There are close parallels between inflammation associated with allergic disease and that caused by infections with helminth parasites. Both allergy and helminth infections are associated with elevated levels of IgE, tissue eosinophilia and mastocytosis, and CD4+ T cells that preferentially secrete the Th2 cytokines IL-4, IL-5, and IL-13 [1, 2]. There is good evidence that the expression of inflammation caused by helminth infections can be modulated by the host immune response [3], and that the failure of the expression of similar mechanisms among individuals predisposed to allergy may be responsible for the clinical expression of allergic disease [4]. Further, there is accumulating evidence that helminth infections, particularly those caused by intestinal helminth parasites (or geohelminths) may be capable of modulating the expression of allergic disease [5–8]. This review will examine the evidence for such a modulatory role of intestinal helminth infections (geohelminths) and will provide evidence that the expression of allergic inflammation in different regions of the Tropics may depend partly on local differences in the endemicity of geohelminth infections.
ATOPY AND ASTHMA
Human allergic disease in Western industrialized countries, commonly manifested as asthma, rhinitis and eczema, is strongly associated with atopy[9–11]. Atopy is characterized by elevated levels of both total IgE and IgE specific for common environmental allergens, and evidence of in vivo IgE-mediated immediate hypersensitivity as determined by skin prick testing with the same allergens [9]. Most researchers consider atopy to be an important determinant of allergic asthma although only 25–30% of atopic individuals in industrialized countries may actually go on to develop clinically relevant allergic disease [12] and an estimated 37% of asthma is attributable to atopy at the population level [13]. The factors that cause only a proportion of atopic individuals to develop clinical disease have not been defined although environmental factors are likely to be important.
EPIDEMIOLOGY AND ENVIRONMENTAL DETERMINANTS OF ASTHMA
Large differences in the prevalence and symptoms of asthma have emerged from the first phase of ISAAC [14]. These studies have shown very large international differences in the prevalence of asthma, allergic rhinoconjunctivitis, and atopic eczema. Further, the prevalence of allergic diseases including asthma appears to be increasing in Western industrialized countries [12,15,16]. The causes of the underlying trend of increased prevalence of allergic diseases within the same populations and the large intercountry differences in prevalence are not clear. Some have attributed the rising prevalence to an increase in atopy [17], although markedly different prevalences of asthma are reported among populations with very similar rates of allergic sensitization [18–20].
The prevalence of allergic disease appears to be much greater in Western industrialized countries than in countries with more traditional agricultural economies [14, 20]. Within Tropical regions, there are large differences in the prevalence of allergy between urban and rural areas with higher rates of asthma [19, 21, 1,2, 3, in urban populations. There is some evidence for a disassociation between atopy and asthma in some regions of the Tropics [19, 24] and in rural agriculture-based populations in Europe [20].
Environmental factors could modulate allergic sensitization to environmental allergens and the expression of allergic disease. Such environmental factors may include high-level exposure to allergens [25,26], air pollution [27], exposure to farm animals [28], and diet [29]. Observations that children that are low in the birth order and that live in large families have a reduced risk of allergic disease has led to the suggestion that multiple and continued exposures to childhood viral and bacterial infections may protect against the development of allergy [30]– the so-called hygiene hypothesis. Several epidemiological studies have demonstrated a protective role for infectious agents against the development of allergy including measles [31], gastrointestinal infections [32] the normal gastrointestinal flora of the gut [28,33], and helminth infections [5,6,8,34].
GEOHELMINTH INFECTIONS AND ALLERGY
The role of gastrointestinal helminth infections as environmental determinants of atopy/allergy is of considerable interest. Geohelminth parasites are ubiquitous world-wide and are estimated to infect approximately one third of the human population. Geohelminth infections are the most prevalent and persistent of all childhood infections and most individuals living in endemic areas are infected at some time during their lives and many are infected continuously from soon after birth into adulthood.
Ascaris lumbricoides, Trichuris trichiura, and Ancylostoma duodenale cause the most prevalent infections. Infection with A.lumbricoides and T.trichiura are acquired at an early age reaching a peak in prevalence and intensity between 5 and 15 years of age. Infections with A.duodenale tend to be delayed until the child is able to walk, and peak prevalence may occur later. A useful indication of the intensity of transmission is the age-prevalence profile that tends to peak earlier in areas of high transmission and later in areas where transmission is less intense. The intensity of transmission of geohelminths and the pattern of transmission throughout the year (i.e. continuous or interrupted) is likely to be an important determinant of the host immune response to the parasite [35] and the nature of the immune interaction between geohelminths and allergy.
EPIDEMIOLOGICAL STUDIES OF GEOHELMINTH INFECTIONS AND ALLERGY
Numerous studies have investigated the relationship between geohelminths and allergy. These studies include anecdotal evidence [36], cross-sectional prevalence surveys [21,37,38] or case-control studies [39–44]. The studies that have determined geohelminth infection by the presence or absence of ova or larvae in stool samples, have provided conflicting evidence showing either no relationship [39, 41–43] or a protective effect of infection [8, 36, 45–49]. Overall, there appears to be a negative association between helminth prevalence and asthma prevalence in Tropical regions at the population level [50].
Probably the most influential studies examining geohelminth–allergy interactions have been a series of studies conducted by Lynch et al.[45–47] in Venezuela. The findings of these studies indicate that the intensity of helminth transmission is an important determinant of the effect of helminth infection on allergic reactivity – in areas where transmission is low or infrequent (e.g. among urban groups of high socio-economic status), allergic reactivity is high, while among urban or rural groups exposed to intense transmission, allergic reactivity is low. Further, treatment of urban children living in a poor and geohelminth endemic environment can lead to increased allergic reactivity [6,48].
