and CD40 or CD154 to determine whether Tnfrsf1 signalling was required for bile duct sclerosis to develop in CP-infected mice.
MATERIALS AND METHODS
Mice
Animal conditions and experimentation were approved by the University of Colorado Institutional Animal Care and Use Committee. C57BL/6 Tnfrsf1a, Tnfrsf1b and double knockout mice were provided by Dr Pippa Marrack (National Jewish Center, Denver) and bred in micro-isolator cages, provided with sterile food, water and bedding as before [1,2]. CD40 and Tnfsf5 knockout mice were originally obtained from Drs Kikutani and Flavell, respectively. They were infected with ‘GCH1’ CP oocysts (McKesson Bioservices, Rockville, MD, USA; NIAID AIDS Research and Reference Reagent Program of the NIAID, NIH) as previously described [1]. Animals were euthanized by CO2 inhalation when (a) they lost 15% of body weight or (b) were 6–13 weeks after infection. The liver, gall bladder and bile ducts were dissected using a microscope. Tissues for RNA extraction were frozen on liquid N2.
Histology
Tissues for conventional histology were fixed in 4% formalin and wax-embedded. Sections were stained with h & E and examined on a Leitz microscope. Images were captured with a Spot camera (Model 1.3.0, Diagnostic Instruments, Sterling Heights, MI, USA) and processed with Adobe PhotoShop 6 (San Jose, CA, USA) software. Samples for frozen section were frozen in Tissue-Tek O.C.T. compound (Sakura-Finetek, Torrance, CA, USA) and sectioned in a cryostat for immunofluorescence. TNFα was stained with FITC-conjugated antibody (Pharmingen, San Jose; rat IgG1 isotype control). Tissues obtained at necropsy for routine histology were paraffin-embedded for sectioning and h & E staining.
CP ELISA
Mouse faeces were collected weekly and stored at –20°C until infection status was determined by faecal CP antigen using a commercial CP antigen ELISA kit (LMD, Alexon, Ramsay, MN, USA). Faeces were resuspended in the homogenization buffer overnight, at 4°C, before testing according to the manufacturer’s instructions. Positive and negative controls were included with each ELISA run as previously described [1].
RNA extraction
Tissues were homogenized in 750 μl Trizol (Gibco BRL, Invitrogen, Carlsbad, CA, USA), the homogenate extracted in chloroform twice and then precipitated by isopropanol. The RNA was quantified by spectrophotometry and cleaned on an RNeasy mini spin column (Qiagen, Valencia, CA, USA).
cDNA synthesis and microarray analysis
The methods, including first strand synthesis using Superscript II RNase H– reverse transcriptase and synthesis of a second strand with DNA polymerase I, are described elsewhere [9]. cDNAs were in vitro transcribed (IVT) to yield biotin-labelled cRNA using the BioArray HighYield Transcript Labelling kit (ENZO Diagnostics, Inc, Farmingdale, NY, USA) according to the manufacturer’s instructions. The cRNAs were cleaned on RNEasy mini columns. cRNA was fragmented at 94°C and applied to the Affymetrix Mu11KsubA array. Following hybridization, the arrays were stained with PE-streptavidin (Vector Laboratories, Burlingame, CA, USA) and biotinylated anti-streptavidin antibody, and scanned using an HP GeneArray Scanner (Hewlett Packard, San Jose, CA, USA). Comparisons between an uninfected animal and animals infected by CP were by MicroArray Suite 4·0 software (Affymetrix, Santa Clara, CA, USA).
RESULTS
Tnfrsf1 and TNF expression
Bile ducts from Tnfsf5–/– knockout C57BL/6 mice, one control and one after 9 weeks of CP infection, were dissected free and processed for oligoarray analysis. The principal identifiable differences were in immune-related transcripts, particularly T- and B-cell receptors. Included amongst the increased transcripts in the CP-infected animal were mRNAs for Tnfrsf1a, Tnfrsf1b, TNFα and TNFβ (Table 1). To determine whether the presence of transcripts was associated with protein production, we stained frozen sections of biliary epithelium with TNFα and isotype control antibody conjugates. The positive staining (Fig. 1) suggested that TNFα was produced, at least in areas of inflammation. No staining was seen in the negative control (not shown). To determine whether signalling through TNF receptors might contribute to bile duct sclerosis, we bred mice with disruptions in their Tnfrsf1a, Tnfrsf1b and Tnfsf5 genes.
