Abstract
The regulatory role of chemokines and chemokine receptors on specific lymphocyte recruitment into periodontal diseased tissue is poorly characterized. We observed that lymphocytes infiltrating inflamed gingival tissue expressed marked levels of CCR6. In periodontal diseased tissue, the expression of MIP-3α mRNA was detected by RT-PCR and further, MIP-3α was distributed in the basal layer of gingival epithelial cells, microvascular endothelial cells and the areas of inflammatory cells as shown by immunohistochemistry. Moreover, CCR6-expressing cells infiltrated into periodontal diseased tissue, and the proportion of CCR6-positive CD4+ T cells was significantly elevated in periodontal diseased tissue compared with peripheral blood in the same patients. Furthermore, gingival lymphocytes isolated from patients showed migration toward MIP-3α in an in vitro chemotaxis assay in which migration was abrogated by specific antibody to CCR6. Thus, these findings suggested that CCR6 and the corresponding chemokine, MIP-3α may have an important regulatory role in specific lymphocyte migration into inflamed periodontal tissue.
Keywords: CCR6, MIP-3α, periodontal disease
INTRODUCTION
Periodontal disease is characterized as chronic inflammation associated with Gram-negative bacteria in the oral cavity [1,2], resulting in soft tissue destruction and periodontal bone resorption. Although host-immune response to these bacteria has been suggested to be associated with alteration or even progress of this disease [3], the role of lymphocytes that infiltrate the gingival tissues of periodontal diseased patients is unknown.
T cells in inflammatory lesions generally express memory/activated marker (CD45RO+/CD29+) in contrast to the naive type marker (CD45RA+/CD29–) [4]. CD45RO+ CD4+ T lymphocytes were also predominant in gingival tissues with periodontal disease [5,6], indicating that memory/activated type CD4+ T cells are localized in the diseased gingival tissue [3]. The memory/activated CD4+ T cells in the effector sites are inferred to be derived from draining lymph nodes where naive CD4+ T cells experience antigen presentation by professional antigen-presenting cells. The locomotion of memory/activated CD4+ T cells to the local effector site from lymph nodes is regulated by chemokines and adhesion molecules [7]. The involvement of adhesion molecules in the lymphocyte migration into gingival tissues was demonstrated by the increased number of mononuclear cells which express α4 and α6 integrins [8] and CD4+ T cells which express LFA-1 [9] in the gingival tissue with periodontal disease. However, the roles of chemokine and chemokine receptor interactions in the migration of CD4+ T cells into the periodontal diseased tissue have not been analysed in detail.
Approximately 40 chemokines and 16 chemokine receptors have been identified at present [10,11], with several studies demonstrating the production of chemokines in gingival tissue [12–14]. Constitutive expression of monocyte chemotactic protein-1 (MCP-1) and interleukin-8 (IL-8), along with lesser expression of growth-related gene product γ (GRO-γ), macrophage inflammatory protein-1α (MIP-1α) and macrophage inflammatory protein-1β (MIP-1β), were measured by RT-PCR in healthy gingivae [12]. Up-regulated production of MCP-1 in diseased tissue was observed to correlate with the degree of inflammation [13,14]. Migration of various types of lymphocytes into effector sites appears to be regulated by specific chemokine receptors expressed on the cells. The preferential expression of specific chemokine receptors on particular leucocyte subsets has been demonstrated [10] (for example, neutrophils; CXCR1, CXCR2, eosinophils; CCR1, CCR3, Th1-type T cells; CCR5, CXCR3, Th2-type T cells; CCR4, CCR8). However, it is unclear which chemokine receptors are expressed on the lymphocytes infiltrating gingival tissue.
CCR6 is the only known receptor for MIP-3α that is chemotactic for lymphocytes and dendritic cells [15,16]. Recently, it was reported that CCR6 is expressed on memory T cells [15] and immature dendritic cells [16], and the ligand MIP-3α preferentially attracts memory T cells [15]. In periodontal diseased tissue, memory T cells [5] and dendritic cells [17] are infiltrated, but the mechanism of infiltration of these cells is uncertain.
