Abstract
Synthetic CpG containing oligodeoxynucleotide (CpG ODN) is recognized for its ability to activate cells to produce several cytokines, such as IL-12 and TNF-α. In the present study we have demonstrated that CpG ODN 1826, known for its immunostimulatory activity in the mouse system could, by itself, induce nitric oxide (NO) and inducible nitric oxide synthase (iNOS) production from mouse macrophage cell line (RAW 264·7). Neutralizing antibody against TNF-α was not able to inhibit NO or iNOS production from the CpG ODN 1826-activated macrophages, suggesting that although the TNF-α was also produced by CpG ODN-activated macrophages, the production of iNOS was not mediated through TNF-α. Although both CpG ODN 1826 and lipopolysaccharide (LPS) were able to stimulate NO and iNOS production, the exposure time required for maximum production of NO and iNOS for the CpG ODN 1826-activated macrophages was significantly longer than those activated with LPS. These results were due probably to a delay of NF-κB translocation, as indicated by the delay of IκBα degradation. Moreover, the fact that chloroquine abolished NO and iNOS production from the cells treated with CpG ODN 1826 but not from those treated with LPS suggested that the induction of NO and iNOS production from the cells stimulated with CpG ODN (1826) also required endosomal maturation/acidification.
Keywords: CpG ODN, iNOS, mouse macrophage, nitric oxide
INTRODUCTION
Bacterial genomic DNA containing unmethylated cytosine followed by guanine (CpG ODN) is well recognized as an immunostimulator. It appears to function as one of the ‘danger signals’ to trigger innate immunity against infection as well as triggering a specific adaptive immune response [1]. For example, both bacterial CpG DNA and synthetic oligonucleotide containing unmethylated cytosine (CpG ODN) can similarly induce the production of proinflammatory cytokines, such as IL-6, IL-12 and TNF-α in both in vitro and in vivo conditions [2–5].
The molecular mechanism of CpG DNA or CpG ODN in immunostimulation is almost identical to that of the more classical inducers of innate immune system, i.e. lipopolysaccharide (LPS) and peptidoglycan (PG) [6]. In fact, both the CpG DNA and CpG ODN, like LPS, can induce septic shock when injected into mice [3]. Toll-like receptor (TLR) is a critical receptor and signal transducer for both bacterial DNA and LPS. TLR4 has been reported to play an important role in LPS signal transducer, while TLR9 is responsible for CpG ODN activation [7,8]. Moreover, endocytosis of TLR9 is a crucial step for signal transduction of CpG ODN [9,10]. Both TLR4 and TLR9 recruit MyD88 which subsequently activates p38 and c-Jun, resulting in activation of transcription factors such as AP-1 and NF-κB [11–13]. However, the LPS could still induce NF-κB and mitogen-activated protein kinase cascades in a MyD88-independent pathway, as MyD88-deficient cells could still respond to LPS but not to CpG DNA [14,15].
Among the mediators produced by LPS-activated mouse macrophages, nitric oxide (NO) produced by inducible nitric oxide synthase (iNOS) plays a crucial role in innate immune response against bacterial infection [16]. However, unlike those treated with the LPS, the macrophages treated with the bacterial DNA by itself failed to produce NO or iNOS [5], but these products were observed only when the cells were first primed with IFN-γ[5,17,18]. Recent data also indicate that CpG DNA or CpG ODN could stimulate NO production, but only in the presence of a subthreshold concentration of LPS [18].
The specific CpG ODN 1826 (5′ TCCATGACGTTCCTGACGTT 3′) used in the present study, is known to be an excellent immunostimulator in the murine model, both in vitro and in vivo[19]. However, whether this specific CpG ODN alone can activate NO and iNOS production has not been illustrated. The data presented herein demonstrated that CpG ODN (1826) induces NO and iNOS production from mouse macrophages by a mechanism different from that induced by LPS.
