Abstract
We have reported recently that mouse liver NK cells and NK1·1+ T cells were activated by bacterial superantigens via the IL-12 production from Kupffer cells. In the present study, we examined the effect of staphyloccoccal enterotoxin A (SEA) on human T cells with NK cell markers, CD56 or CD57 (NK-type T cells). After stimulating peripheral blood mononuclear cells (PBMC) with SEA, PBMC produced a large amount of IFN-γ and acquired a potent antitumour cytotoxicity. The in vitro depletion of either CD56+ TCR– NK cells, CD56+ T cells or 57+ T cells from PBMC significantly inhibited the IFN-γ production from PBMC. When purified NK-type T cells, NK cells and regular T cells were cultured with monocytes and SEA they all produced IFN-γ, while the IFN-γ amounts produced by both NK-type T cells were greater than those produced by NK cells. NK cells as well as CD56+ T cells showed cytotoxicity against NK-sensitive K562 cells, whereas both NK-type T cells showed a more potent cytotoxicity against NK-resistant Raji cells than did NK cells. The IFN-γ production from each population as well as from whole PBMC was greatly inhibited by anti-IL-12 antibody but not by anti-IL-18 antibody. The antitumour cytotoxicity of whole PBMC was also significantly inhibited by anti-IL-12 antibody while the SEA-induced proliferation of PBMC was not affected by anti-IL-12 antibody. Furthermore, SEA-activated NK-type T cells as well as NK cells showed cytotoxicities against vascular endothelial cells. Our findings suggest that human NK-type T cells are thus involved in bacterial superantigen-induced immune response.
Keywords: CD56+ T cells, CD57+ T cells, IFN-γ IL-12, staphylococcal enterotoxin A
INTRODUCTION
Several toxins produced by Staphylococcus aureus or Streptococcus pyrogenes are called bacterial superantigens and are potent stimulators of certain VβT cells and have been reported to cause various clinical features, including multiple organ failure [1–3]. It is believed that superantigens themselves are not cytopathic but the host immune response to superantigens is considered to induce the tissue damage and organ failure. Activated monocytes/macrophages produce proinflammatory cytokines and activate T cells to produce T helper 1 (Th1) cytokines, IFN-γ and IL-2 [1]. TNF-α is often referred to as an effector molecule in shock and organ failure [4,5]. IFN-γ has also been reported to be involved in bacterial superantigen-induced tissue injury and causes mortality in mice [5]. We have reported recently in mice that IL-12 [6–8], one of the Th1 cytokines, produced by monocytes/macrophages stimulated with bacterial infections or bacterial superantigens, activates NK cells and NK1·1+ T cells, thus inducing them to produce IFN-γ in mice [9–11]. This was also the case in LPS-induced shock or generalized Shwartzman reaction in mice [9,12,13].
On the other hand, we have reported recently that CD56+ or CD57+ NK-type T cells in humans produced a greater amount of IFN-γ and acquired a more potent antitumour cytotoxicity than did regular CD8+ T cells by the stimulation with either immobilized anti-CD3 antibody or Th1 cytokines [14]. As human Vα24+ T cells and mouse Vα14+ NK1·1+ T cells have a TCR sequence homology [15] and both human Vα24+ T cells and mouse Vα14+ NK1·1+ T cells respond to α-galactosylceramide CD1 dependently, Vα24+ T cells have been regarded as a counterpart of mouse NK1·1+ T cells [16,17]. However, in contrast to mouse Vα14+ NK1·1+ T cells, the presence of human Vα24+ T cells is extremely rare, both in the peripheral blood and the liver [18]. Therefore, based on the preferential location in the liver, CD161 (NKRP-1) expression, their potent IFN-γ producing capacity and IL-12-induced antitumour cytotoxicity, we proposed that human CD56+ T cells are a functional counterpart of mouse NK1·1+ T cells [9,14,18]. In addition, CD57+ T cells increase with ageing and the IFN-γ producing capacity of PBMC correlated with the proportion of these cells in PBMC, thus suggesting that NK-type T cells play an impotant role in Th1 immune resposes [9,14].
In the present study, we investigated the role of human NK-type T cells in the superantigen-induced immunological response and showed that one of the staphylococcal superantigens, SEA [19], induces not only regular T cells but also NK-type T cells to produce IFN-γ, thereby acquiring a potent antitumour cytotoxicity in an IL-12-dependent manner. We also show that not only NK cells but also NK-type T cells stimulated with SEA are cytotoxic against human vascular endothelial cells from the umbilical vein (HUVEC).
MATERIALS AND METHODS
Reagents
SEA was purchased from Sigma Inc. (St Louis, MO, USA). Anti-IL-12 antibody (goat IgG) and goat IgG as an isotype antibody for anti-IL-12 antibodies were purchased from R&D system (Minneapolis, MN, USA). Anti-IL-18 antibody was purchased from MBL (Nagoya, Japan).