In the present study, we demonstrated by RT-PCR that inflamed gingival tissues express MIP-3α mRNA. Also, immunohistochemical staining revealed that MIP-3α was expressed on the basal layer of gingival epithelial cells, microvascular endothelial cells and an area of inflammatory cells dense in periodontal diseased sections, but little MIP-3α was expressed in normal gingival tissue. Furthermore, we could detect massive infiltration of CCR6-expressing cells in periodontal diseased tissue by immunohistological staining, and the percentage of CCR6-positive CD4+ T cells in inflamed gingivae was greater than in CD4+ peripheral blood T cells from the same patients. Finally, the T cells isolated from inflamed gingivae demonstrated an in vitro chemotactic response to MIP-3α that was inhibited by monoclonal antibody (MoAb) anti-CCR6, suggesting that selective T-cell migration into inflamed gingivae could be regulated by CCR6–MIP-3α interactions.
MATERIALS AND METHODS
Gingival tissue biopsies and preparation of mononuclear cells
Tissue biopsies were sampled from the inflamed gingiva of patients at surgery who were diagnosed with chronic adult periodontitis (four males and 13 females, aged 49 to 82 years old), or from the gingiva of clinically healthy subjects (two males and three females, aged 26 to 40 years old). The site of gingival biopsy of the adult periodontitis group exhibited radiographic evidence of bone destruction, clinical probing depths greater than 6 mm and sulcular bleeding on probing; the patients were otherwise systemically healthy. Subjects in the control group were healthy both systemically and periodontally; the sampled site of gingiva exhibited probing depths less than 3 mm with no attachment loss, clinical inflammation, sulcular bleeding or radiographic bone loss. Informed consent was obtained from all subjects participating in this study. Peripheral blood was also obtained from the patients.
Infiltrating gingival lymphocytes (IGL) were isolated by a method previously described [18]. In brief, the collected tissues were immediately placed in ice-cold RPMI 1640 complete medium (Gibco BRL, Gaithersburg, MD, USA) which contained 10% fetal calf serum (FCS) (BioWhittaker, Walkersville, MD, USA) supplemented with penicillin (50 IU/ml), streptomycin (50 μg/ml),L-glutamine (2 mm) and sodium pyruvate (1 mm). The tissues were washed, diced into 1 mm3 pieces and treated with collagenase (Type IV, 10 mg tissue/300 U/ml; Funakoshi, Tokyo, Japan) in RPMI 1640 complete medium at 37°C for 60 min. After washing the samples twice with RPMI 1640 medium, mononuclear cells were separated by gradient centrifugation (900 g at room temperature for 20 min) with Lympholyte-H (Cederlane Lab., Ontario, Canada). The resulting mononuclear cell fraction was collected and used for the following experiments. Peripheral blood mononuclear cells (PBMC) were separated from the blood by means of Lympholyte-H density gradient centrifugation.
RT-PCR analysis
Total RNA was prepared from gingival biopsies using Isogen (Gibco BRL). Total RNA was reverse transcribed using random 9 primer (TaKaRa Shuzo, Kyoto, Japan) and reverse transcriptase (TaKaRa). The resulting first-strand DNA was amplified in a final volume of 20 μl containing 10 pmol of each primer and 1 U of Taq polymerase (TaKaRa Takara). The primers used were: +5′-TTGCTCCTGGCTGCTTTG-3′ and −5′-ACCCTCCATGATGTGCAAG-3′ for MIP-3α; + 5′-TGAAGGTCGGAGTCAACGGATTTGGT-3′ and 5′-CATGTGGGCCATGAGGTCCACCAC-3′ for GAPDH. Amplification conditions were denaturation at 94°C for 1 min, annealing at 55°C for 1 min and extension at 72°C for 1 min, for 35 cycles. Amplification products (5 μl each) were subjected to electrophoresis on 1·5% agarose gel and stained with ethidium bromide.
Immunohistochemistry
Gingival biopsies were immediately embedded in OCT compound (Miles Laboratories Inc., Elkhart, IN, USA) and quenched and stored in liquid nitrogen. The specimens were cut at 6 μm using a cryostat (SFS, Bright Instrumental Company, Huntingdon, UK) and collected on poly l-lysine-coated slides. Chemokine receptors and chemokines were analysed with specific antibodies: anti-human CCR6 MoAb (clone 45523.111; DAKO, Kyoto, Japan), anti-human CD4 MoAb (clone MT310; DAKO), anti-human CD45RO MoAb (clone VL1; Biosource International, Camarillo, CA, USA) or anti-human MIP-3α goat polyclonal antibody (Peprotech EC, London, UK). We also used isotype-matched control MoAb (DAKO) or non-immune goat serum antibody (DAKO) as the negative control. The sections were reacted with specific antibodies overnight at 4°C. After washing with phosphate-buffered saline (PBS), the sections were incubated with biotinylated anti-mouse and rabbit immunoglobulin (Universal Ab; DAKO), or biotinylated anti-goat immunoglobulin, for 20 min at room temperature; they were washed with PBS to remove unreacted antibodies. The sections were then treated with peroxidase-conjugated streptavidin (DAKO) for 10 min, and washed and reacted with DAB (3,3-diamino-benzidine tetrahydrochrolide; DAKO) in the presence of 3% H2O2 to develop colour. The sections were counter-stained with haematoxylin and mounted with glycerol. Mononuclear cells staining positively in gingival sections were counted in each of three, randomly-selected microscopic fields (at ×200 magnification) from five slides from a gingival tissue sample.
