Abstract
This study investigated the profiles of IFN-γ and its regulatory cytokines (IL-12, IL-18 and IL-10) in response to a purified protein derivative (PPD) antigen in peripheral blood mononuclear cells (PBMC) from 18 HIV-negative patients with multidrug-resistant tuberculosis (MDRTB), and compared them with those from 19 healthy tuberculin reactors (HTR). ELISA results showed that following stimulation with PPD, IFN-γ production was significantly reduced, whereas production of both IL-18 and IL-10 was significantly elevated in MDRTB patients compared with HTR. Three out of 18 patients with MDRTB of greater than 4 years duration showed significantly elevated IL-12 p70 production, induced by in vitro PPD stimulation of their PBMC, when compared with data from HTR. However, when taken as a group, MDRTB patients were similar to HTR in their IL-12 p70-producing capacity. IL-12 p70 protein paralleled IL-12 p40 protein expression. In addition, the production of IL-12 p40 was significantly correlated with IL-10 in all patients, but was not correlated with IFN-γ. Neutralization of IL-10 increased IL-12 p40 about twofold, but did not significantly alter IFN-γ induction in MDRTB. IFN-γ in MDRTB was highly correlated with lymphoproliferation and CD4 counts, but was not correlated with IL-12, IL-18 or IL-10 production. Our findings suggest that patients with MDRTB have dysregulated IL-12, IL-18 and IL-10 production during Mycobacterium tuberculosis infection, and the cytokine profiles are similar to those in patients with drug-sensitive advanced TB previously reported in the literature. In addition, IL-10 may not have a dominant role in defective IFN-γ production in patients with MDRTB.
Keywords: multidrug-resistant pulmonary tuberculosis, interferon-gamma, interleukin-12, interleukin-18, interleukin-10, PPD antigen
INTRODUCTION
Multidrug-resistant tuberculosis (MDRTB) is a significant clinical problem that is associated with high morbidity and mortality, and long-term survival for infected immunocompetent patients is reported to be about 70%[1]. The increased incidence of MDRTB is a significant public health and therapeutic problem that may quickly worsen as the HIV epidemic spreads. In addition, it has been reported that outbreaks of MDRTB are a serious clinical problem among individuals who are HIV-negative [2].
MDRTB is often accompanied by host immunosuppression; consequently, immune response enhancement may be a useful supplement to conventional MDRTB chemotherapy [3]. CD4+ T cells are involved in anti-mycobacterial activity [4], and interferon (IFN)-γ produced by CD4+ T cells plays a critical role in protective immunity [5–7]. In HIV-positive TB patients, the CD4 count is the sole independent predictor of survival [8]. In HIV-negative patients with MDRTB, CD4 lymphocytopenia appears to correlate with the severity of disease and low IL-2/IFN-γ production in response to Mycobacterium tuberculosis and purified protein derivatives (PPD) [9].
Although there is substantial evidence to support a role for cell-mediated immunity in the protective immune response against TB [10], little is known about Th1 regulatory cytokine production in MDRTB. Diverse cytokines, including interleukin (IL)-2, IL-12 and IL-18, are known to play important roles in anti-TB cell-mediated immunity. Two cytokines, IL-12 and IL-18, are currently regarded as the primary inducers of IFN-γ production in inflammatory reactions [11,12]. IL-12 is an inducible, heterodimeric, disulphide-linked cytokine that is composed of 35- and 40-kD subunits encoded by separate genes [13]. IL-12 is the most important cytokine for directing primary Th1 differentiation in CD4+ T cells in vitro and in vivo. Studies have shown that treating mice with IL-12 at the time of TB infection results in increased resistance to infection, illustrating the importance of IL-12 for protective immunity against TB [14,15].
IL-18, initially described as an IFN-γ-inducing factor [16], is a multi-functional cytokine that is produced by a wide variety of cells, including mononuclear phagocytes [17]. IL-18 acts together with IL-12 as an early signal in the development of Th1 responses [11,18]. However, IL-18 alone does not induce IFN-γ production by T lymphocytes [19]. The presence of secondary stimulants, particularly IL-12 or microbial agents, is required for IL-18-induced IFN-γ production [20]. Active pulmonary TB is associated with the enhanced production and activity of immunosuppressive molecules, such as IL-10 and transforming growth factor (TGF)-β1 [21]. IL-10 and TGF-β1 have many overlapping biological effects, including T-cell suppression, macrophage deactivation, modulation of pro-inflammatory cytokines and interference with antigen-presenting cell function [22,23].
This study investigated the PPD-induced production of IL-12, IL-18 and IL-10 in peripheral blood mononuclear cells (PBMC) from patients with MDRTB. We found that IL-18 and IL-10 production was significantly up-regulated after PPD stimulation by PBMC from MDRTB patients, whereas IFN-γ production was greatly reduced compared with production in human tuberculin reactors (HTR). In addition, PPD-induced IL-12 production was highly correlated with IL-10 production in MDRTB, but not with either IFN-γ or IL-18 production.
MATERIALS AND METHODS
Subjects
Patients and healthy volunteers consented to take part in this study. Whole blood was obtained by venipuncture from 18 HIV-negative patients with culture-proven MDRTB at the National Mokpo Tuberculosis Hospital (Mokpo, Chonnam, Korea). These patients were at various stages in their clinical course, and had different treatment durations; minimally, all patients showed resistance to rifampicin and isoniazid. A complete history was taken and a physical examination was performed on each patient by one of the investigators. All patients but one (C3) had remained infected for over 2 years, despite anti-tuberculosis drug therapy. The patients (four women and 14 men) ranged in age from 23 to 73 years, with a mean age of 41·7 years (s.d. = 16). All were classified as having far advanced disease, with massive involvement and multiple cavities (total diameter greater than 4 cm), when assessed by X-ray. The patient profiles are shown in Table 1.