Isolation of peripheral blood mononuclear cells (PBMC), cell sorting and culture
PBMC were obtained from peripheral blood by Lymphocyte Separation Medium (ICN Biomedicals Inc., Aurora, Ohio, USA). For the depletion of NK-type T cells, the whole PBMC were stained with FITC anti-αβTCR antibody and with either PE-anti-CD56 antibody, anti-CD57 antibody or both PE-anti-CD56 antibody and anti-CD57 antibody, and CD56+ T cells, CD57+ T cells or both of them were separated out. Thereafter, 2 × 105 cells of each cell population in 200 μl of RPMI 1640 containing 20% human serum were cultured with SEA (2·5 μg/ml) for 24 h, 48 h and 60 h and then culture supernatants were harvested and maintained at −80°C for ELISA. After a 60-h culture, the cells were harvested and then subjected to cytotoxic assays.
For the purification of lymphocyte subsets, PBMC were stained with FITC-anti-γδ TCR antibody (IMMU510, Beckman Coulter) and γδ T cells were depleted by MACS (Miltenyi Biotec GmbH, Bergisch Gladbach, Germany) using anti-FITC-microbeads. Thereafter, PBMC were stained with FITC-anti-CD57 antibody, PE-anti-αβ TCR antibody and PC5-anti-CD56 antibody. CD56+αβTCR– NK cells, CD56+αβTCR+ cells, CD56– CD57+αβTCR+ cells and CD56– CD57–αβTCR+ cells were purified by EPICS Elite (Beckman Coulter) (purity of each population was more than 95%); 2 × 105 cells of each subset in 200 μl of RPMI containing 20% human serum were cultured with macrophages in the presence of SEA and culture supernatants were harvested and maintained at −80°C for ELISA. After culture for 60 h, the cells were harvested and then subjected to cytotoxic assays. For the preparation of macrophages, 2 × 105 PBMC in 200 μl of RPMI containing 20% human serum were incubated at 37°C for 2h and after gently removing non-adherent cells, adherent cells were regarded as macrophages.
Assays for IFN-γ levels
IFN-γ levels in lymphocyte culture supernatants were evaluated using the cytokine-specific ELISA (OptEIATM, PharMingen).
Cytotoxic assay
NK-sensitive K562 cells or NK-resistant Raji cells were labelled with 100 μCi Na2 (51Cr)O4 for 60 min at 37°C in RPMI 1640 medium containing 10% FCS, washed three times with medium and subjected to cytotoxicity assays. The labelled targets (4 × 103/well) were incubated in a total volume of 200 μl with effector cells which had been stimulated with SEA for 60 h (E/T ratio = 10:1) in RPMI 1640 in 96-well round-bottomed microtitre plates. The plates were centrifuged after incubation for 4 h, after which the supernatant was harvested and counted with a gamma counter. In some experiments, HUVEC were seeded into a 96-well flat-bottomed plate. After achieving confluence, the HUVEC monolayers were labelled with 100 μCi Na2 (51Cr) O4 overnight at 37°C in RPMI 1640 containing 10% FCS, washed three times with medium, and then subjected to a cytotoxic assay. 2 × 105 cells of the purified CD57+ T cells, CD56+ T cells, regular CD8+ T cells or CD56+ NK cells in 200 μl of RPMI containing 20% human serum were cultured with macrophages in the presence of SEA. After a 60-h culture, the cells were harvested and then subjected to a cytotoxic assay. The cytotoxicity was calculated as the percentage of releasable counts after subtracting the spontaneous release. The spontaneous release was less than 15% of the maximum release. Maximum release was determined by the incubation of target cells with 100 μl of 1n hydrochloric acid.
DNA synthesis assay
To test the proliferation of PBMC (2 × 105/200 μl) by stimulation with SEA, the cells were pulsed with 0·5 μCi per well of [3H]thymidine for 12 h before the cells were harvested. The radioactivities of the harvested cells at the indicated culture time-points were assessed by the liquid scintilation counting method.
Addition of the anti-IL-12 antibody, anti-IL-18 antibody or the isotype control antibody for anti-IL-12 antibody in the culture
Ten μg/ml of each antibody was added from the starting culture of whole PBMC or various lymphocyte subsets with SEA.
Statistical analysis
Differences between the groups were analysed by the Mann–Whitney U-test or an anova analysis with Fisher’s PLSD using the StatView program on an Apple computer. Differences were considered to be significant when P was < 0·05.
RESULTS
SEA-stimulated IFN-γ production from either CD56+ T cells or CD57+ T cells
After depletion from the whole PBMC of each cell population by cell sorting, the PBMC were cultured in the presence of SEA (2·5 μg/ml). The results showed that the depletion of each population caused a significant reduction of the IFN-γ production at 48 and 60 h culture (Fig. 1a). These results suggest that each population had an IFN-γ producing capacity. Since the sorting procedure sometimes exerts a negative effect on the function of lymphocytes, whole PBMC were also passed through a cell sorter and were used as a control.