Flow cytometric analyses
IGL and PBMC were incubated with the anti-CCR6 MoAb (DAKO) or IgG1 isotype control antibody (DAKO) on ice for 20 min. After washing twice with PBS, the cells were incubated with the FITC-conjugated rabbit anti-mouse F(ab′)2 fragment (DAKO) for 20 min on ice. The cells were washed twice with PBS, blocked with 10% mouse serum (DAKO) and incubated with phycoerythrin (PE)-conjugated anti-CD4 MoAb (DAKO) for 20 min on ice. After two washing steps with PBS, the cells were immediately analysed by flow cytometry (Epics XL-MCL; Coulter, Hialeah, FL, USA).
To determine CCR6-positive cells in the CD4+ T-cell population, two-colour analysis was employed. The lymphocyte population was first gated using forward and side scatter parameters to exclude debris and dead cells. A second gate was set on the CD4+ lymphocyte subsets, and the percentage of CCR6-positive cells was calculated after defining a cut-off value according to the isotype control.
Chemotaxis assay
Chemotaxis assays were performed in 24-well Transwell culture inserts (Corning Costar, Cambridge, MA, USA) with 5·0 μm pore size polycarbonate membranes, following the method previously described by Ponath et al.[19]. In brief, 100 μl IGL were applied in each upper well chamber. Chemokines diluted in RPMI-1640 (600 μl) were placed in the bottom chamber. The culture plate was then incubated at 37°C in 5% CO2 for 4 h. After incubation, the membrane was removed and migrated cells in the bottom chamber were stained with PE-conjugated anti-CD4 MoAb (DAKO). CD4-positive cells were then counted using flow cytometry, and the chemotactic index (migrated cells divided by the number of cells that migrated without MIP-3α) was calculated. The optimal concentration of chemokine to induce maximal chemotaxis was determined in dose–response experiments by IL-2-stimulated PBMC to be MIP-3α (100 ng/ml). To evaluate the functional role of the chemokine receptor on chemotaxis, lymphocytes were pre-incubated with MoAb (10 μg/ml) anti-CCR6 (10 μg/ml; DAKO) or isotype-matched control mouse antibody (10 μg/ml, IgG1; DAKO) for 30 min at 37°C before chemotaxis assay.
Statistical analysis
Statistical significance was analysed by Student’s t-test. P-values <0·05 were considered significant.
RESULTS
MIP-3α expression by inflamed gingival tissue and normal gingiva
We first examined MIP-3α mRNA expression by whole gingival tissue from periodontal diseased tissue and normal gingival tissue. MIP-3α mRNA was expressed by five of seven periodontal diseased tissues and one of three normal gingival tissues, although one normal gingiva expressed MIP-3α mRNA very weakly (Fig. 1).
Fig. 1.
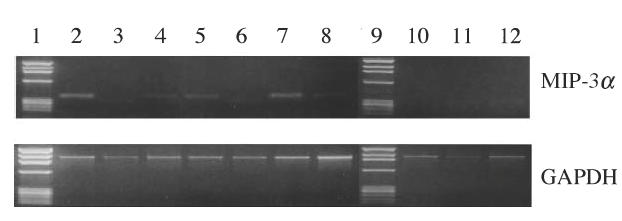
RT-PCR analysis of expression of MIP-3α in human gingiva. Total RNA was prepared from gingival biopsies from three normal donors and seven periodontal disease patients. RT-PCR analysis was carried out for MIP-3α and GAPDH as described in Methods. Lanes 1 and 9: marker; lanes 2–8: gingival biopsies from periodontal diseased patients; lanes 10–12: gingival biopsies from normal donors.