MATERIALS AND METHODS
Oligodeoxynucleotides (ODN)
Nuclease-resistant phosphorothioate ODNs were kindly provided by A. M. Krieg (Coley Pharmaceutical Group, Wellesley, MA, USA). The sequences of the ODNs used are 1826 (5′ TCCATGACGTTCCTGACGTT 3′), 1585 (5′ GGGGTCAACGTTGAGGGGGG 3′), 2006 (5′ TCGTCGTTTTGTCGTTTTGTCGTT 3′), and non-CpG containing ODN 1982 (5′ TCCAGGACTTCTCTCAGGTT 3′). Escherichia coli DNA (strain B) was purchased from Sigma (St Louis, MO, USA) and was purified further by repeated extraction with phenol:chloroform:isoamyl alcohol (25:24:1) or Triton X 114 and ethanol precipitation before made into single stranded by boiling for 10 min. Possible contamination with a trace amount of lipopolysaccharide (LPS) in these samples was determined by a Limulus amoebocyte lysate assay (LAL assay, BioWhittaker, Walkersvill, MD, USA) and expressed as endotoxin units (EU) per ml. The lower limit for the detection of LPS in our laboratory was 0·03 EU/ml. An LPS reference prepared from Salmonella typhimurium (Sigma) had an activity of 4·35 ng/EU. All the ODNs and E. coli DNA used had endotoxic activity less than 0·075 EU/mg.
Cell line and culture condition
Mouse macrophage cell line (RAW 264·7) was obtained from American Type Culture Collection (ATCC, Rockville, MD, USA). If not indicated otherwise, the cells were cultured in 24-well plates with 0·5 ml of Dulbeccco’s modified Eagles’ medium (DMEM) (Gibco Laboratories, Grand Island, NY, USA) supplemented with 10% fetal bovine serum (FBS) (HyClone, Logan, UT, USA) at 37°C under a 5% CO2 atmosphere.
NO assay
The production of NO was determined by measuring the quantity of nitrite in the supernatant from the cells cultured under different conditions by the Griess method, using a standard curve constructed with nitrite ranging from 5 to 40 μM[20]. E. coli LPS (Sigma) at concentration of 10 ng/ml was used as a positive control. Under this condition, the sensitivity limit of the method was 5 μM of nitrite.
TNF-α assay
TNF-α activity was measured by a cytotoxic assay using mouse fibroblast cell line (L-929) [21]. In brief, the supernatant from activated macrophages was appropriately diluted before being added to the mouse fibroblast culture. After 18 h of incubation, the TNF-α-treated fibroblast cells were stained with crystal violet. Change in the absorbance at 540 nm was measured and converted to unit/ml of TNF based on a standard curve using murine TNF-α (Genzyme, Cambridge, MA, USA) as standard.
Immunoblotting for iNOS and IκBα detection
The production of iNOS and IκBα was determined by immunoblotting. Briefly, the mouse macrophages were lysed in lysis buffer containing 62·5 mM Tris pH 6·8, 6 M urea, 10% glycerol, 2% SDS, 0·003% bromphenol blue and 5% 2-mercaptoethanol and followed by sonication on ice for 20 s. The lysates were electrophoresed on 8% polyacrylamide before transferring to polyvinylidene difluoride (PVDF) membrane (Bio-Rad, Hercules, CA). The membrane was blocked in 5% skim milk for 1h before reacting overnight with rabbit polyclonal antibody to mouse iNOS or IκBα (Santa Cruz, Santa Cruz, CA). The blots were then reacted with horseradish peroxidase-conjugated swine antirabbit IgG (Dako, Glostrup, Denmark). Protein bands were detected by enhanced chemiluminescence as recommended by the manufacturer (Pierce, Rockford, IL, USA).
RESULTS
NO production from macrophages activated with CpG ODN
To determine NO production, the mouse macrophage (RAW 264·7) monolayer was treated with various concentrations of CpG ODN 1826. After 24 h, the supernatant was analysed for nitrite by Griess reaction. As shown in Fig. 1, NO was produced by the cells stimulated with CpG ODN 1826 at a concentration as low as 0·05 μg/ml, reaching a plateau production at 1 μg/ml. Conversely, the cells activated with non-CpG containing ODN (1982) which served as a negative control, were unable to produce a significant level of NO, even when used at a concentration as high as 10 μg/ml. Two other CpG ODNs, i.e. 1585 and 2006, and bacterial DNA were also analysed but none of them was able to stimulate NO production from these cells when tested under the same conditions (Fig. 2).
Fig. 1.
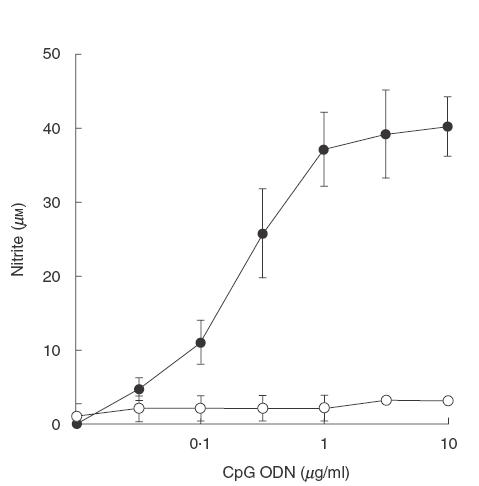
NO production by mouse macrophages stimulated with CpG ODN. RAW 264·7 macrophages (1 × 106 cells/well) were incubated with different concentrations of CpG ODN 1826 (•) or 1982 (○) for 24 h. The supernatant was analysed for nitrite by Griess reaction. Data represent the mean and s.d. of three separate experiments, each carried out in triplicate.