Several studies have demonstrated that anthelmintic treatment of asthmatic subjects living in endemic areas can result in an improvement in asthmatic symptoms [51–53] and/or reduction in skin test reactivity to environmental allergens [53], indicating that intestinal helminth infections may also be capable of enhancing allergic inflammation.
A recent case-control study from Ethiopia explored the effect of different risk factors for wheeze among asthmatics and nonasthmatic controls from both urban and rural populations [34]. The study showed that the effect of house dust mite sensitization on the risk of wheeze was significantly decreased with increasing intensity of parasite infection (with hookworm), but that the rate of sensitization was consistently higher in individuals with the highest parasite burdens (with Trichuris). These findings were interpreted to suggest that while intestinal helminth infections may enhance allergic sensitization to aeroallergens, intestinal helminth infections with a pulmonary phase of larval migration (principally hookworm infection but perhaps also Ascaris), may actually suppress allergic inflammation in the lungs and protect against wheeze. The observation that hepatitis A seroprevalence was not associated with wheeze or atopy suggests that geohelminth infections are not simply a surrogate factor for exposure to a contaminated environment and other enteric pathogens. However, these observations were made principally on adults from an urban area in Ethiopia, and may not be generalizable to children living in rural areas where the pathoaetiology of wheeze [54] and the epidemiology of geohelminth infections [47] may be very different. The findings of increased sensitization to aeroallergens with higher parasite burdens is consistent with observations of increased rates of sensitization to aeroallergens among children who become infected with Ascaris in Eastern Germany [55], and in other areas where geohelminth transmission is likely to be low or intermittent [7,53]. Studies conducted among rural populations indicate a protective effect for helminth infections against atopy [8,45,49]
NATURAL HISTORY OF THE IMMUNE RESPONSE TO HUMAN GEOHELMINTH INFECTIONS
How can such contradictory observations be explained in which helminth infections can both risk factors for atopy/allergy and also protective factors? We have hypothesized that geohelminth infections may alter the immune response to parasite antigens and environmental aeroallergens to either induce or suppress allergic reactivity, and there is good evidence that human helminth infections can alter the immune response to nonparasite antigens to more closely resemble the parasite-specific response [56,57].
A useful paradigm with which to understand the immune response to helminth infections, and the changes that these responses undergo over time is to divide the natural history of helminth infections into ‘acute’ and ‘chronic’ stages. Under conditions of continuous exposure and the maintenance of high parasite burdens, the acute stage will develop into chronic infection over time. The discussion that follows will examine this paradigm using data from helminth infections in general, and then will focus on geohelminths.
THE ‘ACUTE’VERSUS‘CHRONIC’ PARADIGM OF HUMAN HELMINTH INFECTION
‘Acute’ helminth infections may follow a short period of exposure or infrequent exposure [3]. The classic examples of ‘acute’ helminth infections are reported in expatriates with relatively short exposure histories and who frequently develop clinically apparent allergic reactions (e.g. urticarial rashes) [58]. Similar observations have been made among groups with short periods of exposure or infrequent or intermittent exposure such as:
primary infections in experimental volunteers [59,60] or through accidental/malicious exposure [61,62];
populations that have migrated to an endemic area from an nonendemic area [63,65] or populations that become exposed through immigration of infected individuals into a nonendemic area [66];
nonmigrant populations that have become exposed en masse to transmission due to ecological changes [67];
inhabitants of endemic areas where transmission is seasonal or sporadic [68,69].
‘Acute’ infections are associated with parasite-specific immunity that is characterized by a mixed Th1/Th2 (or Th0) cytokine phenotype [66,70,71], marked eosinophilia, and elevated levels of parasite-specific IgE [72]. Acute helminth infections of humans are associated with numerous allergic syndromes [3,58]. These allergic reactions are associated with intense eosinophilic infiltration and may permit the host to immobilize and kill invasive parasite larvae [73].
To sustain transmission, helminths must maintain a state of persistent ‘infectiousness’ within the human host. As host morbidity is closely related to parasite burden, most natural helminth infections of humans are likely to have coevolved, with their hosts, mechanisms to maintain active infections but control parasite numbers. Primarily, there is the need to control the allergic reactions that are so typical of early and acute infections. Allergic phenomena are rare in individuals with long-standing chronic infections, and their immune response differs from the ‘acute’ phenotype by a more polarized Th2 response [66,70,71,74], and the secretion of significant amounts of immunosuppressive cytokines such as IL-10 and TGF-β[66, 74, 76]. Levels of total IgE are significantly higher in chronic infections with proportionately less parasite specific IgE [72]. High levels of polyclonal and parasite specific IgG4 are typical also [77,78].
THE ‘ACUTE’VERSUS‘CHRONIC’ PARADIGM FOR GEOHELMINTH INFECTIONS
All geohelminth parasites with a pulmonary phase of larval migration (i.e. A.lumbricoides, hookworm, and Strongyloides stercoralis) are capable of causing an asthma–like syndrome (Loeffler’s syndrome), that is characterized by breathlessness, cough, and eosinophilia [68]. Ascariasis is also associated with allergic rashes and acute anaphylaxis [79], although the former may be more common with infections in which parasite larvae migrate more widely in the tissues (e.g. S.stercoralis and larva migrans syndromes). In locations where Ascaris infections are seasonal as a result of the failure of eggs to survive throughout the year, symptoms of pulmonary ascariasis may be relatively common. Gelpi and Mustafa [69] reported outbreaks of eosinophilic pneumonitis associated with A.lumbricoides infections occurring every year during and after the short rainy season in Saudi Arabia.