Table 1.
Relative expression of TNF-related mRNAs in bile ducts of an uninfected and a Cryptosporidium parvum-infected Tnfsf5–/–mouse *
| Chip intensity values | ||||
|---|---|---|---|---|
| mRNA species | Accession no. | Uninfected | Infected | Fold increase |
| Tnfrsf1a | M59378 | 921 | 5299 | 5·8 |
| Tnfrsf1b | M59377 | 621 | 483 | 0·7 |
| TNFα | X02611 | 1 | 232 | 26 |
| TNFβ | M17015 | 93 | 391 | 4·2 |
Data are intensity values from Affymetrix Microarray Suite 4·0 using Mu11Ka chip.
Fig. 1.
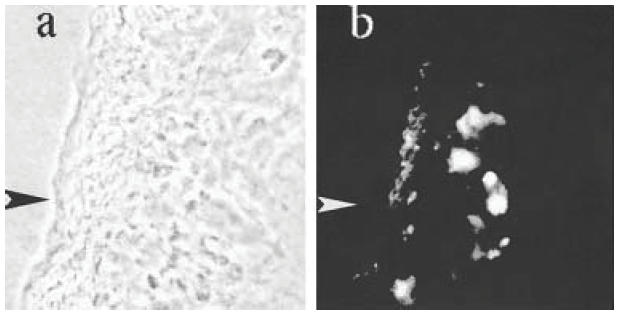
Section of gall bladder of a Tnfsf5–/– mouse infected with Cryptosporidium parvum under (a) phase contrast and (b) incident u.v. and green fluorescence filters (both ×100). The arrow marks the serosal surface of the gall bladder, which is thickened as a result of inflammation. Positive staining for TNFα in (b) is localized principally to the submucosa. No green fluorescence was seen in the same tissue stained with an isotype control or uninfected tissue stained for TNFα (not shown).
CP infection in Tnfrsf1a, Tnfrsf1b and Tnfsf5 knockout mice
The Tnfrsf1a, Tnfrsf1b and Tnfrsf1a & 1b double knockout C57BL/6 mice, whose genes for CD40 and Tnfsf5 were intact, cleared a CP infection within 8 weeks (Table 2). All mice with deletions in either CD40 or Tnfsf5 remained infected through 12 weeks. Two each in the CD40 or Tnfsf5 single knockout groups of eight became visibly jaundiced. The remaining six in these groups appeared well.
Table 2.
Outcome of Cryptosporidium parvum infection in TNFR/Tnfsf5 knockouts *
| Disrupted gene | No. studied | Mean (range) duration of infection | Peak ELISA O.D. | Gross appearance of bile ducts |
|---|---|---|---|---|
| None (control) | 6 | 3 (2–4)weeks | 0·02 | Normal |
| CD40−/− | 8 | >12 weeks | 1·6 | Sclerosed |
| Tnfsf5−/− | 8 | >12 weeks | 2·0 | Sclerosed |
| Tnfrsf1a−/− | 5 | 4 (3–5)weeks | 0·22 | Normal |
| Tnfrsf1b | 4 | 3·5 (3–4) weeks | 0·32 | Normal |
| Tnfrsf1a & 1b | 4 | 4 (3–5)weeks | 0·36 | Normal |
| Tnfrsf1a & Tnfsf5 | 4 | >12 weeks | 2·0 | Sclerosed |
| Tnfrsf1b & Tnfsf5 | 5 | >12 weeks | 2·0 | Sclerosed |
| Tnfrsf1a & 1b & Tnfsf5 | 5 | >12 weeks | 2·0 | Normal |
| Tnfrsf1a & 1b & CD40 | 4 | >12 weeks | 1·0 | Normal |
Mice were infected at 4 weeks of age and followed by weekly ELISA tests on stool. CP infection was defined by an O.D. > 0·1 and the duration of infection indicates the mean number of weeks for the O.D. to return to <0·05. All mice were euthanized 12 weeks after infection and the macroscopic appearance of their extrahepatic bile ducts recorded.