Fig. 2.
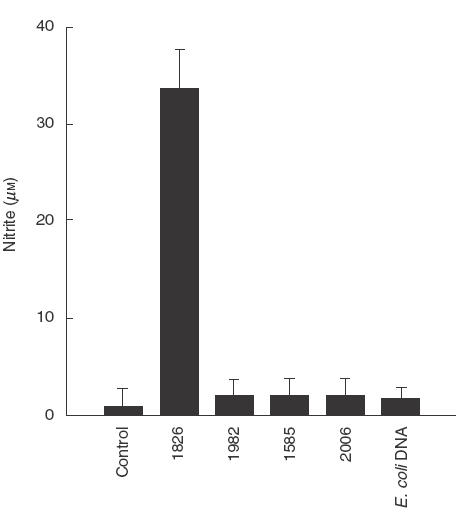
Specificity of CpG ODN sequence in stimulating NO production. RAW 264·7 macrophages (1 × 106 cells/well) were incubated with of CpG ODN 1826, 1982, 1585, 2006 or E. coli DNA at a final concentration of 1 μg/ml for 24 h. Nitrite in the supernatant was determined after 24h of incubation. Unstimulated cells served as control. Data represent means and s.d. of three separate experiments each carried out in triplicate.
In order to rule out the possibility that NO production was due to LPS contamination in the sample, the macrophages were activated with CpG ODN 1826 in the presence of different concentrations of polymyxin B. After 24 h, the supernatant was analysed for nitrite and the cells were subjected to immunoblotting using antibody against iNOS. Results presented in Fig. 3a showed that polymyxin B did not interfere with NO production from the cells activated with CpG ODN 1826. However, under a similar condition, this inhibitor completely abolished NO production from the cells stimulated with 10 ng/ml E. coli LPS. Similarly, polymyxin B at concentration of 10 μg/ml also abrogated iNOS expression completely from the macrophages stimulated with LPS but not with CpG ODN 1826 (Fig. 3b). Together, these results suggest that CpG ODN 1826 was able to stimulate NO and iNOS production and that such a response was not the result of LPS contamination.
Fig. 3.
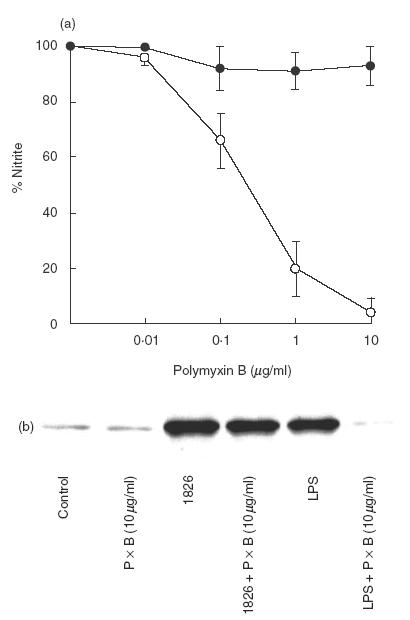
Failure of polymyxin B to inhibit NO production by CpG ODN 1826-activated macrophages. RAW 264·7 macrophages (1 × 106 cells/well) were activated with 1 μg/ml of CpG 1826 (•) or 10 ng/ml of E. coli LPS (○) in the presence of different concentrations of polymyxin B (PxB) for 24 h. The supernatant was analysed for nitrite (a) while the cells were used in the immunoblot for iNOS (b). Control is unstimulated cells. Data (a) represent mean and s.d. of five separate experiments, each carried out in triplicate.
Among the cytokines produced by CpG ODN-activated macrophages, TNF-α is known to be able to stimulate NO and iNOS production [16,22]. The production of TNF-α from the cells activated with CpG ODN 1826 [3] prompted us to investigate further whether NO and iNOS production from the cells treated with CpG ODN 1826 could possibly be mediated through the early TNF-α released. In this experiment, the macrophages were stimulated with CpG ODN 1826 in the presence or absence of neutralizing antibody against TNF-α, and the iNOS production was then measured. The results showed that this antibody was unable to prevent either the NO production (Fig. 4a) or iNOS expression (Fig. 4b). On the contrary, under the same conditions this amount of antibody could neutralize completely the TNF-α activity in the supernatant (Fig. 4c).