In regions where ascariasis is highly endemic, and infections are acquired at an early age, symptomatic pulmonary ascariasis appears to be rare. For example, a large survey conducted over a year in Colombia [80] in communities where the prevalence of ascariasis was between 25 and 85%, was able to identify only 1 typical case of larval ascariasis among over 12000 individuals attending health centres or local hospitals. Therefore, in areas where transmission of Ascaris occurs throughout the year, larval ascariasis is either asymptomatic or is associated with mild and nonspecific symptoms.
RELEVANCE OF THE ACUTE VS. CHRONIC PARADIGM
The acute/chronic paradigm provides a useful framework within which to understand differences in parasite immune responses and clinical disease observed in different countries, and even between different communities within the same region. An important determinant of the expression of geohelminth–associated allergic inflammation may be the epidemiology of geohelminth infections in a particular area (Fig. 1) – where geohelminth transmission is sporadic or seasonal (low prevalence), an acute allergy-enhancing phenotype may predominate while in areas where transmission is continuous (high prevalence), more chronic and allergy-suppressing infections would be expected. In areas where transmission is continuous, such infections would be expected to suppress allergic responses in an age- and infection intensity-dependent fashion. In the case of infections such as A.lumbricoides and T.trichiura that are acquired at an early age, and constant exposure occurs throughout childhood, school age children with the heaviest parasite burdens would be expected to have the lowest rates of atopy and allergic disease.
Fig. 1.
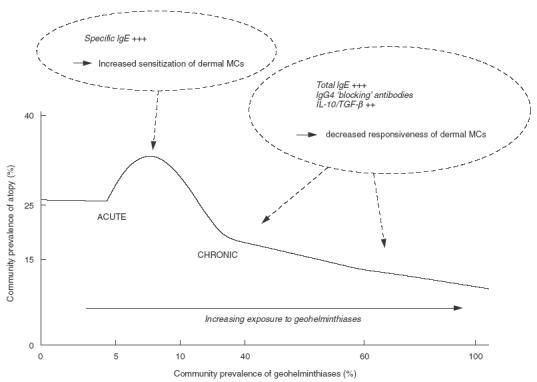
The ‘acute versus chronic’ geohelminth infection paradigm as an environmental determinant of atopy. The Figure shows the relationship between the prevalence of atopy (defined by allergen skin test reactivity) and geohelminth infections in areas of different intensities of geohelminth transmission. Areas of low-level exposure are associated with a low prevalence of geohelminth infections, a predominantly acute geohelminth infection phenotype, and enhanced atopic reactivity while areas of high-level exposure are associated with a high prevalence of infections, a chronic infection phenotype, and suppressed atopic reactivity. The mechanisms by which acute and chronic geohelminth infections may affect atopic reactivity are shown in italics [93], MCs, mastcells.
MECHANISMS OF ALLERGY MODULATION
Geohelminth parasites may modulate allergic disease in two ways: (1) directly – geohelminth parasites may themselves induce allergic disease (e.g. Loeffler’s syndrome); and (2) indirectly – geohelminth parasites may modulate the immune response to environmental allergens. There are several mechanisms by which geohelminth infections can alter the immune response to environmental aeroallergens to either induce or suppress allergic reactivity. Concurrent geohelminth infections may affect immune priming for IgE as well as the development of the pathophysiological changes in the lung that are typical of asthma (airways inflammation and bronchial hyperreactivity), and may act at several levels in the allergic inflammatory pathway by affecting:
the initial development and polarization of Th2 helper cells
Th2 helper cell action in the airways
the level of nonspecific inflammation in the airways
The mechanisms by which acute and chronic infections may modulate allergic inflammation are listed in Fig. 1 and include acute and chronic infections.
Acute infections
The invasive larvae of geohelminth parasites that migrate through the lungs are the primary target of parasite specific immune responses [3], and early during infection induce strong eosinophil-rich inflammation in the lungs [81]. Ascaris larvae secrete large amounts of allergenic substances [82] that are likely to be the primary stimulus for IgE production in infected individuals. Larval antigens are likely to induce strong Th2 responses [83]. During early infections, invasive parasite larvae may not only induce allergic inflammation directly but also may enhance allergic inflammation targeted against nonparasite allergens (such as aeroallergens) through bystander or adjuvant effects as suggested by the findings of experimental animal studies [84]. Enhanced IL-4 and IL-13 production may result in increased synthesis of aeroallergen-specific IgE and sensitize mast cells in a number of tissues including the skin (e.g. resulting in increased atopy).
Chronic infections
Chronic infections with geohelminth parasites may suppress parasite-specific and aeroallergen-specific immune responses through several mechanisms
Mast cell saturation
Geohelminth parasites secrete potent allergens [82,85] and are considered to be the principal explanation for the high levels of polyclonal IgE that are observed in endemic populations [58,86]. Children living in endemic areas often have total IgE levels in excess of 10 000IU/ml [35]. The production of large amounts of polyclonal IgE in helminthiases may modulate immediate hypersensitivity reactions by inhibition of the activity of mast cells by saturation of high affinity FcɛR1 receptors on mast cells and basophils [5,46,87,88]. Saturation of mast cells could explain reduced sensitivity to aeroallergens and also reduced inflammation in the airways (bronchial hyperresponsiveness). Likewise, saturation of low-affinity FcɛR11 with nonspecific IgE on antigen presenting cells may prevent optimal IgE-dependent antigen focusing and presentation to T cells [3].
IgG4 ‘blocking’ antibodies
Polyclonal activation of IgG4 by parasite products and the production of large amounts of IgG4 including IgG4 antibodies specific to IgE-reactive epitopes of environmental allergens, may block IgE-driven inflammation by saturation of available reaginic epitopes [77,89,90].