Table 1.
Profile of the study group
| Number | Sex/Age | Culture severity | BMI* | ID† (years) | TD‡ (months) | CC¶ | IM§ | CXR** |
|---|---|---|---|---|---|---|---|---|
| C1 | M/59 | 1+ | 20·9 | 13·5 | 82 | P | P | FA |
| C2 | M/46 | 1+ | 22·9 | 19·0 | 36 | N | P | FA |
| C3 | M/47 | 2+ | 18·0 (M) | 1·8 | 22 | P | N | FA |
| C4 | M/25 | 1+ | 14·5 (M) | 3·0 | 23 | N | P | FA |
| C5 | M/41 | 3+ | 18·4 (M) | 7·0 | 43 | N | P | FA |
| C7 | M/48 | 1+ | 15·0 (M) | 3·6 | 43 | P | N | FA |
| C8 | M/29 | 1+ | 21·8 | 13·0 | 38 | N | P | FA |
| C9 | F/35 | 1+ | 22·7 | 4·0 | 31 | N | P | FA |
| C10 | F/23 | 1+ | 18·6 (M) | 3·5 | 31 | N | P | FA |
| C11 | M/31 | 3+ | 21·0 | 6·5 | 78 | P | N | FA |
| C12 | F/23 | 3+ | 19·6 (M) | 6·0 | 46 | N | P | FA |
| C13 | M/66 | 3+ | 17·2 (M) | 4·1 | 49 | P | N | FA |
| C14 | M/28 | 4+ | 17·0 (M) | 5·0 | 34 | N | P | FA |
| C15 | M/73 | 2+ | 18·6 (M) | 21·0 | 89 | N | P | FA |
| C17 | M/31 | 3+ | 23·9 | 4·5 | 54 | P | N | FA |
| C18 | M/39 | 1+ | 19·7 (M) | 4·1 | 49 | P | N | FA |
| C19 | F/34 | 1+ | 22·0 | 5·0 | 32 | N | P | FA |
| C20 | M/72 | 3+ | 17·0 (M) | 6·0 | 52 | P | P | FA |
Body mass index (BMI), body weight/height (m)2; M < 20·0, malnutrition.
Duration of infection (years), periods with positive cultures.
Duration of treatment (months).
History of complete cure; P, positive; N, negative.
Intermittent medication; P, positive; N, negative.
Severity by chest X-ray; FA, far advanced.
Nineteen HTR exhibited skin reactions of more than 15 mm after an intradermal test with 5 units of PPD-RT23 (Statens Seruminstitut, Copenhagen, Denmark), within 1–3 years of their PPD skin test examination, and had no previous history of clinical TB. Each of these healthy controls had received Mycobacterium bovis Bacille bilié de Calmette-Guerin (BCG) vaccinations as children.
Antigen and antibodies
Tuberculin PPD for in vitro assay was purchased from the Statens Seruminstitut and was used at a final concentration of 1·0 μg/ml. Endotoxin content was measured by Limulus amoebocyte lysate assay and was below 1·5 pg/ml in PPD antigen. Neutralizing rat anti-human IL-10 antibodies were purchased from R & D Systems (Minneapolis, MN, USA). For flow cytometric analysis, fluorescein isothiocyanate (FITC)-labelled anti-CD4 and phycoerythrin (PE)-labelled anti-CD8 were purchased from PharMingen (San Diego, CA, USA).
Preparation and stimulation of peripheral blood mononuclear cells
Venous blood was drawn from subjects into sterile blood collection tubes, and PBMC were isolated by density sedimentation over Histopaque-1077 (Sigma, St. Louis, MO, USA). FACS analysis of the PBMC fraction was then performed, and a CD4 count was calculated based on a concomitant complete blood count. Additionally, PBMC were suspended at a density of 1 × 106 viable cells/ml in complete medium RPMI 1640 (Gibco-BRL, Gaithersburg, MD, USA) with 10% fetal bovine serum (Gibco-BRL), sodium pyruvate, non-essential amino acids, penicillin G (100 IU/ml) and streptomycin (100 μg/ml)]. Cells were then stimulated with PPD antigen (1·0 μg/ml), phytohaemagglutinin (PHA, 10 μg/ml; Sigma) or lipopolysaccharide (LPS, 0·1 μg/ml; Sigma), and incubated at 37°C in a 5% CO2 humidified air atmosphere until used for either RNA isolation or supernatant fluid collection.
Flow cytometry
Cell suspensions were stained for analysis by flow cytometry in V-bottomed tubes (3 × 106 cells per test). Flow cytometry data were collected using a FACSCalibur machine (Becton Dickinson, Oxnard, CA, USA) and analysed with CELL QUEST software (Becton Dickinson).
Lymphocyte proliferation assay
PBMC (2·5 × 104 per well) were placed in each well of a round-bottomed microtitre tissue culture plate (Falcon Products, Becton Dickinson). The blastogenic response was measured at various PPD antigen concentrations for 5 days at 37°C in a 5% CO2 humidified air atmosphere. Based on dose–response studies, the optimum concentration of PPD antigen in the final culture was 1·0 μg/ml (data not shown). PHA was used at a concentration of 10 μg/ml as a positive control for cell reactivity. Cells were incubated for 5 days at 37°C in a 5% CO2 humidified air atmosphere. PHA-stimulated cultures were incubated for 3 days, and 2 μCi3H] of thymidine (Amersham, UK) were added for the final 18 h. Cells were harvested on fibreglass paper using a cell harvester (Cambridge Technology, Watertown, MA, USA), and the incorporated radioactivity was measured in a liquid scintillation counter (Beckman, Somerset, NJ, USA). The results were expressed as the mean counts per min ± the standard error (s.e.) of triplicate cultures for each donor. The stimulation index (SI) was calculated using this value and the counts per mine obtained in unstimulated cultures.