Augmented expression of MIP-3α and accumulation of CCR6-expressing cells in human periodontal diseased tissue
To examine MIP-3α expression and infiltration of CCR6-expressing cells in periodontal diseased tissue, we carried out immunohistochemical staining of MIP-3α and CCR6 in normal gingival tissues (n = 2) and inflamed gingival tissues (n = 6). The representative results are shown in Fig. 2. In normal gingival tissue, gingival epithelial cells were hardly stained by anti-MIP-3α (Fig. 2g). Furthermore, anti-CCR6 hardly stained any cells present in normal gingival tissue (Fig. 2c), and CD4+ or CD45RO+ cells hardly infiltrated normal gingival tissue (Fig. 2a,b). On the other hand, we observed staining of gingival epithelial cells by anti-MIP-3α in inflamed gingival tissue from periodontal diseased patients (Fig. 2h). The basal layer of gingival epithelial cells was especially stained by anti-MIP-3α. Furthermore, post-capillary and small vascular endothelial cells in periodontal diseased tissues displayed MIP-3α immunoreactivity (Fig. 2i). In addition, CCR6-expressing cells were infiltrated in inflamed gingival tissue. In these samples, we also confirmed that CCR6-expressing cells were mostly co-localized with cells positive for CD4 and CD45RO (Fig. 2d,e).
Fig. 2.
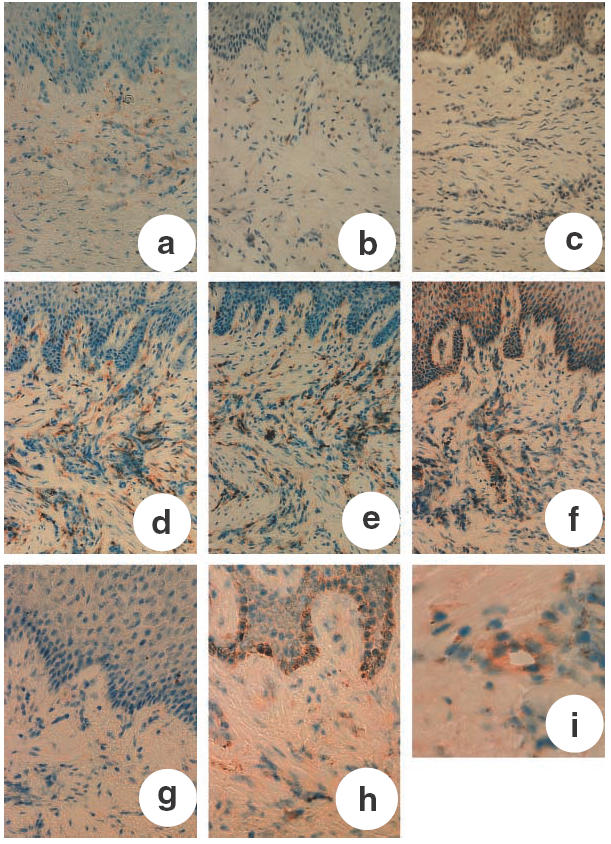
Immunohistochemical analysis of human periodontal tissues. (a) Normal gingiva stained with anti-CD4 MoAb; (b) normal gingiva stained with anti-CD45RO MoAb; (c) normal gingiva stained with anti-CCR6 MoAb; (d) inflamed gingiva stained with anti-CD4 MoAb; (e) inflamed gingiva stained with anti-CD45RO MoAb; (f) inflamed gingiva stained with anti-CCR6 MoAb; (g) normal gingiva stained with MIP-3α MoAb; (h) inflamed gingiva stained with MIP-3α MoAb; (i) endothelial cells stained with MIP-3α MoAb in inflamed gingiva. a–f: magnification ×200; g,h: magnification ×300; i: magnification ×400.
Flow cytometric analysis of CCR6 expression on CD4+ T cells in IGL
To further confirm the results obtained by immunohistochemistry (Fig. 2), we used flow cytometry analyses to measure CCR6 expression on CD4+ lymphocytes (Fig. 3). We examined the expression of CCR6 on CD4+ T cells isolated from inflamed gingival tissue compared with PBMC from the same donors. In PBMC of all patients, only a small proportion of CD4+ lymphocytes expressed CCR6 (Fig. 3). Significantly higher proportions of CCR6-positive CD4+ lymphocytes were detected in the inflamed gingival tissue (Fig. 3).
Fig. 3.