Bystander suppression by anti-inflammatory cytokines
Observations from tissue invasive helminth infections would support the development of cellular immune down-regulatory mechanisms following persistent exposure and chronicity of infection that may suppress allergic inflammation. The principal mechanism by which this occurs appears to be the increased production of anti-inflammatory cytokines (IL-10 and TGF-β) [74–76]. The production of large amounts of anti-inflammatory cytokines such as IL-10 by parasite-antigen stimulated T cells could cause bystander suppression of immune responses to environmental allergens [8] or the induction of T cells specific for environmental allergens that secrete IL-10/TGF-β (e.g. Th3 or Tr-1 cells) and that directly down-regulate allergic responses to environmental allergens [4].
Tolerization
There is some evidence to suggest that ‘tolerization’ to parasite antigens may occur in early infancy or neonatally through the transfer of parasite antigens from infected mothers. Tolerization could occur either peripherally or through thymic deletion of reactive cells [91,92], and could be induced to environmental aeroallergens that are immunologically cross-reactive with parasite allergens [93,94].
CONCLUSION
There are large international differences in the prevalence of allergic disease, that appears to be much lower in Tropical regions, particularly among rural populations. Environmental factors including childhood infections have been implicated as important determinants of the expression of allergic disease. Geohelminth infections are the most prevalent and persistent of all childhood infections and are most prevalent among rural populations in the Tropics. There is evidence from different epidemiological studies that geohelminth infections may modulate the expression of atopy and also of allergic disease, and may be protective against atopy/allergic disease in some populations but risk factors for atopy/allergic disease in others. A partial explanation for such contradictory observations may be provided by a paradigm in which acute geohelminth infections enhance allergic reactivity and chronic infections suppress allergic inflammation (Fig. 1). Acute or early helminth infections appear to enhance allergic inflammation directed against both parasite and nonparasite antigens (e.g. environmental allergens), while chronic infections appear to suppress allergic inflammation. Suppression of atopy and allergic disease among individuals with chronic or long-standing geohelminth infestations may occur through several mechanisms that include mast cell saturation by polyclonal IgE and the enhanced production of anti-inflammatory cytokines (i.e. IL-10 and TGF-β). The overall effect of geohelminth infections on allergic inflammation is likely to vary between different regions and even between different communities in the same area depending on the endemicity of infection with different geohelminth parasites and on the age (and history of infection) of the study group selected. Clearly, the interaction between geohelminth infections and allergy is highly complex, and there remain a number of unanswered questions regarding the modulatory role of geohelminth infections against atopy/allergic disease. Future studies could address the following questions:
What are the important mechanisms of geohelminth-mediated immunomodulation of atopy/allergic disease in populations of different endemicity for geohelminths?
Are atopic individuals more resistant to geohelminth infections or are geohelminth-infected individuals more protected against atopy (i.e. reverse causality)?
Can the suppression of atopy associated with chronic geohelminth infections be reversed by anthelmintic treatment and can the reacquisition of infection after treatment have the reverse effect?
Has the prevalence of atopy/allergy increased in areas where sustained anthelmintic control programmes are in place?
What are the risk factors for allergy in geohelminth-endemic populations and do these differ from nonendemic populations?
REFERENCES
- 1.Maizels RM, Bundy DA, Selkirk ME, Smith DF, Anderson RM. Immunological modulation and evasion by helminth parasites in human populations. Nature. 1993;365:797–805. doi: 10.1038/365797a0. [DOI] [PubMed] [Google Scholar]
- 2.Holt PG, Macaubas C, Stumbles PA, Sly PD. The role of allergy in the development of asthma. Nature. 1999;402:B12–B17. doi: 10.1038/35037009. [DOI] [PubMed] [Google Scholar]
- 3.Cooper PJ, Nutman TB. IgE and its role in parasitic helminth infection: implications for anti-IgE based therapies. In: Fick RB, Jardieu P, editors. IgE and Anti-IgE Therapy in Asthma and Allergic Disease. New York: Marcel Dekker 2002: in press; [Google Scholar]