Enzyme-linked immunosorbent assay for IFN-γ, IL-12, IL-18 and IL-10
Supernatant fluids were collected from cultures of PBMC stimulated with PPD antigen at 16 (for IL-12 and IL-18), 48 (for IL-10) and 96 (for IFN-γ) h, and were then frozen at –80°C. The frozen supernatant fluids were thawed at room temperature, and cytokine levels were measured with commercial assay kits for IFN-γ, IL-12 p70, IL-12 p40, IL-10 (PharMingen) and IL-18 (R & D Systems), according to the manufacturers’ instructions. Cytokine concentrations in the samples were calculated with standard curves generated from recombinant cytokines, and the results were expressed in picograms per millilitre. The difference between duplicate wells was consistently less than 100% of the mean.
Statistical methods
The results are presented as the mean ± s.d. Statistical significance was calculated using either Student’s t-test or linear regression analysis.
RESULTS
CD4-positive T-cell counts, PPD-stimulated lymphoproliferative responses and IFN-γ production in patients with MDRTB compared with HTR
CD4+ T cell counts and lymphoproliferative responses. As shown in Fig. 1(a), the CD4+ T-cell counts of MDRTB patients were significantly lower than those of HTR (mean 1409·9 ± 1038·1 versus 2044·9 ± 695·3 μl–1, P < 0·05). In addition, the lymphoproliferative responses to PPD antigen were significantly lower in PPD-stimulated PBMC from MDRTB patients than in cells from HTR (mean 4118·1 ± 4658·8 versus 18 601·7 ± 8315·5, P < 0·001; Fig. 1b). A majority of the MDRTB patients did not recognize (SI < 4·0) either of the antigens (12 of 18 64·7%]). All of the non-reactors showed either no stimulation (n = 7; SI < 2·0), or only a marginal increase (n = 5; SI = 2·0–4·0) in the lymphocyte response to PPD. Furthermore, three MDRTB patients with CD4+ T-cell counts below 500 μl–1 had no stimulation (SI < 2·0) of lymphoproliferative responses to PPD.
Fig.1.
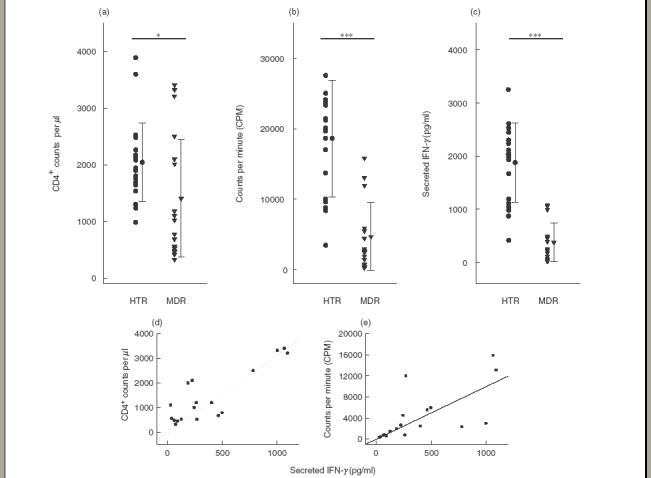
Lymphoproliferative responses, CD4 counts and IFN-γ production in PBMC from patients with MDRTB and HTR in response to the PPD antigen of Mycobacterium tuberculosis. (a) PBMC were isolated and FACS analysis performed. CD4 count was calculated based on a concomitant complete blood count. (b) PBMC were stimulated for 5 days with the PPD antigen of M. tuberculosis at a concentration of 1·0 μg/ml. Proliferative responses were assessed as 3H]-thymidine incorporation in PBMC from healthy controls and TB patients. Incorporation of 3H]-thymidine occurred during the last 18 h of a 5 day culture; unstimulated PBMC served as controls. (c) PBMC were stimulated for 96 h with PPD antigen at a concentration of 1·0 μg/ml. Supernatant fluids were prepared following a 96 h stimulation with PPD, and IFN-γ production was measured using ELISA. Values are the mean ± s.d. of triplicate supernatant samples. *P < 0·05; **P < 0·01; ***P < 0·001 (Student’s t-test). Significant correlations were found between (d) IFN-γ and CD4 counts (n = 18, r = 0·85, P < 0·001), and (e) between IFN-γ and lymphoproliferative responses in TB patients (n = 18, r = 0·68, P < 0·01).
In HTR, the background CPM incorporated was 920·1 ± 350·5, and positive responses ranged from 3459·0 to 38 986·0. To assess the proliferative response under maximal conditions, PBMC were stimulated with the polyclonal mitogen PHA. Stimulation of PBMC with PHA resulted in greater lymphoproliferation (50 000–200 000) than PPD in both groups tested (data not shown).
IFN-γ. Individual data on IFN-γ production by PBMC were obtained following 96 h stimulation with PPD (Fig. 1c). The mean IFN-γ concentrations of MDRTB patients were significantly lower than corresponding values in HTR (mean 403·1 ± 352·1 versus 1877·2 ± 745·6 pg/ml, P < 0·001). Production of IFN-γ in all patients was significantly correlated with CD4+ T-cell counts (n = 18, r = 0·85, P < 0·001; Fig. 1d). Furthermore, PPD-induced IFN-γ production was significantly correlated with lymphoproliferative responses in MDRTB patients (n = 18, r = 0·68, P < 0·01; Fig. 1d).
Stimulation of PBMC with PHA resulted in the secretion of IFN-γ (3000–15 000 pg/ml), and the mean IFN-γ production in response to PHA was similar to HTR (data not shown), indicating that there is no absolute qualitative defect in IFN-γ production in these patients.