The histology of the liver, bile ducts and gall bladder shown in Fig. 2 is selected from CD40 and Tnfsf5 knockouts, with and without disruptions of the TNF receptors. As before [2], we found that proliferation of biliary epithelium, fibrosis and sclerosis were present around bile ducts and gall bladders of CD40 and Tnfsf5 knockout mice (Fig. 2a,b,c). No differences were seen between the bile ducts and gall bladders of uninfected and CP-infected C57BL/6 mice, whether wild-type or with disruptions of Tnfrsf1a, Tnfrsf1b or both (not shown). The bile ducts and gall bladder of the Tnfsf5 mice with disruption of either Tnfrsf1a or Tnfrsf1b showed the proliferative and sclerotic changes of the CD40 or Tnfsf5 single knockout controls (Fig. 2d,e,f), and CP forms were readily seen at the epithelial border. These mice still had positive CP ELISA results when they were euthanized after 12 weeks of infection.
Fig. 2.
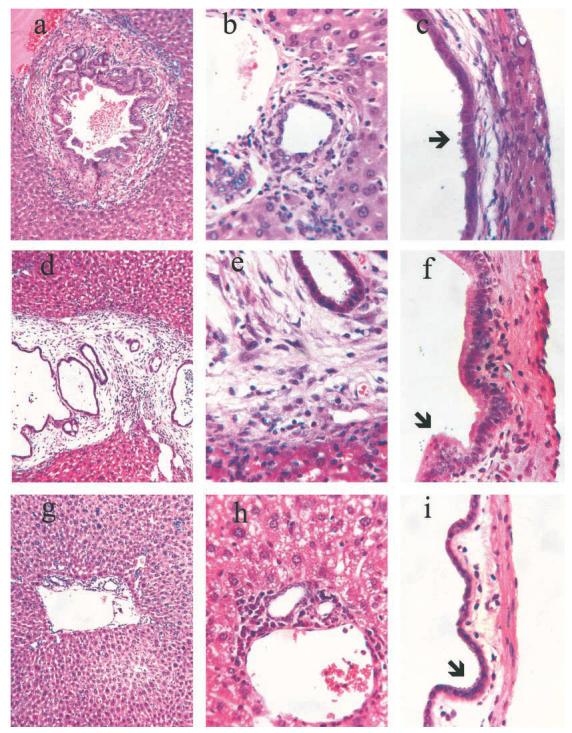
Sections of liver showing bile ducts (a, b, d, e, g and h) and gall bladder (c, f and i) of Cryptosporidium parvum-infected mice. (a) CD40–/–; (b, c) Tnfsf5–/– mice after 12 weeks of infection showing inflammation and fibrosis surrounding large and small bile ducts (a and b) and beneath the biliary epithelium of the gall bladder (c). (d) Double knockouts Tnfsf5/Tnfrsf1a and (e, f) Tnfsf5/Tnfrsf1b after 12 weeks of C. parvum infection showing fibrosis and inflammation comparable with the Tnfsf5–/– single knockouts in (a–c). (g) Triple knockout mice Tnfsf5/Tnfrsf1a/Tnfrsf1b; (h) CD40/Tnfrsf1a/Tnfrsf1b and (i) Tnfsf5/Tnfrsf1a/Tnfrsf1b infected by C. parvum for 12 weeks. Arrows in (c), (f) and (i) indicate C. parvum trophozoites. Magnifications: (a), (d) and (g) = 10×; remainder = 100×. Sections are representative of groups of animals defined in Table 2.
Triple knockout mice with disruption of Tnfrsf1a & 1b, plus either Tnfsf5 or CD40, also remained infected throughout the 12 weeks of follow up. After euthanasia, all had CP forms associated with biliary epithelial cells, but there was little, if any, fibrosis or sclerosis associated with the infection (Fig. 2g,h,i).