Fig. 4.
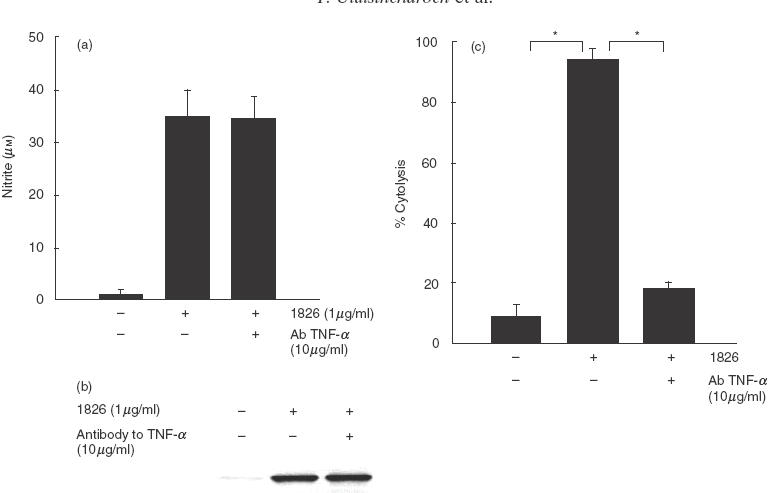
NO and iNOS production by mouse macrophages activated with CpG ODN 1826 is independent of TNF-α production. RAW 264·7 macrophages (1 × 106 cells/well) were stimulated with CpG ODN 1826 (1 μg/ml) in the presence or absence of neutralizing antibody (10 μg/ml) against TNF-α. After 24 h, the supernatant was analysed for nitrite (a) and the cells were analysed for iNOS by immunoblotting (b). The ability of antibody to neutralize biological activity of TNF-α was also determined by cytolysis of mouse fibroblasts (L929) (c). Data (a and c) represent mean and s.d. of three separate experiments, each carried out in duplicate. *P < 0·05 according to Student’s t-test.
Exposure time required for maximum stimulation of NO production by CpG ODN 1826-activated macrophages
To achieve this, the macrophages were pulsed with a precalibrated concentration of CpG ODN 1826 for different time intervals before analysis for NO and iNOS production. After pulsing, the cells were washed and then cultured further for a total of 24h, when the supernatant was collected and analysed for NO and the cells were analysed for iNOS. The results showed that a minimum of 8 h of exposure was required for the cells to be fully activated by CpG ODN 1826 (Fig. 5a). In contrast, the pulsing time required for the LPS to activate these cells fully was less than 1 h. It should be noted that at 1 h, very low NO production was detected when the cells were pulsed with CpG ODN 1826. A similar conclusion could also be obtained with the production of iNOS from the cells activated with these two stimulators (Fig. 5b), although it could be argued that a trace amount of LPS might still be present on the cells (and plastic plates), which would lead to the macrophages activation. This possibility is unlikely to alter the result presented, as we had evidence (unpublished) that polymyxin B added after pulsing gave similar results.
Fig. 5.
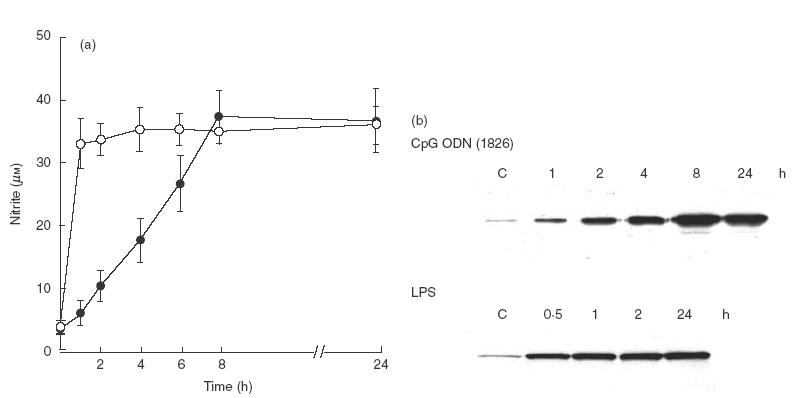
Time required for maximal stimulation of NO in CpG ODN 1826 or LPS activated macrophages. RAW 264·7 macrophages (1 × 106 cells/well) were activated with CpG ODN 1826 (1 μg/ml) (•) or LPS (10 ng/ml) (○) for different time intervals. At times indicated, the supernatant was discarded, washed three times with PBS before incubating further in fresh media. At 24 h, the supernatant was analysed for nitrite (a). Data represent mean and s.d. for three separated experiments, each carried out in duplicate. The cells were analysed for iNOS by immunoblotting (b).