IL-18 and IL-10 production after in vitro PPD stimulation in patients with MDRTB compared with HTR
IL-18. PBMC from MDRTB and HTR produced comparable concentrations of IL-18, as determined by ELISA. As shown in Fig. 2(a), the mean concentration of IL-18 in PBMC from MDRTB patients was significantly increased after an 18h PPD stimulation compared with those from HTR (mean 405·4 ± 252·6 versus 159·9 ± 63·1 pg/ml, P < 0·01) (Fig. 2a). We found that unstimulated PBMC also produced comparative values of IL-18 (121·8 ± 66·7 pg/ml). Therefore, IL-18 production in HTR was not shown to be up-regulated following 18 h of PPD stimulation compared with other stimuli, such as adherence alone. An insignificant correlation between IL-18 production and IFN-γ production was observed in culture supernatant fluids from MDRTB patients (n = 18, r = −0·06, P > 0·05; Fig. 2c). Stimulation of PBMC with LPS resulted in the secretion of IL-18 (1000–2000 pg/ml). LPS induced similar IL-18 titres in the healthy controls and TB patients (data not shown).
Fig.2.
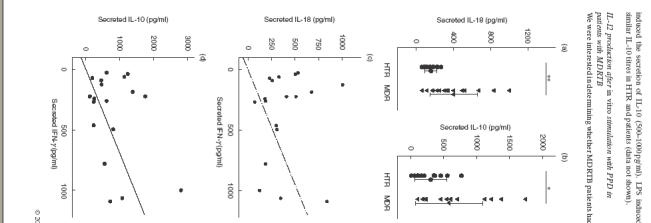
IL-18 and IL-10 production in PBMC from patients with MDRTB in response to the PPD antigen of M. tuberculosis. (a) IL-18 and (b) IL-10 production in PBMC were determined after in vitro stimulation with PPD. Supernatant fluids were prepared following a 96 h stimulation with PPD, and cytokine production was measured using ELISA. Values are the mean ± s.d. of triplicate supernatant samples. *P < 0·05; **P < 0·01; ***P < 0·001 (Student’s t-test). No significant correlations were found (c) between IFN-γ and IL-18 (n = 18, r = –0·06, P > 0·05), or (d) between IFN-γ and IL-10 production in TB patients (n = 18, r = 0·34, P > 0·05).
IL-10. Although there was a substantial heterogeneity in the production of IL-10 in PBMC from MDRTB patients, PBMC from MDRTB patients produced significantly more IL-10 after stimulation with PPD, compared with HTR (mean 588·1 ± 509·2 versus 305·4 ± 244·7 pg/ml, P < 0·05) (Fig. 2b). An insignificant correlation was observed between IL-18 and IFN-γ production in culture supernatant fluids from MDRTB patients (n = 18, r = 0·34, P > 0·05; Fig. 2d). Stimulation of PBMC with LPS preferentially induced the secretion of IL-10 (500–1000 pg/ml). LPS induced similar IL-10 titres in HTR and patients (data not shown).
IL-12 production after in vitro stimulation with PPD in patients with MDRTB
We were interested in determining whether MDRTB patients had a lower endogenous IL-12 production that was correlated with their deficient IFN-γ response. As shown in Fig. 3(a), there was no statistically significant difference between the MDRTB patients and the HTR group, although the mean IL-12 p40 production was higher in MDRTB than in HTR (mean 1886·9 ± 2123·2 versus 603·3 ± 511·7 pg/ml, P > 0·05).
Fig. 3.
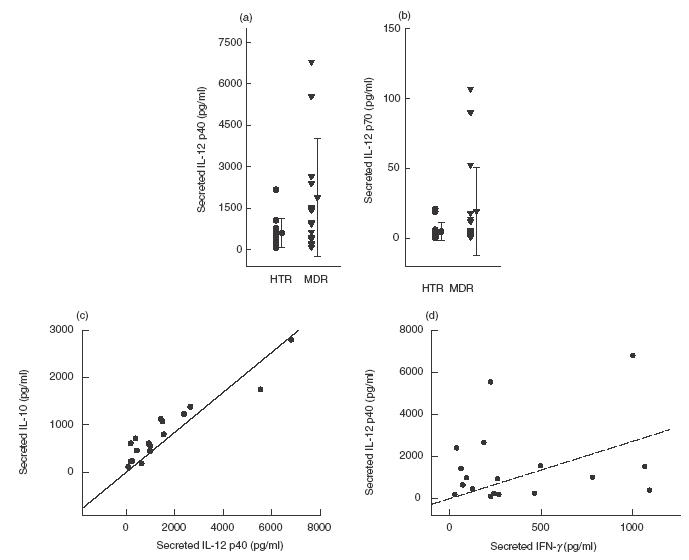
IL-12 production in PBMC from patients with MDRTB in response to the PPD antigen of M. tuberculosis. IL-12 p40 and p70 production in PBMC were determined after in vitro stimulation with PPD antigen. Supernatant fluids were prepared after 18 h and cytokine concentrations were measured using ELISA. Values are the mean ± s.d. of triplicate supernatant samples. (a) IL-12 p40 production in MDRTB patients. (b) IL-12 p70 production in MDRTB patients. A significant correlation was found between (c) IL-12 p40 and IL-10 (n = 18, r = 0·94, P < 0·001), whereas no significant correlation was found between (d) IFN-γ and IL-12 p40 production in TB patients (n = 18, r = 0·24, P > 0·05).
In addition, we measured the release of IL-12 p70, which is a more reliable indicator of biologically active IL-12 production (Fig. 3b). Although excess IL-12 p70 protein (from 20·0 to 110·0 pg/ml) was detected in some donors with IL-12 p40 protein levels exceeding 1000 pg/ml, the PPD-induced IL-12 p70 levels were very low. The PPD-stimulated PBMC from MDRTB patients did not show any significant difference compared with those from HTR (mean 19·4 ± 31·4 versus 4·7 ± 6·3 pg/ml, P > 0·05).