Fig.1.
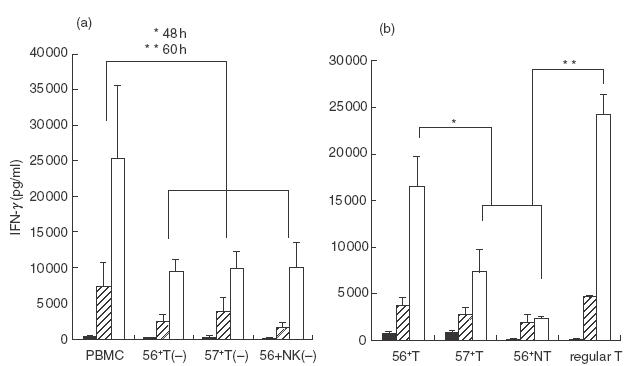
IFN-γ production from human NK-type T cells stimulated with SEA. (a) Decreased IFN-γ production from PBMC by the depletion of either CD56+ T cells (56+ T (−)), CD57+ T cells (57+ T (−)) or CD56+ NK cells (56+ NK (−)). CD56+ T cells, CD57+ T cells or CD56+ NK cells were depleted from whole PBMC by a cell sorter and were cultured with SEA for the indicated hour and culture supernatants were subjected to ELISA. Data are the means ± s.e. from five independent experiments. (b) The IFN-γ production from various lymphocyte subsets. CD56+ T cells, CD57+ T cells, CD56+ NK cells and regular T cells were purified by a cell sorter and were cultured with SEA for the indicated hour and culture supernatants were subjected to ELISA. The data are the means ± s.e. from five independent experiments. *P < 0·05; **P < 0·01. ▪, 24 h;  , 48 h; □, 60h.
, 48 h; □, 60h.
When CD56+ T cells, CD57+ T cells and CD56+ NK cells were purified by cell sorting and then cultured with plastic adherent macrophages in the presence of SEA (2·5 μg/ml), each population produced a substantial amount of IFN-γ (Fig. 1b), especially in the early phase of the culture (24 h and 48 h). In contrast, regular T cell produced a large amount of IFN-γ in the later phase (60 h) (Fig. 1b). Of note, both CD56+ T cells and CD57+ T cells produced larger amounts of IFN-γ than did NK cells (Fig. 1b).
Antitumour cytotoxic activity of SEA-stimulated CD56+ T cells, CD57+ T cells and CD56+ NK cells in comparison with that of regular T cells
After a 60-h culture of each cell population with monocytes in the presence of SEA (2·5 μg/ml), CD56+ T cells and NK cells, but neither CD57+ T cells nor regular T cells, acquired a potent cytotoxicity against NK-sensitive K562 cells (Fig. 2a). The cytotoxic activities against K562 cells of control NK cells cultured with medium alone were usually less than one-third of those of the NK cells cultured with SEA, while the percentage cytotoxicity of control CD56+ T cells against K562 cells was less than 5% (data not shown). In addition, although all populations cultured with SEA showed potent cytotoxicities against NK-resistant Raji cells, both NK-type T cells and T cells showed more potent cytotoxicities than did NK cells (Fig. 2b). When these populations were cultured without SEA, % cytotoxicities of these cells against Raji cells were all less than 5% (not shown).
Fig.2.
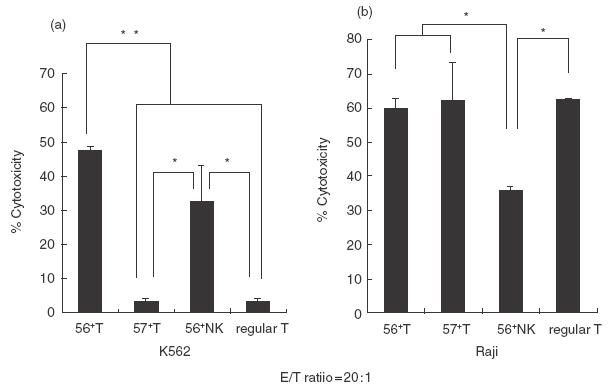
The antitumour cytotoxic activities of various lymphocyte subsets stimulated with SEA. CD56+ T cells, CD57+ T cells, CD56+ NK cells and regular T cells were purified by a cell sorter and were cultured with SEA in the presence of monocytes for 60 h and cytotoxic activities of harvested cells against K562 cells (a) and Raji cells (b) were measured. The data are the means ± s.e. from five independent experiments. *P < 0·05; **P < 0·01.