Kinetics of IκBα degradation in CpG ODN 1826-activated macrophages
The macrophages were activated with CpG ODN 1826 at a concentration of 1 μg/ml for 5, 15, 30, 45 and 60 min and IκBα in the lysate of activated cells was determined by immunoblotting. Degradation of IκBα from the CpG ODN 1826-treated cells could not be observed until 45 min after exposure (Fig. 6). In contrast, IκBα degradation from the cells activated with LPS was detected within 15 min. These results suggested that comparing with the LPS, the IκBα degradation in the macrophages activated with CpG ODN 1826 was significantly delayed.
Fig. 6.
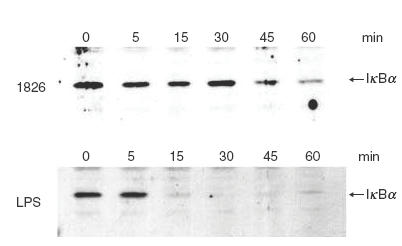
Kinetics of IκBα degradation in the CpG ODN 1826 activated macrophages. RAW 264·7 macrophages (1 × 106 cells/well) were activated with 1μg/ml of CpG ODN 1826 (a) or 10 ng/ml of LPS for different time intervals before the cells were lysed in lysis buffer and analysed for IκBα by immunoblotting.
CpG ODN 1826-mediated NO and iNOS production is dependent on a chloroquine sensitive step
Chloroquine is an endosomal maturation/acidification inhibitor known to abolish CpG ODN-mediated cytokine production and cell activation [9,23]. However, no information on NO and iNOS is currently available. Therefore, in the present study, NO and iNOS production was determined when the macrophages were activated with CpG ODN 1826 or LPS in the presence or absence of chloroquine. As to be expected from its molecular mechanism of action, this inhibitor did not have any effect on NO (Fig. 7a) or iNOS (Fig. 7b) production from the cells stimulated with LPS. However, the chloroquine was able to abrogate both the NO and iNOS production by CpG ODN 1826-activated cells (Fig. 6) suggesting that the cells activated with CpG ODN 1826 required an endosomal maturation/acidification step which was not essential for LPS stimulation.
Fig. 7.
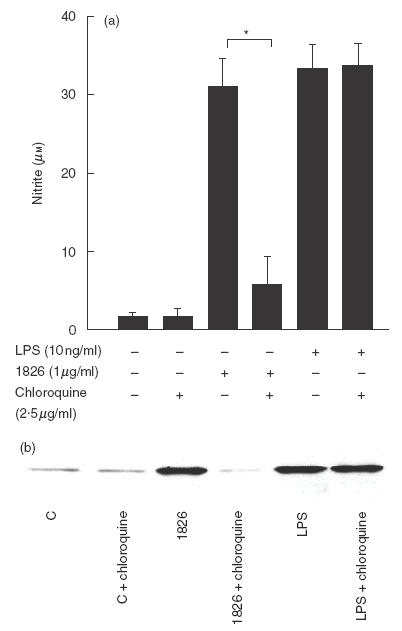
Endosomal maturation/acidification is required for CpG ODN 1826 to activate NO and iNOS production. RAW 264·7 macrophages (1 × 106 cells/well) were preincubated with or without chloroquine (2·5 μg/ml) for 2 h before activated with CpG ODN 1826 (1 μg/ml) or E. coli LPS (10 ng/ml). After 24 h, the supernatant was analysed for nitrite (a) and the cells were analysed for iNOS (b). Control represents the unstimulated cells. Data (a) represent mean and s.d. of three separate experiments, each carried out in duplicate. *P < 0·05 according to Student’s t-test.