In addition, IL-12 p70 release was significantly correlated with the release of IL-12 p40 (n = 15, r = 0·83, P < 0·001; data not shown). Unstimulated PBMC showed no detectable levels of either IL-12 p40 (<100·0 pg/ml) or IL-12 p70 (<0·2 pg/ml, data not shown). Stimulation of PBMC with LPS resulted in the secretion of IL-12 p40 (1200–2500 pg/ml). LPS induced similar IL-12 titres in MDRTB and HTR (data not shown).
Although we did not observe a significant correlation between IL-12 p40 and IFN-γ production in culture supernatant fluids from MDRTB patients (n = 18, r = 0·24, P > 0·05; Fig. 3d), there was a significant correlation between IL-12 p40 and IL-10 production (n = 18, r = 0·94, P < 0·001; Fig. 3c). This relationship was also observed in HTR (n = 9, r = 0·75, P < 0·001; data not shown).
Some MDRTB patients exhibited IL-12 p40 production more than two s.d. above the mean IL-12 p40 concentration in HTR (>1626·8 pg/ml; n = 4; mean 3406·3 ± 2030·6 pg/ml). Although there were no significant differences between these patients and others (age, nutrition, chest X-ray findings, IFN-γ levels and CD4 counts), these patients had a history of infection of less than 4 years. Patients with lower IL-12 had a longer history of infection (>10 years; n = 4).
Effect of neutralization of endogenous IL-10 on PPD-induced IL-12 p40 and IFN-γ production
Next, we assessed the effect of endogenous IL-10 neutralization on PPD-induced IL-12 p40 and IFN-γ production. PBMC from MDRTB patients were cultured in complete RPMI, with or without PPD (1 μg/ml), in the presence or absence of neutralizing antibodies to IL-10 (2 μg/ml). In preliminary experiments, the amounts of neutralizing antibodies were found to abrogate the bioactivity of 300 pg of IL-10 per ml. Culture supernatant fluids were collected at 18 and 96 h and assayed for IL-12 p40 and IFN-γ immunoreactivity. PPD-induced IL-12 p40 levels doubled in cultures containing neutralizing antibody to IL-10 in HTR and MDRTB patients, as shown in Fig. 4 (P < 0·001, HTR; P < 0·01, MDRTB). In addition, co-culture with neutralizing antibody to IL-10 led to a significant increase in IFN-γ production in HTR (1·6-fold, P < 0·05; Fig. 4). However, there was no significant increase in IFN-γ production after co-culture with neutralizing antibody to IL-10 in MDRTB patients (1·2-fold, P > 0·05; Fig. 4).
Fig.4.
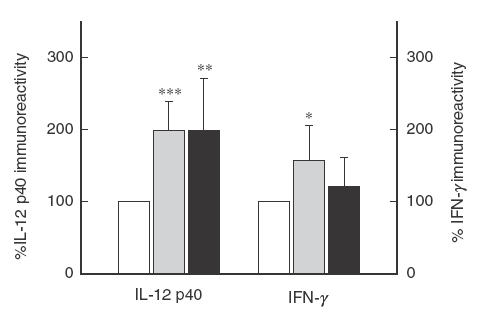
Effect of endogenous IL-10 on PPD-induced IL-12 p40 and IFN-γ production by PBMC. PBMC from HTR (n = 10) and MDRTB patients (n = 9) were cultured with or without neutralizing antibody to IL-10 (2 μl). PPD (1 μg/ml) was added to all cultures. The immunoreactivity for IL-12 p40 and IFN-γ was assessed in culture supernatant fluids at 18 and 96 h, respectively. The percent increase in IFN-γ and IL-12 p40 immunoreactivity compared with those of PPD alone (100%) is shown. (□) PPD only; (▪) a-IL-10 (HTR); (▪) a-IL-10 (MDRTB).
DISCUSSION
Our previous work and several other studies showed that PBMC proliferation and IFN-γ production in PBMC were reduced in patients with active TB and advanced disease [24–27]. In addition, M. tuberculosis-stimulated IFN-γ production by patients with pulmonary TB was found to deteriorate as their disease worsened [27,28]. Our study found that HIV-negative MDRTB patients, who had been infected for at least 2 years, also exhibited depressed IFN-γ production after mycobacterial antigen stimulation. Our findings support earlier indications that HIV-negative MDRTB patients with CD4 counts below 500 per μl have markedly decreased lymphoproliferation and IFN-γ production in response to PPD or M. tuberculosis[9]. One possibility is that the diminished IFN-γ responses in MDRTB patients are due to the loss of M. tuberculosis-reactive T cells through apoptotic mechanisms [29]. A previous study of M. tuberculosis-induced apoptosis in active pulmonary TB patients showed a reduction in the rate of cell apoptosis and increased IFN-γ production after successful anti-tuberculosis chemotherapy [29]. Thus, apoptotic pathways may contribute to T-cell hyporesponsiveness through prolonged deletion of mycobacterial antigen-sensitive T cells in these chronic TB patients with treatment failure.
Protective immunity against M. tuberculosis requires both activated mononuclear phagocytes and T cells. IL-12 may provide a crucial link between these two cell populations by regulating IFN-γ production and the cytotoxic effector function of mycobacterial antigen-specific T cells [30]. Our previous data demonstrated that depressed IL-12 and IFN-γ production recovered in active pulmonary TB patients after anti-tuberculosis therapy [24]. We investigated whether IL-12 depression is associated with the reduced IFN-γ production seen in MDRTB patients. However, the patients in this study showed extremely variable patterns of IL-12 production, and no significant correlation was observed between IFN-γ and IL-12. Although we could not observe any overall correlation between IL-12 and various clinical factors (age, nutrition, chest X-ray findings, IFN-γ levels and CD4 counts), the patient group with higher IL-12 levels (p40, >1630 pg/ml; n = 4) had a shorter history of infection (<4 years), whereas others with lower IL-12 levels (p40, <300 pg/ml; n = 4) had a longer history (>10 years).