- 4.Wills-Karp M, Santeliz J, Karp CL. The germless theory of allergic disease: revisiting the hygiene hypothesis. Nature Rev Immunol. 2001;1:69–75. doi: 10.1038/35095579. [DOI] [PubMed] [Google Scholar]
- 5.Hagel I, Lynch NR, Perez M, Di Prisco MC, Lopez R, Rojas E. Modulation of the allergic reactivity of slum children by helminthic infection. Parasite Immunol. 1993;15:311–5. doi: 10.1111/j.1365-3024.1993.tb00615.x. [DOI] [PubMed] [Google Scholar]
- 6.Lynch NR, Hagel I, Perez M, Di Prisco MC, Lopez R, Alvarez N. Effect of anthelmintic treatment on the allergic reactivity of children in a tropical slum. J Allergy Clin Immunol. 1993;92:404–11. doi: 10.1016/0091-6749(93)90119-z. [DOI] [PubMed] [Google Scholar]
- 7.Lynch NR, Hagel IA, Palenque ME, et al. Relationship between helminthic infection and IgE response in atopic and nonatopic children in a tropical environment. J Allergy Clin Immunol. 1998;101:217–21. doi: 10.1016/S0091-6749(98)70386-0. [DOI] [PubMed] [Google Scholar]
- 8.van den Bigelaar AHJ, van Ree R, Rodrigues LC, Lell B, Deelder AM, Kremsner PG, Yazdanbakhsh M. Decreased atopy in children infected with Schistosoma hematobium: a role for parasite-induced interleukin-10. Lancet. 2000;356:1723–7. doi: 10.1016/S0140-6736(00)03206-2. [DOI] [PubMed] [Google Scholar]
- 9.Burrows B, Martinez FD, Halonen M, Barbee RA, Cline MG. Association of asthma with serum IgE levels and skin-test reactivity to allergens. N Engl J Med. 1989;320:271–7. doi: 10.1056/NEJM198902023200502. [DOI] [PubMed] [Google Scholar]
- 10.Von Mutius E, Martinez FD, Fritsch C, Nicolai T, Reotmeir P, Thiemann HH. Skin test reactivity and number of siblings. BMJ. 1994;308:692–5. doi: 10.1136/bmj.308.6930.692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Remes ST, Korrpi M. Asthma and atopy in schoolchildren in a defined population. Acta Paediatrica. 1996;85:965–70. doi: 10.1111/j.1651-2227.1996.tb14195.x. [DOI] [PubMed] [Google Scholar]
- 12.Woolcock AJ, Peat JK, Trevillion LM. Is the increase in asthma prevalence linked to increase in allergen load. Allergy. 1995;50:935–40. doi: 10.1111/j.1398-9995.1995.tb02504.x. [DOI] [PubMed] [Google Scholar]
- 13.Pearce N, Pekkanen J, Beasley R. How much asthma is really attributable to atopy? Thorax. 1999;54:268–72. doi: 10.1136/thx.54.3.268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.International Study of Asthma and Allergies in Childhood (ISAAC) Steering Committee. Worldwide variation in prevalence of symptoms of asthma, allergic rhinoconjunctivitis, and atopic eczema: ISAAC Lancet. 1998;351:1225–32. [PubMed] [Google Scholar]
- 15.Carlsen KH. Epidemiology of childhood asthma. Eur Resp Rev. 1994;4:5–9. [Google Scholar]
- 16.Anonymous. Surveillance for asthma – United States 1960–95. Morb Mort Wkly Rep. 1998;47:1–28. [PubMed] [Google Scholar]
- 17.Lewis S. ISAAC – a hypothesis generator for asthma? Lancet. 1998;351:1220–1. doi: 10.1016/s0140-6736(98)22017-4. [DOI] [PubMed] [Google Scholar]
- 18.Leung R, Ho P, Lam CW, Lai CK. Sensitisation to inhaled allergens as a risk factor for asthma and allergic diseases in Chinese population. J Allergy Clin Immunol. 1997;99:594–9. doi: 10.1016/s0091-6749(97)70018-6. [DOI] [PubMed] [Google Scholar]
- 19.Yemanerbhan H, Bekele Z, Venn A, Lewis S, Parry E, Britton J. Prevalence of wheeze and asthma and relation to atopy in urban and rural Ethiopia. Lancet. 1997;350:85–90. doi: 10.1016/S0140-6736(97)01151-3. [DOI] [PubMed] [Google Scholar]
- 20.Pritanji A, Strachan D, Burr M, Sinamati J, Shkurti A, Grabocka E, Kaur B, Fitzpatrick S. Asthma and allergy in Albania and the UK. Lancet. 2001;358:1426–7. doi: 10.1016/S0140-6736(01)06521-7. [DOI] [PubMed] [Google Scholar]
- 21.Van Niekerk CH, Weinberg WG, Shore SC, Heese HD, Van Shalkwyk DJ. Prevalence of asthma: a comparative study of urban and rural Xhosa children. Clin Allergy. 1979;9:319–24. doi: 10.1111/j.1365-2222.1979.tb02489.x. [DOI] [PubMed] [Google Scholar]
- 22.Turner KJ. Is the prevalence of allergy related to parasitic disease. In: Oehling A, editor. Advances in Allergology and Immunology. Oxford: Pergamon Press; 1980. pp. 279–87. [Google Scholar]
- 23.Brabin BJ, Kelly Y. Prevalence of childhood asthma in the tropics. Ann Trop Med Parasitol. 1998;18:S33–S69. doi: 10.1080/02724936.1998.11747978. [DOI] [PubMed] [Google Scholar]
- 24.Penny ME, Sterne JAC, Murad S, et al. Respiratory symptoms, asthma, exercise test spirometry, and atopy in schoolchildren from a Lima shanty town. Respiratory symptoms, asthma, exercise test spirometry, and atopy in schoolchildren from a Lima shanty town. Thorax. 2001;56:607–12. doi: 10.1136/thorax.56.8.607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sporik K, Holgate ST, Platts-Mills TAE, Cogswell JJ. Exposure to housedust mite allergen (Der p 1) and the development of asthma in childhood. N Engl J Med. 1990;323:502–7. doi: 10.1056/NEJM199008233230802. [DOI] [PubMed] [Google Scholar]