DISCUSSION
During the past decade, there have been many independent studies demonstrating the immunomodulatory potential of prokaryotic DNA, especially of bacterial origin. The genomic DNA of bacteria is discriminated from that of the host derived by a pattern recognition system of the innate immune system [24]. Unmethylated CpG oligodeoxynucleotides in the context of particular flanking sequences (CpG motif) are required structure for this specific recognition [25]. In addition, a short synthetic oligonucleotide containing a CpG motif can also serve as a danger signal for the vertebrate immune system [19]. It is well documented that both CpG DNA and synthetic CpG ODN can induce macrophage activation with the production of several proinflammatory cytokines similar to that induced by the classical inducers of the innate immune system, e.g. LPS and peptidoglycan. However, unlike LPS, the bacterial DNA by itself cannot activate NO production from mouse macrophages unless it is given together with IFN-γ or a subthreshold concentration of LPS [5,17,18]. In the present study, we investigated and characterized in more detail NO and iNOS production from mouse macrophages stimulated with CpG ODN 1826. The results indicated that the presence of CpG ODN sequence 1826 alone was sufficient to activate NO and iNOS production by mouse macrophages. This specific ODN, which contains CpG motif flanked by two 5′ purine and two 3′ pyrimidine and containing two optimal murine CpG motif (5′ GACGTT 3′), is also known to have high immunostimulatory activity in the murine system, both in vitro and in vivo[19]. Other CpG ODNs such as 1585 (stimulator for both mouse and human cells) or bacteria DNA can also stimulate cytokine production from mouse macrophages, but neither of them were able to activate NO or iNOS production [5,19] (Fig. 2). Similarly, the CpG ODN 2006 (which is potent for cells of human origin) or non-CpG containing ODN (1982) also failed to stimulate NO or iNOS production (Fig. 2). On the other hand, it can be argued that the NO and iNOS production from the cells used in the present study could be due to a trace contamination of CpG ODN with LPS. This appears not to be the case as polymyxin B, which is a known LPS inhibitor for macrophage activation, had no effect on NO and iNOS production by CpG ODN 1826-activated cells (Fig. 3). Moreover, analysis of these CpG ODNs for endotoxic activity by LAL assay shows only a trace amount, if any, of LPS in these samples. It appears therefore that the DNA or synthetic ODN containing CpG motif alone is sufficient to activate the production of not only cytokines but also of NO and iNOS.
The time-pulsed study employed here suggested that NO and iNOS production by the macrophages activated with CpG ODN 1826 required a minimum of 8 h exposure time for the cells to be activated fully (Fig. 5). This result was strikingly different from that of the LPS stimulation, which required a much shorter pulsing interval. The macrophage response to LPS stimulation is initiated by the binding of LPS to the lipopolysaccharide binding protein (LBP) and this complex then interacts with CD 14. Within 1 min of exposure, the LPS is able to bind to the CD 14 on the cell surface [26]. Phosphorylation of intracellular signalling proteins such as p38 also occurred within 5 min after activation with the LPS [27,28]. However, the phosphorylation of intracellular signalling proteins such as p38 and JNK appeared later when the cells were activated with CpG DNA [19,29]. The slower rate of phosphorylation of the signalling protein may cause a delay of the NF-κB translocation in the CpG ODN 1826-activated cells, judging from a delay of IκBα degradation (Fig. 7). Taken together, these data may explain partially why a longer time is required for the CpG ODN 1826 to activate NO and iNOS production fully.
Endocytosis of CpG DNA is required for its immunostimulatory activity [23,29]. To initiate signalling, the CpG DNA-stimulated cells recruit MyD88 to endosome-like vesicular structure where it co-localizes with CpG DNA. Inhibition of endosomal maturation/acidification such as chloroquine can prevent cytokine production by inhibiting phosphorylation of p38 and JNK [13]. This inhibitor could also inhibit NO and iNOS production from the cells stimulated with CpG ODN 1826 (Fig. 7). In contrast, LPS did not require endocytosis for signalling and cytokine production [13]. Chloroquine also did not interfere with NO and iNOS production from the LPS stimulated macrophages (Fig. 6). These results indicate, therefore, that the NO and iNOS production by CpG ODN 1826-stimulated macrophages is generated by a mechanism similar to that of other cytokines, i.e. requiring endocytosis but possibly different from that of the LPS.
The data currently available on the mediators produced by macrophages stimulated with synthetic CpG ODN and bacterial DNA suggest that they may have similar effect on macrophage activation. However, in this communication we have demonstrated clearly that only the synthetic CpG ODN and not the bacterial DNA in general could stimulate NO production in macrophages. Moreover, our data suggest that a particular sequence structure of synthetic CpG ODN is essential for activating NO production. Previous reports by other investigators have shown that bacterial DNA could stimulate NO production only when the macrophages were pretreated with IFN-γ[5,17,18]. However, Zhu et al. claimed recently that the bacterial DNA could stimulate NO production in the absence of IFN-γ[30]. Their conclusion is inconsistent with the data presented by other investigators [5,17,18]. Their limited results on the effect of CpG ODN (which possessed a sequence similar to the 1826 used herein) on NO production are compatible with the more extensive investigation presented in this communication. More information on the biological effect of synthetic CpG ODN on macrophage and other cell types should be elucidated in order to explain its beneficial effect in the in vivo condition in both experimental animals and human model.