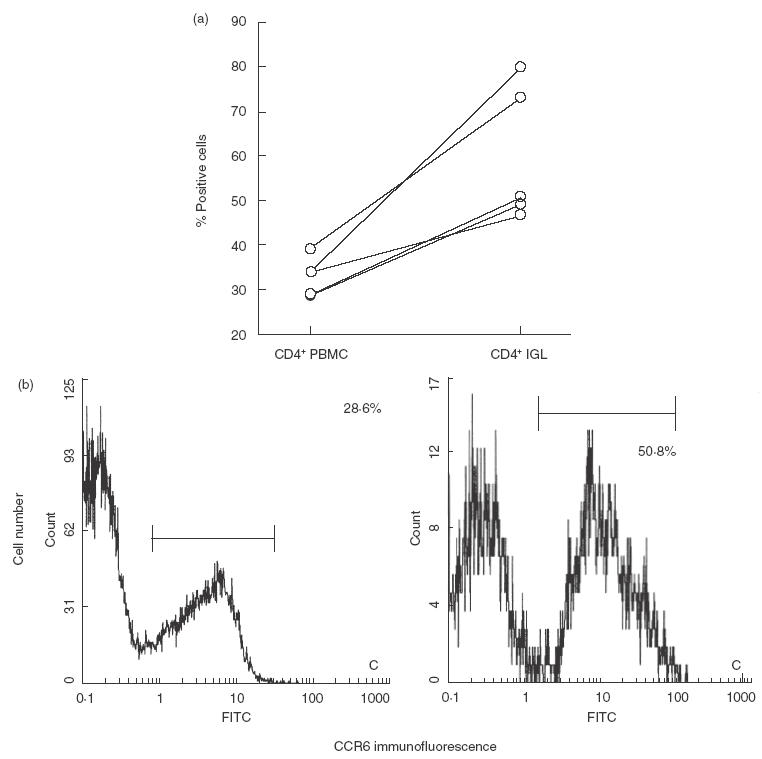
Flow cytometric analysis for CCR6 from patients’ CD4+ IGL and CD4+ PBMC. The same patient’s IGL and PBMC were analysed for the expression of CCR6. Compensation was made for each colour with positively and negatively stained control cells. Lymphocytes were gated according to their side scatter properties, and 104 cells in the gate were monitored for two-colour analysis. Isotype-matched antobodies were used to define the background threshold. (a) Percentage of CCR6-positive cells in CD4+ IGL and CD4+ PBMC of five patients with periodontal disease. (b) Flow cytometry results showing CCR6 expression on CD4+ IGL (right panel) and CD4+ PBMC (left panel) of one patient with periodontal disease.
Function of CCR6 expressed on IGL
To analyse whether the CCR6 expressed on IGL was functional, we investigated chemotaxis in an in vitro assay of IGL towards MIP-3α. IGL (5 × 105) was added to the upper chamber of a 5·0μm pore size polycarbonate transwell culture insert and recombinant MIP-3α (100 ng/ml) was added to the lower chamber. After 4 h of incubation, lymphocytes that had transmigrated into the lower chamber were counted by flow cytometry as described in Materials and Methods. As shown in Fig. 4, IGL migrated towards MIP-3α. The IGL chemotaxis towards MIP-3α was blocked by anti-CCR6 MoAb. Therefore, the CCR6 expressed on IGL were functional in chemotaxis towards MIP-3α.
Fig. 4.
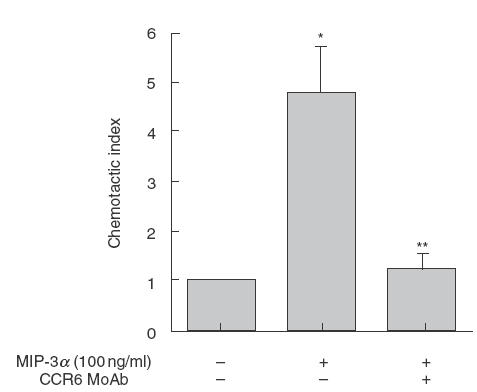
Chemotaxis assay of IGL to MIP-3α. MIP-3α (100 ng/ml) was applied to the lower compartment of the transwell chemotaxis system. Each group of IGL was pre-incubated with anti-CCR6 MoAb, isotype-matched antibody or medium alone for 30 min, before chemotaxis assay. Then, IGL were added to the upper well chamber and incubated for 4 h. The migrated cells were counted using a flow cytometer. *Significantly different from medium control, by Student’s t-test,P < 0·01; **significantly different from control IgG, by Student’s t-test,P < 0·01.