A recent study of TB patients with differing pulmonary involvement revealed that IL-12 synthesis was only augmented in advanced TB cases, who also displayed lower IFN-γ production, when compared with moderate cases and HTR [28]. Since all the patients in our study were classified as very advanced disease as assessed by X-ray, the results indicate that patients with advanced disease have augmented IL-12 levels. In addition, our data increase the knowledge of IL-12 depression in chronic TB patients with long-standing infection. Long-term follow-up of the patients will clarify the picture of IL-12 secretion and its role in the pathogenesis of chronic progressive TB.
The heterogenous IL-12 profile seen in MDRTB patients does not appear to be directly involved in the individual variation in IL-12 producing cells. The number of monocyte-derived macrophages, the main source of IL-12, was normal in most of these patients, and they showed similar IL-12 production in response to LPS when compared with HTR (data not shown). However, we could not examine whether monocyte-derived or CD83(+) blood dendritic cells were normal. Further experiments on dendritic cells in MDRTB patients should clearly demonstrate whether the very depressed IL-12 secretion in some patients is mediated by depressed dendritic cell function.
Our findings of augmented IL-12 levels in MDRTB patients with disease for less than 3 years may be due to restored IFN-γ production. A previous report suggests that reduced IFN-γ production by PBMC in TB patients is correlated with poor IL-12Rβ1 and IL-12Rβ2 expression [31]. In addition, there are reports of the significance of IL-12Rβ2 in leprosy patients. IL-12Rβ2 was more strongly expressed in lesions from tuberculoid patients (the resistant form of leprosy) than in those from lepromatous patients (the susceptible form of leprosy), whose cells do not respond to IL-12 [32]. Previous studies and our data led us to hypothesize that depressed IL-12R production may lead to IL-12 hyporesponsiveness in the CD4+ T cells of these patients.
Since IL-12 production was significantly correlated with IL-10 levels, we examined whether a concomitant increase in IL-10 plays a role in the down-regulation of IFN-γ production. Interestingly, IFN-γ was not significantly increased in MDRTB patients, in spite of IL-12 p40 increases after endogenous IL-10 neutralization. Several studies have reported increased IL-10 production in active pulmonary TB patients [24,26]. Recent data showed that TGF-β1 and IL-10 together potentiate the modulatory effect on M. tuberculosis-induced T-cell production of IFN-γ, and TGF-β1 alone enhances IL-10 production. At sites of active M. tuberculosis infection, these interactions might be conducive to the suppression of mononuclear cell function [33]. We did not test the effect of adding anti-TGFβ1 or anti-IL-4 to cultures to determine whether IFN-γ or IL-12 was increased in MDRTB patients. Studies in TGF-β1 indicate that expression of both IL-12 p40 and p35 is suppressed by TGF-β1, and that TGF-β1 interferes with the bioactivity of IL-12 in enhancing M. tuberculosis-induced IFN-γ production [34]. Thus, we cannot rule out a TGF-β1 effect on IFN-γ reduction in MDRTB patients.
Earlier studies in patients with MDRTB have revealed impaired Th1 responses; however, little is known about IL-18 production. We found that both IL-18 and IL-10 production were significantly elevated in MDRTB after stimulation with PPD, and these elevations were not correlated with IFN-γ production. IL-18 is a pro-inflammatory cytokine, initially isolated from liver cells [17,35], that has a pleiotropic function which participates in the induction of IFN-γ and other cytokines [11]. In animals, IL-18 contributes to protective immunity against a variety of pathogens, including Cryptococcus, Leishmania, Salmonella and M. tuberculosis [20,36–39]. In humans, the role of IL-18 is controversial. IL-18 production in response to mycobacterial antigens correlates strongly with IFN-γ production and with protective immunity to mycobacteria [40,41]. However, PBMC from active pulmonary TB patients showed significantly enhanced IL-18 proteins after 96 h of stimulation [24], suggesting a pro-inflammatory role in TB. Furthermore, circulating IL-18 correlated with the extent of disease in pulmonary TB; it was significantly higher in far advanced pulmonary TB than in patients with minimal TB or HTR [42]. We found that MDRTB patients with advanced disease have increased levels of IL-18, suggesting its role in immunopathogenesis.
In conclusion, these data demonstrate that MDRTB patients showed significantly decreased IFN-γ production compared with HTR, although their IL-18 and IL-10 levels were higher after stimulation with PPD antigen. Furthermore, the IL-12 response to PPD was correlated with IL-10 production, but not with IFN-γ production. These results suggest that production of IL-12, IL-18 and IL-10 may be dysregulated in MDRTB patients, and further characterization of the cytokine modulation may provide clarification of the possible application of adjunctive immunotherapy to the treatment of MDRTB.
Acknowledgments
This work was supported by Grant R04-2000–00029 from the Korea Research Foundation for the 2000 programme year.