Dependence on IL-12 of IFN-γ production and cytotoxicity but not of proliferation of SEA-activated PBMC
Although anti-IL-12 antibody did not inhibit the SEA (2·5 μg/ml)-induced proliferation of whole PBMC (Fig. 3a), anti-IL-12 antibody greatly decreased SEA-activated IFN-γ production (Fig. 3b) and cytotoxicity (Fig. 3c) in whole PBMC while anti-IL-18 antibody did not (Fig. 3b,c). The IFN-γ production from whole PBMC without antibody was larger than that from whole PBMC presented in Fig. 1, probably because the IFN-γ data in Fig. 1 were from whole PBMC which had been passed through a cell sorter.
Fig.3.
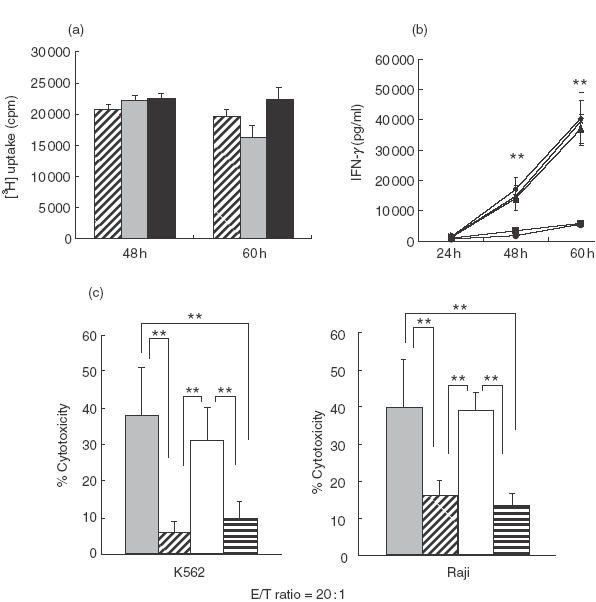
The effect of anti-IL-12 antibody and/or anti-IL-18 antibody on the proliferation, IFN-γ production and antitumour cytotoxicity of PBMC stimulated with SEA. (a) Whole PBMC were cultured with SEA in the presence of anti-IL-12 antibody or an isotype antibody for the indicated times and their proliferations [3H] uptake) at indicated time-points were measured. The data are the means ± s.e. from three independent experiments.  , SEA;
, SEA;  , SEA + anti-IL-12; ▪, SEA + isotype control. (b) Whole PBMC were cultured with SEA in the presence of anti-IL-12 antibody (goat IgG as an isotype control), anti-IL-18 antibody or both anti-IL-12 antibody and anti-IL-18 antibody and IFN-γ levels in the culture supernatants at indicated time-points were compared with those of control without antibody. The data are the means ± s.e. from four independent experiments. **P < 0·01. ♦▴×versus▪•. ♦, SEA; ▪, SEA + anti-IL-12; ▴, SEA + anti-IL-18; •, SEA + anti-IL-12, 18; ×, SEA + isotype control. (c) Whole PBMC were cultured with SEA for 60 h as described above and antitumour cytotoxicities were examined. The data are the means ± s.e. from four independent experiments. **P < 0·01.
, SEA + anti-IL-12; ▪, SEA + isotype control. (b) Whole PBMC were cultured with SEA in the presence of anti-IL-12 antibody (goat IgG as an isotype control), anti-IL-18 antibody or both anti-IL-12 antibody and anti-IL-18 antibody and IFN-γ levels in the culture supernatants at indicated time-points were compared with those of control without antibody. The data are the means ± s.e. from four independent experiments. **P < 0·01. ♦▴×versus▪•. ♦, SEA; ▪, SEA + anti-IL-12; ▴, SEA + anti-IL-18; •, SEA + anti-IL-12, 18; ×, SEA + isotype control. (c) Whole PBMC were cultured with SEA for 60 h as described above and antitumour cytotoxicities were examined. The data are the means ± s.e. from four independent experiments. **P < 0·01.  , SEA;
, SEA;  , SEA + anti-IL-12; □, SEA + anti-IL-18;
, SEA + anti-IL-12; □, SEA + anti-IL-18;  , SEA + anti-IL-12+18.
, SEA + anti-IL-12+18.
Inhibition by anti-IL-12 antibody of IFN-γ production from various lymphocyte subsets stimulated with SEA
The SEA (2·5 μg/ml)-stimulated production of IFN-γ from CD56+ T cells, CD57+ T cells, NK cells and regular T cells was greatly inhibited by anti-IL-12 antibody but not by anti-IL-18 antibody (Fig. 4). Although the IFN-γ amounts produced by all lymphocyte subsets were relatively low, this may also be due to the long sorting time of the each subset before starting the culture.
Fig.4.