Acknowledgments
This work was supported by research grants from the Thailand Research Fund (TRF) and Chulabhorn Research Institute (Thailand). We are grateful to A. M. Krieg (Coley Pharmaceutical Group, Wellesley, MA, USA) for providing CpG ODN.
REFERENCES
- 1.Krieg AM, Yi AK, Schorr J, Davis HL. The role of CpG dinucleotides in DNA vaccine. Trends Microbiol. 1998;6:23–7. doi: 10.1016/S0966-842X(97)01145-1. [DOI] [PubMed] [Google Scholar]
- 2.Messina JP, Gilkeson GS, Pisetsky DS. Stimulation of in vitro murine lymphocyte proliferation by bacterial DNA. J Immunol. 1991;147:1759–64. [PubMed] [Google Scholar]
- 3.Sparwasser T, Miethke T, Lipford G, Erdmann A, Hacker H, Heeg K, Wagner H. Macrophages sense pathogens via DNA motive. induction of tumor necrosis factor-α mediated shock. Eur J Immunol. 1997;27:1671–9. doi: 10.1002/eji.1830270712. [DOI] [PubMed] [Google Scholar]
- 4.Sparwasser T, Koch ES, Vabulas RM, et al. Bacterial DNA and immunostimulatory CpG oligonucleotides trigger maturation and activation of murine dendritic cells. Eur J Immunol. 1998;28:2045–54. doi: 10.1002/(SICI)1521-4141(199806)28:06<2045::AID-IMMU2045>3.0.CO;2-8. 10.1002/(sici)1521-4141(199806)28:06<2045::aid-immu2045>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 5.Stacey KJ, Sweet MJ, Hume DA. Macrophages ingest and are activated by bacterial DNA. J Immunol. 1996;157:2116–22. [PubMed] [Google Scholar]
- 6.Wagner H. Toll meets bacterial CpG DNA. Immunity. 2001;14:499–502. doi: 10.1016/s1074-7613(01)00144-3. 10.1016/s1074-7613(01)00144-3. [DOI] [PubMed] [Google Scholar]
- 7.Rock FL, Hardiman G, Timans C, Kastelein RA, Bazan JF. A family of human receptors structurally related to Drosophila Toll. Proc Natl Acad Sci USA. 1998;95:588–93. doi: 10.1073/pnas.95.2.588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Medzhitov R, Preston-Hurlburt P, Janeway CA. A human homologue of the Drosophila Toll protein signals activation of adaptive immunity. Nature. 1997;388:394–7. doi: 10.1038/41131. [DOI] [PubMed] [Google Scholar]
- 9.Yi AK, Tuetken R, Redford T, Kirch J, Krieg AM. CpG motif in bacterial DNA activates leukocytes through the pH-dependent generation of reactive oxygen species. J Immunol. 1998;160:4755–61. [PubMed] [Google Scholar]
- 10.Hemmi H, Takeuchi O, Kaisho T, et al. A Toll-like receptor recognizes bacterial DNA. Nature. 2000;408:740–5. doi: 10.1038/35047123. [DOI] [PubMed] [Google Scholar]
- 11.Schnare M, Holt AC, Takeda K, Akira S, Medzhitov R. Recognition of CpG DNA is mediated by signaling pathways dependent on the adaptor MyD88. Curr Biol. 2000;10:1139–42. doi: 10.1016/s0960-9822(00)00700-4. [DOI] [PubMed] [Google Scholar]
- 12.Hacker H, Vabulas RM, Takeuchi O, Hoshino K, Akira S, Wagner H. Immune cell activation by bacterial CpG-DNA through myeloid differentiation marker 88 and tumor necrosis factor receptor-associated factor (TRAF) 6. J Exp Med. 2000;192:595–600. doi: 10.1084/jem.192.4.595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yi AK, Krieg AM. Rapid induction of mitogen-activated protein kinase by immune stimulatory CpG DNA. J Immunol. 1998;161:4493–7. [PubMed] [Google Scholar]
- 14.Kawai T, Adachi O, Ogawa T, Takeda K, Akira S. Unresponsiveness of MyD88-deficient mice to endotoxin. Immunity. 1999;11:115–22. doi: 10.1016/s1074-7613(00)80086-2. [DOI] [PubMed] [Google Scholar]
- 15.Kaisho T, Takeuchi O, Kawai T, Hoshino K, Akira S. Endotoxin induced maturation of MyD88-deficient dendritic cell. J Immunol. 2001;166:5688–94. doi: 10.4049/jimmunol.166.9.5688. [DOI] [PubMed] [Google Scholar]