REFERENCES
- 1.Goble M, Iseman MD, Madsen LA, Waite D, Ackerson L, Horsburgh CR., Jr Treatment of 171 patients with pulmonary tuberculosis resistant to isoniazid and rifampin. N Engl J Med. 1993;328:527–32. doi: 10.1056/NEJM199302253280802. [DOI] [PubMed] [Google Scholar]
- 2.Raviglione MC, Snider DE, Jr, Kochi A. Global epidemiology of tuberculosis. Morbidity and mortality of a worldwide epidemic. JAMA. 1995;273:220–6. [PubMed] [Google Scholar]
- 3.Tsuyuguchi I. Immunotherapy for MDR-TB (multi-drug resistant tuberculosis) – its feasibility. Kekkaku. 1999;74:479–91. [PubMed] [Google Scholar]
- 4.Barnes PF, Modlin RL, Ellner JJ. T-cell responses and cytokines. In: Bloom BR, editor. Tuberculosis: pathogenesis, protection, and control. Washington DC: ASM Press; 1994. pp. 418–23. [Google Scholar]
- 5.Orme IM, Roberts AD, Griffin JP, Abrams JS. Cytokine secretion by CD4 T lymphocytes acquired in response to Mycobacterium tuberculosis infection. J Immunol. 1993;151:518–25. [PubMed] [Google Scholar]
- 6.Cooper AM, Dalton DK, Stewart TA, Griffin JP, Russell DG, Orme IM. Disseminated tuberculosis in interferon gamma gene-disrupted mice. J Exp Med. 1993;178:2243–7. doi: 10.1084/jem.178.6.2243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Flynn JL, Chan J, Triebold KJ, Dalton DK, Stewart TA, Bloom BR. An essential role for interferon gamma in resistance to Mycobacterium tuberculosis infection. J Exp Med. 1993;178:2249–54. doi: 10.1084/jem.178.6.2249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shafer RW, Bloch AB, Larkin C, et al. Predictors of survival in HIV-infected tuberculosis patients. AIDS. 1996;10:269–72. doi: 10.1097/00002030-199603000-00005. [DOI] [PubMed] [Google Scholar]
- 9.McDyer JF, Hackley MN, Walsh TE, Cook JL, Seder RA. Patients with multidrug-resistant tuberculosis with low CD4+ T cell counts have impaired Th1 responses. J Immunol. 1997;158:492–500. [PubMed] [Google Scholar]
- 10.Modlin RL, Barnes PF. IL-12 and the human response to mycobacteria. Res Immunol. 1995;146:526–31. doi: 10.1016/0923-2494(96)83027-6. [DOI] [PubMed] [Google Scholar]
- 11.Dinarello CA, Novick D, Puren AJ, et al. Overview of interleukin-18: more than an interferon-gamma inducing factor. J Leukoc Biol. 1998;63:658–64. [PubMed] [Google Scholar]
- 12.Trinchieri G, Gerosa F. Immunoregulation by interleukin-12. J Leukoc Biol. 1996;59:505–11. doi: 10.1002/jlb.59.4.505. [DOI] [PubMed] [Google Scholar]
- 13.Wolf SF, Temple PA, Kobayashi M, et al. Cloning of cDNA for natural killer cell stimulatory factor, a heterodimeric cytokine with multiple biologic effects on T and natural killer cells. J Immunol. 1991;146:3074–81. [PubMed] [Google Scholar]
- 14.Cooper AM, Roberts AD, Rhoades ER, Callahan JE, Getzy DM, Orme IM. The role of interleukin-12 in acquired immunity to Mycobacterium tuberculosis infection. Immunology. 1995;84:423–32. [PMC free article] [PubMed] [Google Scholar]
- 15.Flynn JL, Goldstein MM, Triebold KJ, Sypek J, Wolf S, Bloom BR. IL-12 increases resistance of BALB/c mice to Mycobacterium tuberculosis infection. J Immunol. 1995;155:2515–24. [PubMed] [Google Scholar]
- 16.Nakamura K, Okamura H, Wada K, Nagata K, Komatsu T, Tamura T. Endotoxin-induced serum factor that stimulates gamma interferon production. Infect Immun. 1989;38:590–5. doi: 10.1128/iai.57.2.590-595.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Klein SA, Ottmann OG, Ballas K, et al. Quantification of human interleukin 18 mRNA expression by competitive reverse transcriptase polymerase chain reaction. Cytokine. 1999;11:451–8. doi: 10.1006/cyto.1998.0424. [DOI] [PubMed] [Google Scholar]
- 18.Kohno K, Kataoka J, Ohtsuki T, et al. IFN-gamma-inducing factor (IGIF) is a costimulatory factor on the activation of Th1 but not Th2 cells and exerts its effect independently of IL-12. J Immunol. 1997;158:1541–50. [PubMed] [Google Scholar]
- 19.Okamura H, Tsutsi H, Komatsu T, et al. Cloning of a new cytokine that induces IFN-gamma production by T cells. Nature. 1995;378:88–91. doi: 10.1038/378088a0. [DOI] [PubMed] [Google Scholar]
- 20.Yoshimoto T, Takeda K, Tanaka T, et al. IL-12 up-regulates IL-18 receptor expression on T cells, Th1 cells, and B cells: synergism with IL-18 for IFN-gamma production. J Immunol. 1998;161:3400–7. [PubMed] [Google Scholar]
- 21.Ellner JJ. Review: the immune response in human tuberculosis-implications for tuberculosis control. J Infect Dis. 1997;176:1351–9. doi: 10.1086/514132. [DOI] [PubMed] [Google Scholar]
- 22.de Waal Malefyt R, Yssel H, Roncarolo MG, Spits H, de Vries JE. Interleukin-10. Curr Opin Immunol. 1992;4:314–20. doi: 10.1016/0952-7915(92)90082-p. [DOI] [PubMed] [Google Scholar]
- 23.Wahl SM. Transforming growth factor beta (TGF-beta) in inflammation. a cause and a cure. J Clin Immunol. 1992;12:61–74. doi: 10.1007/BF00918135. [DOI] [PubMed] [Google Scholar]