- 26.Platts-Mills TAE, Wheatley LM, Aalberse RC. Indoor versus outdoor allergens in allergic respiratory disease. Curr Opin Immunol. 1998;10:634–9. doi: 10.1016/s0952-7915(98)80081-2. [DOI] [PubMed] [Google Scholar]
- 27.Strachan DP. The role of environmental factors in asthma. Br Med Bull. 2000;56:865–82. doi: 10.1258/0007142001903562. [DOI] [PubMed] [Google Scholar]
- 28.Von Ehrenstein OS, Von Mutius E, Illi S, Baumann L, Von Kries R. Reduced risk of hay fever and asthma among children of farmers. Clin Exp Allergy. 2000;30:187–93. doi: 10.1046/j.1365-2222.2000.00801.x. 10.1046/j.1365-2222.2000.00801.x. [DOI] [PubMed] [Google Scholar]
- 29.Newman-Taylor A. Environmental determinants of asthma. Lancet. 1995;345:296–9. doi: 10.1016/s0140-6736(95)90280-5. [DOI] [PubMed] [Google Scholar]
- 30.Strachan DP. Hay fever, hygiene, and household size. BMJ. 1989;299:1259–60. doi: 10.1136/bmj.299.6710.1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shaheen SO, Aaby P, Hall AJ, Barker DJ, Heyes CB, Shiell AW, Goudiaby A. Measles and atopy in Guinea-Bissau. Lancet. 1996;347:1792–6. doi: 10.1016/s0140-6736(96)91617-7. [DOI] [PubMed] [Google Scholar]
- 32.Matricardi PM, Rosmini F, Riondino S, et al. Exposure to foodborne and orofecal microbes versus airborne viruses in relation to atopy and allergic asthma: epidemiological study. BMJ. 2000;320:412–7. doi: 10.1136/bmj.320.7232.412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gereda JE, Leung DYM, Thatayatikom A, et al. Relation between house-dust endotoxin exposure, type 1 T-cell development, and allergen sensitisation in infants at high risk of asthma. Lancet. 2000;355:1680–3. doi: 10.1016/s0140-6736(00)02239-x. [DOI] [PubMed] [Google Scholar]
- 34.Scrivener S, Yemanerbhan H, Zebenigus M, et al. Independent effects of intestinal parasite infection and domestic allergen exposure on risk of wheeze in Ethiopia: a nested case-control study. Lancet. 2001;358:1493–9. doi: 10.1016/S0140-6736(01)06579-5. [DOI] [PubMed] [Google Scholar]
- 35.Cooper PJ. Immunity in humans – Ascaris. In: Kennedy MW, Holland CV, editors. Ascaris – World Class Parasites. Amsterdam: Kluwer Academic Press; 2002. in press. [Google Scholar]
- 36.Kok A, Robinson MJ. IgE, parasites, and allergy. Lancet. 1976;2:633. doi: 10.1016/s0140-6736(76)90703-0. [DOI] [PubMed] [Google Scholar]
- 37.Godfrey RC. Asthma and IgE levels in the Gambia. Clin Allergy. 1975;5:201–7. doi: 10.1111/j.1365-2222.1975.tb01853.x. [DOI] [PubMed] [Google Scholar]
- 38.Knight R, Merrett TG. Hookworm infection in rural Gambia. seasonal changes, morbidity and total IgE levels. Ann Trop Med Parasitol. 1981;75:299–314. doi: 10.1080/00034983.1981.11687444. [DOI] [PubMed] [Google Scholar]
- 39.Warrell DA, Fawcett IW, Harrison BD, Agamah AJ, Ibu JO, Pope HM, Maberly DJ. Bronchial asthma in the Nigerian Savannah Region. A clinical and laboratory study of 106 patients with a review of the literature on asthma in the Tropics. Q J Med. 1975;174:325–47. [PubMed] [Google Scholar]
- 40.Merrett T, Merrett J, Cookson J. Allergy and parasites: the measurement of total and specific IgE levels in urban and rural communities in Rhodesia. Clin Allergy. 1976;6:131–4. doi: 10.1111/j.1365-2222.1976.tb01890.x. [DOI] [PubMed] [Google Scholar]
- 41.Carswell F, Meakins RH, Harland PS. Parasites and asthma in Tanzanian children. Lancet. 1976;II:706–7. doi: 10.1016/s0140-6736(76)90004-0. [DOI] [PubMed] [Google Scholar]
- 42.Wolstenholme RJ. Bronchial asthma in the Southern Maldives. Clin Allergy. 1979;9:325–32. doi: 10.1111/j.1365-2222.1979.tb02490.x. [DOI] [PubMed] [Google Scholar]
- 43.Macfarlane JT, Bachelor M, Ridyard JB, et al. Asthma, IgE, and environment in northern Nigeria. Clin Allergy. 1979;9:333–7. doi: 10.1111/j.1365-2222.1979.tb02491.x. [DOI] [PubMed] [Google Scholar]
- 44.Joubert JR, de Klerk HC, Malan C. Ascaris lumbricoides and allergic asthma. South Afr Med J. 1979;56:599–602. [PubMed] [Google Scholar]
- 45.Lynch NR, Di Medouze LPF, Verde O, Lopez RI, Malave C. Incidence of atopic disease in a tropical environment: partial independence from intestinal helminthiasis. J Allergy Clin Immunol. 1984;73:229–33. doi: 10.1016/s0091-6749(84)80012-3. [DOI] [PubMed] [Google Scholar]
- 46.Lynch NR, Lopez RI, Di Prisco-Fuenmayor MC, Hagel I, Medouze L, Viana G, Ortega C, Prato G. Allergic reactivity and socio-economic level in a tropical environment. Clin Allergy. 1987;17:199–207. doi: 10.1111/j.1365-2222.1987.tb02004.x. [DOI] [PubMed] [Google Scholar]
- 47.Lynch NR. Influence of socio-economic level on helminthic infection and allergic reactivity in tropical countries. In: Moqbel R, editor. Allergy and Immunity to Helminths. London: Taylor & Francis; 1992. pp. 51–62. [Google Scholar]