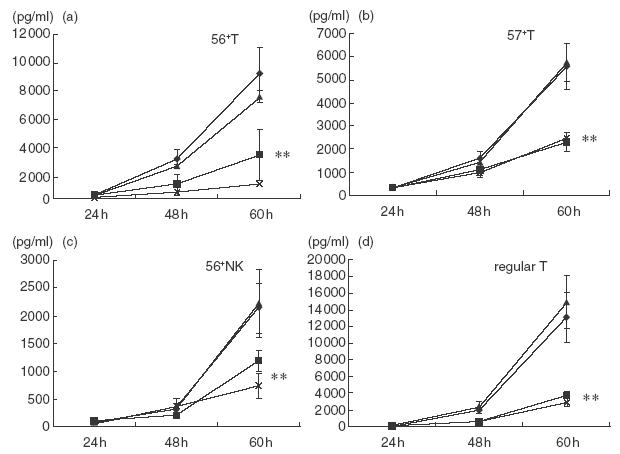
The effect of anti-IL-12 antibody on the IFN-γ production from various lymphocyte subsets. Purified CD56+ T cells, CD57+ T cells, NK cells and regular T cells were stimulated with macrophages and SEA in the presence of anti-IL-12 antibody, anti-IL-18 antibody or both anti-IL-12 antibody and anti-IL-18 antibody and IFN-γ levels in the culture supernatants at the indicated time-points were compared with those of control without antibody. The data are the means ± s.e. from four independent experiments. ♦, SEA; ▪, SEA + anti-IL-12; ▴, SEA + anti-IL-18; ×, SEA + anti-IL-12 + 18. **P < 0·01.
SEA-stimulated CD8+ T cells as well as CD4+ T cells produce IFN-γ and CD8+ T cells exert a potent antitumour cytotoxicity
When purified CD56– CD57– CD4+ T cells and CD56– CD57– CD8+ T cells were stimulated with SEA (2·5 μg/ml) in the presence of monocytes, both subsets produced large amounts of IFN-γ. Notably, CD8+ T cells produced a larger amount of IFN-γ than CD4+ T cells by the 48 h culture (Fig. 5a). In addition, CD8+ T cells showed a more potent cytotoxicity than did the CD4+ T cells against either K562 cells or Raji cells (Fig. 5b,c).
Fig.5.
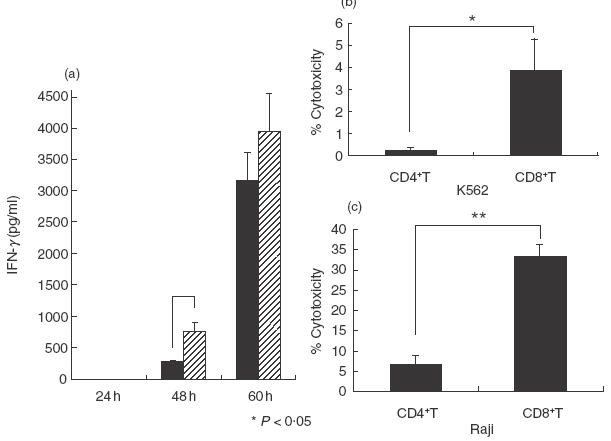
The IFN-γ production and antitumour cytotoxicity of regular CD4+ T cells and CD8+ T cells. (a) Purified CD56– CD57– CD4+ T cells and CD56– CD57– CD8+ T cells were cultured with monocytes in the presence of SEA for the indicated times and the IFN-γ levels in the culture supernatants were measured. ▪, CD4+ T;  , CD8+ T. (b, c) Purified CD56– CD57– CD4+ T cells and CD56– CD57– CD8+ T cells were cultured with monocytes in the presence of SEA for 60 h and cytotoxic activities of these cells were examined. The data are the means ± s.e. from three independent experiments. *P < 0·05; **P < 0·01. E:T ratio = 20:1.
, CD8+ T. (b, c) Purified CD56– CD57– CD4+ T cells and CD56– CD57– CD8+ T cells were cultured with monocytes in the presence of SEA for 60 h and cytotoxic activities of these cells were examined. The data are the means ± s.e. from three independent experiments. *P < 0·05; **P < 0·01. E:T ratio = 20:1.
SEA-activated NK cells as well as NK-type T cells but not regular CD8+ T cells are cytotoxic against HUVEC
In addition to NK cells, CD56+ T cells and CD57+ T cells, especially CD56+ T cells, were found to be cytotoxic against HUVEC after 60 h culture with SEA (2·5 μg/ml) and monocytes (Table 1). However, regular CD8+ T cells showed a minimum cytotoxicity (Table 1). All of these populations cultured with macrophages in the absence of SEA showed less than a 3% cytotoxicity against HUVEC (not shown).
Table 1.
SEA-induced cytotoxic activities of various lymphocyte subsets against HUVEC
| Subsets | % Cytotoxicities against HUVEC† |
|---|---|
| Regular CD8+ T cells | 3·2 ± 1·9 |
| CD57+ T cells | 10·7 ± 4·1* |
| CD56+ T cells | 20·8 ± 7·7* |
| CD56+ NK cells | 46·3 ± 16·2* |
Data are the means ± s.e. from three independent experiments.