DISCUSSION
We demonstrate here that the CC chemokine MIP-3α, recently re-named CCL20 according to a new systematic chemokine nomenclature, and its receptor, CCR6, were significantly up-regulated in periodontal diseased tissue. We detected foci of MIP-3α-expressing gingival epithelial cells co-localizing with gingiva-infiltrating T cells, and gingiva-homing CD4+ T cells from periodontal diseased patients showed chemotaxis to MIP-3α.
We initially used RT-PCR to confirm an association between the expression of MIP-3α mRNA in normal gingiva and periodontal diseased gingiva. These findings suggested that MIP-3α is significantly up-regulated in periodontal diseased tissue. Immunohistochemical analysis consistently confirmed these findings. Inflamed gingiva-infiltrating CD4+CD45RO+ T cells expressed CCR6, and these cells were detected directly adjacent to foci of MIP-3α-expressing gingival epithelial cells. Moreover, normal gingiva-infiltrating CD4+CD45RO+ T cells were very few, and small numbers of T cells expressed CCR6. These findings, together with the observation of Liao et al.[15] that MIP-3α specifically attracts the memory subset of T cells in vitro, strongly suggest that MIP-3α may play a role in T-cell recruitment to periodontal diseased tissue. Flow cytometric analysis revealed that the proportion of CCR6+CD4+ T cells infiltrated in periodontal diseased tissue was higher than PBMC in the same patients. The significantly increased expression of CCR6 on inflamed gingiva-infiltrating CD4+ T cells, and their responsiveness towards MIP-3α gradients, may demonstrate that CCR6 has a role of selective lymphocyte recruitment to periodontal diseased tissue.
We revealed that the basal layer of epithelial cells was a potent producer of MIP-3α in inflamed gingival tissue. Recently, Charbonnier et al. demonstrated that the epidermal keratinocytes from clinically normal skin were immunologically strongly positive for LARC/CCL20, while epidermis-derived Langerhans cells, as well as in vitro differentiated CD34+ haematopoietic precursor cell-derived Langerhans-like cells, expressed CCR6 [20]. From these observations, the authors proposed a scenario that epidermal keratinocytes attract CCR6-expressing Langerhans cells into the epidermal layer by constitutively producing MIP-3α. On the other hand, Nakayama et al. demonstrated that epidermal keratinocytes were positive for MIP-3α in lesional skin tissues in atopic patients, but not in normal tissues [21]. The present findings revealed that gingival epithelial cells were positive for MIP-3α in periodontal diseased tissues but not in normal gingival tissues, and MIP-3α mRNA was significantly detected only from inflamed gingival tissue. Therefore, it is suggested that the production of MIP-3α by gingival epithelial cells appears to be closely related to pro-inflammatory responses of the gingiva.
Recently, a non-chemokine ligand for CCR6 was identified. Yang et al. showed that human β-defensin-2 is able to bind CCR6-transfected cells and to induce chemotaxis. However, its chemotactic activity was considerably lower than that of MIP-3α[22]. It was demonstrated that human β-defensin-2 mRNA was also expressed in gingival tissue samples [23]. We suggest that not only MIP-3α but also human β-defensin-2 may control infiltration of memory T cells in periodontal diseased tissue.
Localization of both CD4+ and CD8+ T cells in periodontal diseased tissue has been confirmed by several studies [24,25], and it is suggested that both T cells are associated with the progress of periodontal disease. It was reported that expression of CCR6 was found in both CD4+ and CD8+ T cells, but a higher percentage of the former expressed CCR6 [15]. we then considered that the CCR6–MIP-3a interaction was important for infiltration of CD4+ T cells, but further studies are required to determine the role of CCR6 and MIP-3a in periodontal diseased tissue, especially with respect to CD8+ T cells.
Recently, several studies have demonstrated expression of chemokines and chemokine receptors in periodontal diseased tissue [26,27]. For example, it was reported that CCR5- or CXCR3-positive CD4+ T cells in gingivae were significantly elevated in diseased tissue compared with healthy tissue, and the expression of the chemokines RANTES (CCR5 ligand), MIP-1alpha (CCR5 ligand) and IP-10 (CXCR3 ligand) was markedly elevated in inflamed periodontal tissue [26]. Therefore, not only MIP-3α and CCR6 but also some other chemokines and chemokine receptors may control selective recruitment of lymphocytes in periodontal diseased tissue.