- 48.Hagel I, Lynch NR, DiPrisco MC, Lopez RI, Garcia NM. Allergic reactivity of children of different socioeconomic levels in Tropical populations. Int Arch Allergy Immunol. 1993;101:209–14. doi: 10.1159/000236521. [DOI] [PubMed] [Google Scholar]
- 49.Araujo MI, Lopes AA, Medeiros M, Cruz AA, Sousa-Atta L, Sole D, Carvalho EM. Inverse association between skin response to aeroallergens and Schistosoma mansoni infection. Int Arch Allergy Immunol. 2000;123:145–8. doi: 10.1159/000024433. [DOI] [PubMed] [Google Scholar]
- 50.Masters M, Barrett-Connor E. Parasites and asthma- predictive or protective. Epidemiol Rev. 1985. p. 7. [DOI] [PubMed]
- 51.Khedr MS, Al-Shishtawi MM, El Kafass EA. Bronchial asthma among school children with gastrointestinal parasitic infection. East Med Reg Health Serv J. 1989;7:29–35. [Google Scholar]
- 52.Alshishtawy MM, Abdella AM, Gelber LE, Chapman MD. Asthma in Tanta, Egypt. serologic analysis of total and specific IgE antibody levels and their relationship to parasite infection. Int Arch Allergy Appl Immunol. 1991;96:348–54. doi: 10.1159/000235520. [DOI] [PubMed] [Google Scholar]
- 53.Lynch NR, Palenque M, Hagel I, DiPrisco MC. Clinical improvement of asthma after anthelmintic treatment in a tropical situation. Am J Respir Crit Care Med. 1997;156:50. doi: 10.1164/ajrccm.156.1.9606081. [DOI] [PubMed] [Google Scholar]
- 54.Dowse GK, Turner KJ, Stewart GA, Alpers MP, Woolcock AJ. The association between Dermatophagoides mites and the increasing prevalence of asthma in village communities within the Papua New Guinea highlands. J Allergy Clin Immunol. 1985;75:75–83. doi: 10.1016/0091-6749(85)90016-8. [DOI] [PubMed] [Google Scholar]
- 55.Dold S, Heinrich J, Wichmann HE, Wjst M. Ascaris-specific IgE and allergic sensitisation in a cohort of school children in the former East Germany. J Allergy Clin Immunol. 1998;102:414–20. doi: 10.1016/s0091-6749(98)70129-0. [DOI] [PubMed] [Google Scholar]
- 56.Cooper PJ, Espinel I, Paredes W, Guderian RH, Nutman TB. Impaired tetanus-specific cellular and humoral responses following tetanus vaccination in human onchocerciasis: a probable role for IL-10. J Infect Dis. 1998;178:1133–8. doi: 10.1086/515661. [DOI] [PubMed] [Google Scholar]
- 57.Cooper PJ, Chico M, Sandoval C, Espinel I, Guevara A, Levine MM, Griffin GE, Nutman TB. Human infection with Ascaris lumbricoides is associated with suppression of the interleukin-2 response to recombinant cholera toxin B subunit following vaccination with the live oral cholera vaccine CVD 103-HgR. Infect Immun. 2001;69:1574–80. doi: 10.1128/IAI.69.3.1574-1580.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Jarrett EEE, Miller HRP. Production and activities of IgE in helminth infection. Prog Allergy. 1982;31:178–233. [PubMed] [Google Scholar]
- 59.Vogel H, Mining W. Contributions to clinical knowledge of lung ascariasis and to the question of transient eosinophilic lung infiltrates. Beitrage zur Klinik Tuberkulose. 1942;98:620–54. [Google Scholar]
- 60.Maxwell C, Hussain R, Nutman TB, Poindexter RW, Little MD, Schad GA, Ottesen EA. The clinical and immunologic responses of normal human volunteers to low dose hookworm (Necator americanus) infection. Am J Trop Med Hyg. 1987;37:126–34. doi: 10.4269/ajtmh.1987.37.126. [DOI] [PubMed] [Google Scholar]
- 61.Barlow JB, Pocock WA, Tabatznick BA. An epidemic of ‘acute eosinophilic pneumonia’ following ‘beer drinking’ and probably due to infestation with Ascaris lumbricoides. S Afr Med J. 1961;35:390. [PubMed] [Google Scholar]
- 62.Phills JA, Harold AJ, Whiteman GV, Perlmutter L. Pulmonary infiltrates, asthma, and eosinophilia due to Ascaris suum infestation in man. N Engl J Med. 1972;286:965–70. doi: 10.1056/NEJM197205042861802. [DOI] [PubMed] [Google Scholar]
- 63.Butterworth AE, Corbett EL, Dunne DW, et al. Frontiers of Infectious Diseases: New Strategies in Parasitology. Edinburgh: Churchill Livingstone; 1989. Immunity and morbidity in human schistosomiasis; pp. 193–210. [Google Scholar]
- 64.Luty AJ, Downham MD, Whitworth JA, Morgan D, McNicholas A, Taylor DW. Immunological studies on onchocerciasis in Sierra Leone. 1. Pretreatment baseline data. Trop Med Parasitol. 1990;41:371–5. [PubMed] [Google Scholar]
- 65.Partono F, Pribadi PW, Soewarta A. Epidemiological and clinical features of Brugia timori in a newly established village, Karakuak, West Flores. Indonesia Am J Trop Med Hyg. 1978;27:910–5. doi: 10.4269/ajtmh.1978.27.910. [DOI] [PubMed] [Google Scholar]
- 66.Cooper PJ, Mancero T, Espinel M, Sandoval C, Lovato R, Guderian RH, Nutman TB. Early human infection with Onchocerca volvulus is associated with an enhanced parasite-specific cellular immune response. J Infect Dis. 2001;183:1662–8. doi: 10.1086/320709. [DOI] [PubMed] [Google Scholar]