E:T ratio = 20:1
P < 0.05 versus CD8+ T cells.
DISCUSSION
In the present study, we have demonstrated that human NK-type T cells produce IFN-γ and acquire an antitumour cytotoxicity by stimulation with SEA mainly via IL-12 production from monocytes/macophages. Furthermore, NK-type T cells produced greater amounts of IFN-γ than did NK cells. In addition, SEA-stimulated regular CD8+ T cells produced an even greater amount of IFN-γ and showed a more potent antitumour cytotoxicity than did regular CD4+ T cells. Whereas SEA-stimulated NK cells exhibited a potent cytotoxicity against HUVEC, NK-type T cells but not regular CD8+ T cells were also cytotoxic against HUVEC.
We proposed recently that CD56+ T cells are a human functional counterpart of mouse NK1·1+ T cells while CD57+ T cells are a human functional counterpart of mouse CD8+ IL-2Rβ (CD122)+ T cells (both increase with age), especially in Th1 immune responses [9,14,20]. The CD56+ T cells activated by IL-2 and IL-12 or anti-CD3 antibody produce a large amount of IFN-γ and exert a potent antitumour cytotoxicity in vitro[14,21]. NK cells and CD56+ T cells are abundant in human livers [21] and these cells in the liver were cytotoxic against hepatocellular carcinoma [18]. These findings revealed that human CD56+ T cells are indeed antitumour effectors similar to mouse NK1·1+ T cells [9,22]. On the other hand, the proportion of CD57+ T cells increases with age in PBMC [23] and also has a potent capacity to produce IFN-γ and a substantial antitumour cytotoxicity [14], thus suggesting that CD57+ T cells play an important role in the Th1 responses against infections and tumours of aged humans [9].
Our present study suggests that not only regular T cells but also NK-type T cells may be involved in immunopathological states induced by bacterial superantigens. Bacterial superantigens sometimes induce multiple organ failure including the kidney, lung and liver [1,2]. The finding that SEA-activated NK-type T cells as well as NK cells produced IFN-γ and were cytotoxic against HUVEC and that activated mouse NK1·1+ T cells are also cytotoxic against hepatocytes [13,24], thus suggests that NK-type T cells induce damage in normal cells under certain conditions. Although the multiple organ failure induced by bacterial superantigens may be due primarily to cardiovascular shock and disseminated intravascular coagulation [1,2] and such cytokines as TNF-α and IFN-γ may play a crucial role in these phenomena [4,5,25,26], it could be hypothesized that endothelial damage induced by superantigen-activated NK cells and NK-type T cells may contribute to the capillary leakage that leads to shock and organ failure.
IL-18 reportedly synergizes with IL-12 and stimulates NK cells and T cells to produce IFN-γ[27,28]. However, anti-IL-18 antibody did not inhibit the SEA-activated IFN-γ production and cytotoxicity of PBMC. Therefore, unlike LPS [29], the SEA-induced activation of PBMC is not dependent on IL-18. Consistent with this finding, a recent study reported that IL-18-deficient mice did not produce IFN-γ in response to LPS but they produce IFN-γ in response to SEB [30]. These findings suggest strongly that staphylococcal superantigens (enterotoxins) activate PBMC mainly through IL-12 produced by the macrophages/dendritic cells.
The finding that, although SEA activates both regular CD4+ T cells and regular CD8+ T cells, CD8+ T cells exerted a more potent cytotoxicity and produce a greater amount of IFN-γ than did regular CD4+ T cells suggests that CD8+ T cells should also be considered as effectors in immunopathological states induced by bacterial superantigens. These results correlate with those of a previous report, which showed not only CD4+ T cells but also CD8+ T cells to be activated by SEB [31].
It should be noted that the depletion of NK cells and either NK-type T cells from PBMC greatly decreased the SEA-induced IFN-γ production from PBMC to a similar degree despite the fact that purified subsets, especially NK cells and CD57+ T cells, produced relatively lower amounts of IFN-γ in response to SEA. However, we recently encountered a similar situation in which the depletion of mouse CD8+ IL-2Rβ (CD122)+ T cells from whole splenocytes reduced the anti-CD3-stimulated IFN-γ production from splenocytes greater than that estimated by the IFN-γ producing capacity of the purified CD8+ IL-2Rβ (CD122)+ T cells themselves [20]. Furthermore, CD8+ IL-2Rβ (CD122)+ T cells induced the IFN-γ production from CD4+ T cells, thus indicating that CD8+ IL-2Rβ (CD122)+ T cells may induce further IFN-γ production from other T cell populations [20]. These findings suggest that the interaction of NK cells and NK-type T cells with regular T cells is required for the effective activation of PBMC as a whole in response to various stimuli.