In conclusion, the high proportion of CCR6-positive lymphocytes and expression of their corresponding chemokine, MIP-3α, in periodontal diseased tissue appears to have an important role in the selective recruitment of T cells in the context of periodontal inflammation.
Acknowledgments
We thank Dr Akiko Kawasaki (Department of Conservative Dentistry, University of Tokushima in Japan) for her assistance in collecting clinical samples and data.
REFERENCES
- 1.Dzink JL, Tanner ACR, Haffajee AD, Socransky SS. Gram negative species associated with active destructive periodontal lesions. J Clin Periodontol. 1985;12:648–59. doi: 10.1111/j.1600-051x.1985.tb00936.x. [DOI] [PubMed] [Google Scholar]
- 2.Socransky SS, Haffajee AD, Cugini MA, Smith C, Kent RLJ. Microbial complexes in subgingival plaque. J Clin Periodontol. 1998;25:134–44. doi: 10.1111/j.1600-051x.1998.tb02419.x. [DOI] [PubMed] [Google Scholar]
- 3.Taubman MA, Eastcott JW, Shimauchi H, Takeichi O, Smith DJ. Modulatory role of T lymphocytes in periodontal inflammation. In: Genco RJ, Hamada S, Mergenhagen SE, et al., editors. Molecular pathogenesis of periodontal disease. Washington DC: American Society for Microbiology; 1994. pp. 147–57. [Google Scholar]
- 4.Pitzalis C, Kingsley G, Haskard D, Panayi G. The preferential accumulation of helper inducer T lymphocytes in inflammatory lesions: evidence for regulation by selective endothelial and homotypic adhesion. Eur J Immunol. 1988;18:1397–404. doi: 10.1002/eji.1830180915. [DOI] [PubMed] [Google Scholar]
- 5.Yamazaki K, Nakajima T, Aoyagi T, Hara K. Immunohistological analysis of memory T lymphocytes and activated B lymphocytes in tissue with periodontal disease. J Periodont Res. 1993;28:324–34. doi: 10.1111/j.1600-0765.1993.tb01076.x. [DOI] [PubMed] [Google Scholar]
- 6.Gemmell E, Feldner B, Seymour GJ. CD45RA and CD45RO positive CD4 cells in human peripheral blood and periodontal disease tissue before and after stimulation with periodontopathic bacteria. Oral Microbiol Immunol. 1992;7:84–8. doi: 10.1111/j.1399-302x.1992.tb00514.x. [DOI] [PubMed] [Google Scholar]
- 7.Butcher EC, Picker LJ. Lymphocyte homing and homeostasis. Science. 1996;272:60–6. doi: 10.1126/science.272.5258.60. [DOI] [PubMed] [Google Scholar]
- 8.Del Castillo LF, Gomez RS, Pelka M, Hornstein OP, Johannessen AC, von den Driesch P. Immunohistochemical localization of very late activation integrins in healthy and diseased human gingivae. J Periodont Res. 1996;31:36–42. doi: 10.1111/j.1600-0765.1996.tb00461.x. [DOI] [PubMed] [Google Scholar]
- 9.Takeuchi Y, Sakurai K, Ike I, Yoshie H, Kawasaki K, Hara K. ICAM-1-expressing pocket epithelium, LFA-1-expressing T cells in gingival tissue and gingival crevicular fluid as features characterizing inflammatory cell invasion and exudation in adult periodontitis. J Periodont Res. 1995;30:426–35. doi: 10.1111/j.1600-0765.1995.tb01297.x. [DOI] [PubMed] [Google Scholar]
- 10.Sallusto F, Mackay CR, Lanzavecchia A. The role of chemokine receptors in primary, effector, and memory immune responses. Annu Rev Immunol. 2000;18:593–620. doi: 10.1146/annurev.immunol.18.1.593. [DOI] [PubMed] [Google Scholar]
- 11.Rossi D, Zlotnik A. The biology of chemokines and their receptors. Annu Rev Immunol. 2000;18:217–42. doi: 10.1146/annurev.immunol.18.1.217. [DOI] [PubMed] [Google Scholar]
- 12.Zehnder M, Greenspan JS, Greenspan D, Bickel M. Chemokine gene expression in human oral mucosa. Eur J Oral Sci. 1999;107:231–5. doi: 10.1046/j.0909-8836.1999.eos107401.x. [DOI] [PubMed] [Google Scholar]
- 13.Yu X, Graves DT. Fibroblasts, mononuclear phagocytes and endothelial cells express MCP-1 in inflamed human gingivae. J Periodontol. 1995;66:80–8. doi: 10.1902/jop.1995.66.1.80. [DOI] [PubMed] [Google Scholar]