- 24.Song CH, Kim HJ, Park JK, et al. Depressed IL-12, but not IL-18 production in response to a 30- and 32-kDa mycobacterial antigen in patients with active pulmonary tuberculosis. Infect Immun. 2000;68:4477–84. doi: 10.1128/iai.68.8.4477-4484.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jo EK, Kim HJ, Lim JH, et al. Dysregulated production of interferon-gamma, interleukin-4 and interleukin-6 in early tuberculosis patients in response to antigen 85B of Mycobacterium tuberculosis. Scand J Immunol. 2000;51:209–17. doi: 10.1046/j.1365-3083.2000.00663.x. [DOI] [PubMed] [Google Scholar]
- 26.Torres M, Herrera T, Villareal H, Rich EA, Sada E. Cytokine profiles for peripheral blood lymphocytes from patients with active pulmonary tuberculosis and healthy household contacts in response to the 30-kilodalton antigen of Mycobacterium tuberculosis. Infect Immun. 1998;66:176–80. doi: 10.1128/iai.66.1.176-180.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dlugovitzky D, Bay ML, Rateni L, et al. In vitro synthesis of inter-feron-gamma, interleukin-4, transforming growth factor-beta and interleukin-1 beta by peripheral blood mononuclear cells from tuberculosis patients: relationship with the severity of pulmonary involvement. Scand J Immunol. 1999;49:210–7. doi: 10.1046/j.1365-3083.1999.00492.x. 10.1046/j.1365-3083.1999.00492.x. [DOI] [PubMed] [Google Scholar]
- 28.Dlugovitzky D, Bay ML, Rateni L, et al. Influence of disease severity on nitrite and cytokine production by peripheral blood mononuclear cells (PBMC) from patients with pulmonary tuberculosis (TB) Clin Exp Immunol. 2000;122:343–9. doi: 10.1046/j.1365-2249.2000.01394.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hirsch CS, Toossi Z, Vanham G, et al. Apoptosis and T cell hyporesponsiveness in pulmonary tuberculosis. J Infect Dis. 1999;179:945–53. doi: 10.1086/314667. [DOI] [PubMed] [Google Scholar]
- 30.Trinchieri G. Interleukin-12. a cytokine produced by antigen-presenting cells with immunoregulatory functions in the generation of T-helper cells type 1 and cytotoxic lymphocytes. Blood. 1994;84:4008–27. [PubMed] [Google Scholar]
- 31.Zhang M, Gong J, Presky DH, Xue W, Barnes PF. Expression of the IL-12 receptor beta 1 and beta 2 subunits in human tuberculosis. J Immunol. 1999;162:2441–7. [PubMed] [Google Scholar]
- 32.Kim J, Uyemura K, Van Dyke MK, Legaspi AJ, Rea TH, Shuai K, Modlin RL. A role for IL-12 receptor expression and signal transduction in host defense in leprosy. J Immunol. 2001;167:779–86. doi: 10.4049/jimmunol.167.2.779. [DOI] [PubMed] [Google Scholar]
- 33.Othieno C, Hirsch CS, Hamilton BD, Wilkinson K, Ellner JJ, Toossi Z. Interaction of Mycobacterium tuberculosis-induced transforming growth factor beta 1 and interleukin-10. Infect Immun. 1999;67:5730–5. doi: 10.1128/iai.67.11.5730-5735.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Toossi Z, Mincek M, Seeholtzer E, Fulton SA, Hamilton BD, Hirsch CS. Modulation of IL-12 by transforming growth factor-beta (TGF-beta) in Mycobacterium tuberculosis-infected mononuclear phagocytes and in patients with active tuberculosis. J Clin Lab Immunol. 1997;49:59–75. [PubMed] [Google Scholar]
- 35.Ushio S, Namba M, Okura T, et al. Cloning of the cDNA for human IFN-gamma-inducing factor, expression in Escherichia coli, and studies on the biologic activities of the protein. J Immunol. 1996;156:4274–9. [PubMed] [Google Scholar]
- 36.Kawakami K, Qureshi MH, Zhang T, Okamura H, Kurimoto M, Saito A. IL-18 protects mice against pulmonary and disseminated infection with Cryptococcus neoformans by inducing IFN-gamma production. J Immunol. 1997;159:5528–34. [PubMed] [Google Scholar]
- 37.Wei XQ, Leung BP, Niedbala W, et al. Altered immune responses and susceptibility to Leishmania major and Staphylococcus aureus infection in IL-18-deficient mice. J Immunol. 1999;163:2821–8. [PubMed] [Google Scholar]
- 38.Mastroeni P, Clare S, Khan S, et al. Interleukin 18 contributes to host resistance and gamma interferon production in mice infected with virulent Salmonella typhimurium. Infect Immun. 1999;67:478–83. doi: 10.1128/iai.67.2.478-483.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sugawara I, Yamada H, Kaneko H, Mizuno S, Takeda K, Akira S. Role of interleukin-18 (IL-18) in mycobacterial infection in IL-18-gene-disrupted mice. Infect Immun. 1999;67:2585–9. doi: 10.1128/iai.67.5.2585-2589.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vankayalapati R, Wizel B, Weis SE, Samten B, Girard WM, Barnes PF. Production of interleukin-18 in human tuberculosis. J Infect Dis. 2000;182:234–9. doi: 10.1086/315656. [DOI] [PubMed] [Google Scholar]
- 41.Garcia VE, Uyemura K, Sieling PA, et al. IL-18 promotes type 1 cytokine production from NK cells and T cells in human intracellular infection. J Immunol. 1999;162:6114–21. [PubMed] [Google Scholar]
- 42.Yamada G, Shijubo N, Shigehara K, Okamura H, Kurimoto M, Abe S. Increased levels of circulating interleukin-18 in patients with advanced tuberculosis. Am J Respir Crit Care Med. 2000;161:1786–9. doi: 10.1164/ajrccm.161.6.9911054. [DOI] [PubMed] [Google Scholar]