Although the proportions of CD56+ T cells and CD57+ T cells in PBMC in adults are relatively small (2–5% and 3–10%, respectively), the NK-type T cells, especially CD57+ T cells, increase constantly in proportion with age and CD3-stimulated IFN-γ production from PBMC correlated with the proportion of CD57+ T cells in PBMC [14]. NK cells also increase proportionally with age [14]. It is therefore possible that NK cells as well as NK-type T cells may play a greater role in the superantigen-induced immune responses than previously thought in elderly adults.
REFERENCES
- 1.Scherer MT, Ignatowicz L, Winslow GM, Kappler JW, Marrack P. Superantigens: bacterial and viral proteins that manipulate the immune system. Annu Rev Cell Biol. 1993;9:101–28. doi: 10.1146/annurev.cb.09.110193.000533. [DOI] [PubMed] [Google Scholar]
- 2.Larkin SM, Williams DN, Osterholm MT, Tofte RW, Posalaky Z. Toxic shock syndrome. clinical, laboratory, and pathologic findings in nine fatal cases. Ann Intern Med. 1982;96:858–64. doi: 10.7326/0003-4819-96-6-858. [DOI] [PubMed] [Google Scholar]
- 3.Cone LA, Woodard DR, Byrd RG, Schulz K, Kopp SM, Schlievert PM. A recalcitrant, erythematous, desquamating disorder associated with toxin-producing staphylococci in patients with AIDS. J Infect Dis. 1992;165:638–43. doi: 10.1093/infdis/165.4.638. [DOI] [PubMed] [Google Scholar]
- 4.Doherty GM, Lange JR, Langstein HN, Alexander HR, Buresh CM, Norton JA. Evidence for IFN-gamma as a mediator of the lethality of endotoxin and tumor necrosis factor-alpha. J Immunol. 1992;149:1666–70. [PubMed] [Google Scholar]
- 5.Nagaki M, Muto Y, Ohnishi H, et al. Hepatic injury and lethal shock in galactosamine-sensitized mice induced by the superantigen staphylococcal enterotoxin B. Gastroenterology. 1994;106:450–8. doi: 10.1016/0016-5085(94)90604-1. [DOI] [PubMed] [Google Scholar]
- 6.Kobayashi M, Fitz L, Ryan M, et al. Identification and purification of natural killer cell stimulatory factor (NKSF), a cytokine with multiple biologic effects on human lymphocytes. J Exp Med. 1989;170:827–45. doi: 10.1084/jem.170.3.827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wolf SF, Temple PA, Kobayashi M, et al. Cloning of cDNA for natural killer cell stimulatory factor, a heterodimeric cytokine with multiple biologic effects on T and natural killer cells. J Immunol. 1991;146:3074–81. [PubMed] [Google Scholar]
- 8.Schoenhaut DS, Chua AO, Wolitzky AG, et al. Cloning and expression of murine IL-12. J Immunol. 1992;148:3433–40. [PubMed] [Google Scholar]
- 9.Seki S, Habu Y, Kawamura T, et al. The liver as a crucial organ in the first line of host defense: the roles of Kupffer cells, natural killer (NK) cells and NK1.1 Ag+ T cells in T helper 1 immune responses. Immunol Rev. 2000;174:35–46. doi: 10.1034/j.1600-0528.2002.017404.x. [DOI] [PubMed] [Google Scholar]
- 10.Dobashi H, Seki S, Habu Y, et al. Activation of mouse liver natural killer cells and NK1.1 (+) T cells by bacterial superantigen-primed Kupffer cells. Hepatology. 1999;30:430–6. doi: 10.1002/hep.510300209. [DOI] [PubMed] [Google Scholar]
- 11.Seki S, Osada S, Ono S, et al. Role of liver NK cells and peritoneal macrophages in gamma interferon and interleukin-10 production in experimental bacterial peritonitis in mice. Infect Immun. 1998;66:5286–94. doi: 10.1128/iai.66.11.5286-5294.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ozmen L, Pericin M, Hakimi J, et al. Interleukin 12, interferon gamma, and tumor necrosis factor alpha are the key cytokines of the generalized Shwartzman reaction. J Exp Med. 1994;180:907–15. doi: 10.1084/jem.180.3.907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ogasawara K, Takeda K, Hashimoto W, et al. Involvement of NK1+ T cells and their IFN-gamma production in the generalized Shwartzman reaction. J Immunol. 1998;160:3522–7. [PubMed] [Google Scholar]