- 16.MacMicking J, Xie QW, Nathan C. Nitric oxide and macrophage function. Annu Rev Immunol. 1997;15:323–50. doi: 10.1146/annurev.immunol.15.1.323. [DOI] [PubMed] [Google Scholar]
- 17.Shoda LKM, Kegerreis KA, Suarez CE, et al. DNA from protozoa parasites Babesia bovis,Trypanosoma cruzi, and T. brucei is mitogenic for B lymphocytes and stimulates macrophage expression of interleukin-12, tumor necrosis factor alpha, and nitric oxide. Infect Immun. 2001;69:2162–71. doi: 10.1128/IAI.69.4.2162-2171.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gao JJ, Zuvanich EG, Xue Q, Horn DL, Silverstein R, Morrison DC. Bacterial DNA and LPS act in synergy in inducing nitric oxide production in RAW 264.7 macrophages. J Immunol. 1999;163:4095–9. [PubMed] [Google Scholar]
- 19.Hartmann G, Weeratna RD, Ballas ZK, et al. Delineation of a CpG phosphorothioate oligonucleotide for activating primate immune response in vitro and in vivo. J Immunol. 2000;164:1617–24. doi: 10.4049/jimmunol.164.3.1617. [DOI] [PubMed] [Google Scholar]
- 20.Green LC, Wagner DA, Glogowski J, Skipper PL, Wishnok JS, Tannenbaum SR. Analysis of nitrate, nitrite, and [15N] nitrate in biological fluids. Anal Biochem. 1982;126:131–8. doi: 10.1016/0003-2697(82)90118-x. [DOI] [PubMed] [Google Scholar]
- 21.Ruff MR, Gifford GE. Purification and physiochemical characterization of rabbit tumor necrosis factor. J Immunol. 1980;125:1671–7. [PubMed] [Google Scholar]
- 22.Nathan C. Nitric oxide as a secretory product of mammalian cells. FASEB J. 1992;6:3051–64. [PubMed] [Google Scholar]
- 23.Macfarlan DE, Manzel L. Antagonism of immunostimulatory CpG-oligodeoxynucleotides by quinacrine, chloroquine and structurally related compound. J Immunol. 1998;160:1122–31. [PubMed] [Google Scholar]
- 24.Wagner H. Bacterial CpG DNA activates immune cells to signal infectious danger. Adv Immunol. 1999;73:329–68. doi: 10.1016/s0065-2776(08)60790-7. [DOI] [PubMed] [Google Scholar]
- 25.Krieg AM, Yi AK, Hartman G. Mechanisms and therapeutic applications of immune stimulatory CpG DNA. Pharmacol Ther. 1999;84:113–20. doi: 10.1016/s0163-7258(99)00023-6. [DOI] [PubMed] [Google Scholar]
- 26.Gallay P, Jongeneel CV, Barras C, et al. Short time exposure to lipopolysaccharide is sufficient to activate human monocyte. J Immunol. 1993;150:5086–93. [PubMed] [Google Scholar]
- 27.Han J, Lee JD, Tobis PS, Ulevitch RJ. Endotoxin induces rapid protein tyrosine phosphorylation in 70Z/3 cells expressing CD14. J Biol Chem. 1993;268:25009–14. [PubMed] [Google Scholar]
- 28.Utaisincharoen P, Tangthawornchaikul N, Kespichayawattana W, Anuntagool N, Chaisuriya P, Sirisinha S. Kinetic studies of the production of nitric oxide (NO) and tumor necrosis factor alpha (TNF-α) in macrophages stimulated with Burkholderia pseudomallei endotoxin. Clin Exp Immunol. 2000;122:324–9. doi: 10.1046/j.1365-2249.2000.01386.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hacker H, Mischak H, Miethke T, et al. CpG DNA specific activation of antigen-presenting cells requires stress kinase activity and is preceded by non-specific endocytosis and endosomal maturation. EMBO. 1998;17:6230–40. doi: 10.1093/emboj/17.21.6230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhu F, Pisetskty DS. Role of the heat shock protein 90 in immune response stimulation by bacterial DNA and synthetic oligonucleotides. Infect Immun. 2001;69:5546–52. doi: 10.1128/IAI.69.9.5546-5552.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]