DISCUSSION
Biliary epithelial cells both make and respond to a large number of cytokines including IL1, TGF-β, IL6 and TNF [10,11]. Injury to biliary epithelial cells from obstructed bile flow or from infection is associated with proliferation and, subsequently, with fibrosis, leading to a sclerosing cholangitis and the appearance of large cystic structures. A role for TNF in the process that leads to sclerosing cholangitis has been proposed on the grounds of local production but has not previously been tested with knockout mice. We selected mice with disrupted CD40 or Tnfsf5 genes for study because we had previously shown that these animals are unable to clear a CP infection. After 3 or more months of infection, the animals typically develop a sclerosing cholangitis. When deletion for either Tnfrsf1a or Tnfrsf1b was added to a CD40 or Tnfsf5 deletion, the mice continued to develop inflammatory and sclerotic bile duct changes typical of their CP-infected CD40/Tnfsf5 deleted parents.
Triple knockout Tnfrsf1a–/–Tnfrsf1b–/–CD40–/– and Tnfrsf1a–/–Tnfrsf1b–/–Tnfsf5–/– mice also remained chronically infected by CP. However, at least in the 12 weeks that follow a CP infection, these mice were significantly spared from sclerosis of both their bile ducts and the epithelium of their gall bladders. The difference did not appear to be due to a lesser infection of the Tnfrsf knockouts, as CP forms were identified on the epithelium of these and the wild-type mice. The ability of either Tnf receptor to mediate the inflammation that leads to sclerosis is of interest because TNF inflammatory responses are sometimes mediated through Tnfrsf1a alone, while the Tnfrsf1b receptor is restricted to endothelial and marrow-derived cells. Disruption of either of these receptors is sufficient to interfere with activation and proliferation in cultured macrophages [12], while the prevention of biliary sclerosis in our study required the disruption of both receptors. Similarly, disruption of Tnfrsf1a alone was insufficient to prevent NF-kappaB activation in cultured hepatocytes [13].
The sclerosing cholangitis that occurs in boys with mutations in their Tnfsf5 gene is important because it may result in liver failure and require transplant treatment. Cryptosporidium parvum has been implicated in some affected boys, although chronic infection of the biliary tree by other pathogens, possibly including Helicobacter species [14], might also contribute. Sclerosing cholangitis can be reproduced in mice with a disrupted Tnfsf5 gene by infection with CP, provided that the infection persists for 6 weeks or longer [2]. Dense inflammatory infiltrates surround the intra- and extra-hepatic bile ducts during this interval, and it seems likely that inflammatory cytokines contribute to the damage.
This finding has potential clinical significance. Monoclonal antibodies to TNF are being used successfully in the management of rheumatoid arthritis and are being evaluated in inflammatory bowel disease [15]. If TNF plays the same role in infection-induced sclerosing cholangitis in man as it does in CP-infected mice, then anti-TNF antibody may have some role in the prevention or slowing of bile duct damage in CP-infected XHIM patients.
Acknowledgments
We thank Mary Cosyns for outstanding perseverance and technical support and Michelle Jones for genotyping the double and triple knockouts. We thank Dr Pippa Marrack for making the Tnfrsf1a + Tnfrsf1b knockout mice available to us. This research was funded in part by grants form the March of Dimes (6-FY99–427) and from the NIH (R01 AI40870).