- 14.Yu X, Antoniades H, Graves DT. Expression of monocyte chemoattractant protein-1 in human inflamed gingival tissue. Infect Immun. 1993;61:4622–8. doi: 10.1128/iai.61.11.4622-4628.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liao F, Rabin RL, Smith CS, Sharma G, Nutman TB, Farber JM. CC-chemokine receptor 6 is expressed on diverse memory subsets of T cells and determines responsiveness to macrophage inflammatory protein 3 alpha. J Immunol. 1999;162:186–94. [PubMed] [Google Scholar]
- 16.Dieu MC, Vanbervliet B, Vicari A, et al. Selective recruitment of immature and mature dendritic cells by distinct chemokines expressed in different anatomic sites. J Exp Med. 1998;188:373–86. doi: 10.1084/jem.188.2.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yuan ZN, Schreurs O, Gjermo P, Helgeland K, Schenck K. Topical distribution of Fc gamma R1, Fc gamma R2 and Fc gamma R3 in inflamed human gingiva. J Clin Periodontol. 1999;26:441–7. doi: 10.1034/j.1600-051x.1999.260705.x. [DOI] [PubMed] [Google Scholar]
- 18.Stoufi ED, Taubman MA, Ebersole JL, Smith DJ, Stashenko PP. Phenotypic analyses of mononuclear cells recovered from healthy and diseased human periodontal tissues. J Clin Immunol. 1987;7:235–45. doi: 10.1007/BF00915729. [DOI] [PubMed] [Google Scholar]
- 19.Ponath PD, Qin S, Ringler DJ, et al. Cloning of the human eosinophil chemoattractant, eotaxin; Expression, receptor binding, and functional properties suggest a mechanism for the selective recruitment of eosinophils. J Clin Invest. 1996;97:604–12. doi: 10.1172/JCI118456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Charbonnier AS, Kohrgruber N, Kriehuber E, Stingl G, Rot A, Maurer D. Macrophage inflammatory protein 3alpha is involved in the constitutive trafficking of epidermal langerhans cells. J Exp Med. 1999;190:1755–68. doi: 10.1084/jem.190.12.1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nakayama T, Fujisawa R, Yamada H, et al. Inducible expression of a CC chemokine liver- and activation-regulated chemokine (LARC)/ macrophage inflammatory protein (MIP)-3 alpha/CCL20 by epidermal keratinocytes and its role in atopic dermatitis. Int Immunol. 2001;13:95–103. doi: 10.1093/intimm/13.1.95. [DOI] [PubMed] [Google Scholar]
- 22.Yang D, Chertov O, Bykovskaia SN, et al. Beta-defensins: linking innate and adaptive immunity through dendritic and T cell CCR6. Science. 1999;286:525–8. doi: 10.1126/science.286.5439.525. [DOI] [PubMed] [Google Scholar]
- 23.Krisanaprakornkit S, Kimbell JR, Weinberg A, Darveau RP, Bainbridge BW, Dale BA. Inducible expression of human beta-defensin 2 by Fusobacterium nucleatum in oral epithelial cells: multiple signaling pathways and role of commensal bacteria in innate immunity and the epithelial barrier. Infect Immun. 2000;68:2907–15. doi: 10.1128/iai.68.5.2907-2915.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yamazaki K, Nakajima T, Hara K. Immunohistological analysis of T cells functional subsets in chronic inflammatory periodontal disease. Clin Exp Immunol. 1995;99:384–91. doi: 10.1111/j.1365-2249.1995.tb05562.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Malberg K, Molle A, Streuer D, Gangler P. Determination of lymphocyte populations and subpopulations extracted from chronically inflamed human periodontal tissues. J Clin Periodontol. 1992;19:155–8. doi: 10.1111/j.1600-051x.1992.tb00631.x. [DOI] [PubMed] [Google Scholar]
- 26.Taubman MA, Kawai T. Involvement of T-lymphocytes in periodontal disease and in direct and indirect induction of bone resorption. Crit Rev Oral Biol Med. 2001;12:125–35. doi: 10.1177/10454411010120020301. [DOI] [PubMed] [Google Scholar]
- 27.Gemmel E, Carter CL, Seymour GJ. Chemokines in human periodontal disease tissue. Clin Exp Immunol. 2001;125:134–41. doi: 10.1046/j.1365-2249.2001.01511.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