- 14.Ohkawa T, Seki S, Dobashi H, et al. Systematic characterization of human CD8+ T cells with natural killer cell markers in comparison with natural killer cells and normal CD8+ T cells. Immunology. 2001;103:281–90. doi: 10.1046/j.1365-2567.2001.01248.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lantz O, Bendelac A. An invariant T cell receptor alpha chain is used by a unique subset of major histocompatibility complex class I-specific CD4+ and CD4−8−T cells in mice and humans. J Exp Med. 1994;180:1097–106. doi: 10.1084/jem.180.3.1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kawano T, Cui J, Koezuka Y, et al. CD1d-restricted and TCR-mediated activation of valpha14 NKT cells by glycosylceramides. Science. 1997;278:1626–9. doi: 10.1126/science.278.5343.1626. [DOI] [PubMed] [Google Scholar]
- 17.Brossay L, Chioda M, Burdin N, et al. D1d-mediated recognition of an alpha-galactosylceramide by natural killer T cells is highly conserved through mammalian evolution. J Exp Med. 1998;188:1521–8. doi: 10.1084/jem.188.8.1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kawarabayashi N, Seki S, Hatsuse K, et al. Decrease of CD56 (+) T cells and natural killer cells in cirrhotic livers with hepatitis C may Be involved in their susceptibility to hepatocellular carcinoma. Hepatology. 2000;32:962–9. doi: 10.1053/jhep.2000.19362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yagi J, Baron J, Buxser S, Janeway CA., Jr Bacterial proteins that mediate the association of a defined subset of T cell receptor: CD4 complexes with class II MHC. J Immunol. 1990;144:892–901. [PubMed] [Google Scholar]
- 20.Takayama E, Seki S, Ohkawa T, et al. Mouse CD8+ CD122+ T cells with intermediate TCR increasing with age provide a source of early IFN-gamma production. J Immunol. 2000;164:5652–8. doi: 10.4049/jimmunol.164.11.5652. [DOI] [PubMed] [Google Scholar]
- 21.Satoh M, Seki S, Hashimoto W, et al. Cytotoxic gammadelta or alphabeta T cells with a natural killer cell marker, CD56, induced from human peripheral blood lymphocytes by a combination of IL-12 and IL-2. J Immunol. 1996;157:3886–92. [PubMed] [Google Scholar]
- 22.Hashimoto W, Takeda K, Anzai R, et al. Cytotoxic NK1.1 Ag+ alpha beta T cells with intermediate TCR induced in the liver of mice by IL-12. J Immunol. 1995;154:4333–40. [PubMed] [Google Scholar]
- 23.Miyaji C, Watanabe H, Minagawa M, et al. Numerical and functional characteristics of lymphocyte subsets in centenarians. J Clin Immunol. 1997;17:420–9. doi: 10.1023/a:1027324626199. [DOI] [PubMed] [Google Scholar]
- 24.Nakagawa R, Nagafune I, Tazunoki Y, et al. Mechanisms of the antimetastatic effect in the liver and of the hepatocyte injury induced by alpha-galactosylceramide in mice. J Immunol. 2001;166:6578–84. doi: 10.4049/jimmunol.166.11.6578. [DOI] [PubMed] [Google Scholar]
- 25.Arad G, Hillman D, Levy R, Kaempfer R. Superantigen antagonist blocks Th1 cytokine gene induction and lethal shock. J Leukoc Biol. 2001;69:921–7. [PubMed] [Google Scholar]
- 26.Saha B, Jaklic B, Harlan DM, Gray GS, June CH, Abe R. Toxic shock syndrome toxin × 1-induced death is prevented by CTLA4Ig. J Immunol. 1996;157:3869–75. [PubMed] [Google Scholar]
- 27.Okamura H, Tsutsi H, Komatsu T, et al. Cloning of a new cytokine that induces IFN-gamma production by T cells. Nature. 1995;378:88–91. doi: 10.1038/378088a0. [DOI] [PubMed] [Google Scholar]
- 28.Okamura H, Tsutsui H, Kashiwamura S, Yoshimoto T, Nakanishi K. Interleukin-18: a novel cytokine that augments both innate and acquired immunity. Adv Immunol. 1998;70:281–312. doi: 10.1016/s0065-2776(08)60389-2. [DOI] [PubMed] [Google Scholar]
- 29.Tsutsui H, Matsui K, Kawada N, et al. IL-18 accounts for both TNF-alpha- and Fas ligand-mediated hepatotoxic pathways in endotoxin-induced liver injury in mice. J Immunol. 1997;159:3961–7. [PubMed] [Google Scholar]
- 30.Hochholzer P, Lipford GB, Wagner H, Pfeffer K, Heeg K. Role of interleukin-18 (IL-18) during lethal shock: decreased lipopolysaccharide sensitivity but normal superantigen reaction in IL- 18-deficient mice. Infect Immun. 2000;68:3502–8. doi: 10.1128/iai.68.6.3502-3508.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Herrmann T, Baschieri S, Lees RK, MacDonald HR. In vivo responses of CD4+ and CD8+ cells to bacterial superantigens. Eur J Immunol. 1992;22:1935–8. doi: 10.1002/eji.1830220739. [DOI] [PubMed] [Google Scholar]