REFERENCES
- 1.Cosyns M, Tsirkin S, Jones M, Flavell R, Kikutani H, Hayward AR. Requirement for CD40–CD154 interaction for elimination of Cryptosporidium parvum from mice. Inf Immun. 1998;66:603–7. doi: 10.1128/iai.66.2.603-607.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stephens J, Cosyns M, Jones M, Hayward A. Liver and bile duct pathology following Cryptosporidium parvum infection of immunodeficient mice. Hepatology. 1999;30:27–35. doi: 10.1002/hep.510300138. [DOI] [PubMed] [Google Scholar]
- 3.Hayward AR, Levy J, Facchetti F, Notarangelo L, Ochs HD, Etzioni A. Cholangiopathy and tumors of the pancreas, liver and biliary tree in boys with X-linked immunodeficiency with hyper-IgM (XHIM) J Immunol. 1997;158:977–83. [PubMed] [Google Scholar]
- 4.Orth T, Neurath M, Schirmacher P, Galle PR, Mayet WJ. A novel rat model of chronic fibrosing cholangitis induced by local administration of a hapten reagent into the dilated bile duct is associated with increased TNF-alpha production and autoantibodies. J Hepatol. 2000;33:862–72. doi: 10.1016/s0168-8278(00)80116-6. [DOI] [PubMed] [Google Scholar]
- 5.Jackson GD, Dai Y, Sewell WA. Bile mediates intestinal pathology in endotoxemia in rats. Infection Immunity. 2000;68:4714–9. doi: 10.1128/iai.68.8.4714-4719.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Aggarwal BB, Natarajan K. Tumor necrosis factors: developments during the last decade. Euro Cytokine Netw. 1996;7:93–124. [PubMed] [Google Scholar]
- 7.Loffreda S, Rai R, Yang SQ, Lin HZ, Diehl AM. Bile ducts and portal and central veins are major producers of tumor necrosis factor alpha in regenerating rat liver. Gastroenterology. 1997;112:2089–98. doi: 10.1053/gast.1997.v112.pm9178702. [DOI] [PubMed] [Google Scholar]
- 8.Seydel KB, Zhang T, Champion GA, et al. Cryptosporidium parvum infection of human intestinal xenografts in SCID mice induces production of human tumor necrosis factor alpha and interleukin-8. Infect Immun. 1998;66:2379–82. doi: 10.1128/iai.66.5.2379-2382.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ponnuraj EM, Hayward AR. Intact mRNAs of intestinal epithelial cells but not C. parvum, reach mesenteric lymph nodes. J Immunol. 2001;167:5321–8. doi: 10.4049/jimmunol.167.9.5321. [DOI] [PubMed] [Google Scholar]
- 10.Reynoso-Paz S, Coppel RL, Mackay IR, Bass NM, Ansari AA, Gershwin ME. The immunobiology of bile and biliary epithelium. Hepatology. 1999;30:351–7. doi: 10.1002/hep.510300218. [DOI] [PubMed] [Google Scholar]
- 11.Matsumoto K, Fujii H, Michalopoulos G, Fung JJ, Demetris AJ. Human biliary epithelial cells secrete and respond to cytokines and hepatocyte growth factors in vitro: interleukin-6, hepatocyte growth factor and epidermal growth factor promote DNA synthesis in vitro. Hepatology. 1994;20:376–82. [PubMed] [Google Scholar]
- 12.Mukhopadhyay A, Suttles J, Stout RD, Aggarwal BB. Genetic deletion of the tumor necrosis factor receptor p60 or p80 abrogates ligand-mediated activation of nuclear B kappa B and of mitogen-activated protein kinases in macrophages. J Biol Chem. 2001;276:31906–12. doi: 10.1074/jbc.M105252200. [DOI] [PubMed] [Google Scholar]
- 13.Miyoshi H, Rust C, Guicciardi ME, Gores GJ. NF-kappaB is activated in cholestasis and functions to reduce liver injury. Am J Path. 2001;158:967–75. doi: 10.1016/s0002-9440(10)64043-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nilsson HO, Taneera J, Castedal M, Glatz E, Olsson R, Wadstrom T. Identification of Helicobacter pylori and other Helicobacter species by PCR, hybridization and partial DNA sequencing in human liver samples from patients with primary sclerosing cholangitis or primary biliary cirrhosis. J Clin Microbiol. 2000;38:1072–6. doi: 10.1128/jcm.38.3.1072-1076.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Taylor PC. Anti-tumor necrosis factor therapies. Curr Opin Rheumatol. 2001;13:164–9. doi: 10.1097/00002281-200105000-00003. [DOI] [PubMed] [Google Scholar]