- 67.Stelma FF, Talla I, Polman K, Niang M, Sturrock RF, Deelder AM, Gryseels B. Epidemiology of Schistosoma mansoni infection in a recently exposed community in northern Senegal. Am J Trop Med Hyg. 1993;49:701–6. doi: 10.4269/ajtmh.1993.49.701. [DOI] [PubMed] [Google Scholar]
- 68.Loeffler P. Transient lung infiltrations with blood eosinophilia. Arch Int Allergy. 1956;8:54–9. [PubMed] [Google Scholar]
- 69.Gelpi AP, Mustafa A. Seasonal pneumonitis with eosinophilia. Am J Trop Med Hyg. 1967;16:646–57. [PubMed] [Google Scholar]
- 70.Soboslay PT, Geiger SM, Weiss N, et al. The diverse expression of immunity in humans at distinct states of Onchocerca volvulus infection. Immunol. 1997;90:592–9. doi: 10.1046/j.1365-2567.1997.00210.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ribeiro de Jesus A, Silva A, Santana LB, et al. Clinical and immunological evaluation of 31 patients with acute Schistosomiasis mansoni. J Infect Dis. 2002;185:98–105. doi: 10.1086/324668. [DOI] [PubMed] [Google Scholar]
- 72.McCarthy JS, Ottesen EA, Nutman TB. Onchocerciasis in endemic and nonendemic populations: differences in clinical presentation and immunologic findings. J Infect Dis. 1994;170:736–41. doi: 10.1093/infdis/170.3.736. [DOI] [PubMed] [Google Scholar]
- 73.Butterworth AE. Cell-mediated damage to helminths. Adv Parasitol. 1984;23:143–235. doi: 10.1016/s0065-308x(08)60287-0. [DOI] [PubMed] [Google Scholar]
- 74.King CL, Medhat A, Malhotra I, et al. Cytokine control of parasite-specific anergy in human urinary schistosomiasis. IL-10 modulates lymphocyte reactivity. J Immunol. 1996;156:4715–21. [PubMed] [Google Scholar]
- 75.Soboslay PT, Luder CGK, Riesch S, Geiger SM, Banla M, Batchassi E, Stadler A. Regulatory effects of Th1-type (IFN-γ, L-12) and Th2-type (IL-10, IL-13) cytokines on parasite-specific cellular responsiveness in Onchocerca volvulus-infected humans and exposed endemic controls. Immunol. 1999;97:219–25. doi: 10.1046/j.1365-2567.1999.00018.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Doetze A, Satoguina J, Burchard G, Rau T, Fleischer B, Hoerauf A. Antigen-specific cellular hyporesponsiveness in a chronic human helminth infection is mediated by Th3/Tr1-type cytokines IL-10 and TGF-β but not by a Th1 to Th2 shift. Int Immunol. 2000;12:623–30. doi: 10.1093/intimm/12.5.623. [DOI] [PubMed] [Google Scholar]
- 77.Hussain R, Poindexter RW, Ottesen EA. Control of allergic reactivity in human filariasis: predominant localization of blocking antibodies to the IgG4 subclass. J Immunol. 1992;148:2731–7. [PubMed] [Google Scholar]
- 78.Mahanty S, Day KP, Alpers MP, Kazura JW. Antifilarial IgG4 antibodies in children from Filaria-endemic areas correlate with duration of infection and are dissociated from antifilarial IgE antibodies. J Infect Dis. 1994;170:1339–43. doi: 10.1093/infdis/170.5.1339. [DOI] [PubMed] [Google Scholar]
- 79.Odunjo EO. Helminthic anaphylactic syndrome (HAS) in children. Pathol Microbiol. 1970;35:220. doi: 10.1159/000162233. [DOI] [PubMed] [Google Scholar]
- 80.Spillman RK. Pulmonary ascariasis in tropical communities. Am J Trop Med Hyg. 1975;24:791–800. doi: 10.4269/ajtmh.1975.24.791. [DOI] [PubMed] [Google Scholar]
- 81.Arean VM, Crandall CA. Ascariasis. In: Marcial-Rojas RA, editor. Pathology of Protozoal and Helminthic Diseases. New York: Williams & Wilkins; 1971. pp. 769–807. [Google Scholar]
- 82.Kennedy MW, Qureshi F. Stage-specific secreted antigens of the parasitic larval stages of the nematode Ascaris. Immunol. 1986;58:515–22. [PMC free article] [PubMed] [Google Scholar]
- 83.Cooper PJ, Chico ME, Sandoval C, et al. Human infection with Ascaris lumbricoides is associated with a polarized cytokine response. J Infect Dis. 2000;182:1207–13. doi: 10.1086/315830. [DOI] [PubMed] [Google Scholar]
- 84.Holland MJ, Harcus YM, Riches PL, Maizels RM. Proteins secreted by the parasitic nematode Nippostrongylus brasiliensis act as adjuvants for Th2 responses. Eur J Immunol. 2000;30:1977–87. doi: 10.1002/1521-4141(200007)30:7<1977::AID-IMMU1977>3.0.CO;2-3. 10.1002/1521-4141(200007)30:7<1977::aid-immu1977>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 85.Fraser EM, Christie JF, Kennedy MW. Heterogeneity amongst infected children in IgE antibody repertoire to the antigens of parasitic nematode Ascaris. Int Arch Allergy Immunol. 1993;100:283–6. doi: 10.1159/000236425. [DOI] [PubMed] [Google Scholar]
- 86.Johansson SG, Melbin T, Vahlquist B. Immunoglobulin levels in Ethiopian preschool children with special reference to high concentrations of immunoglobulin E (IgND) Lancet. 1968;I:1118–21. doi: 10.1016/s0140-6736(68)90187-6. [DOI] [PubMed] [Google Scholar]
- 87.Bazaral M, Orgel HA, Hamburder RN. The influence of serum IgE levels of selected recipients, including patients with allergy, helminthiasis and tuberculosis, on the apparent P-K titer of a reaginic serum. Clin Exp Immunol. 1973;14:117–25. [PMC free article] [PubMed] [Google Scholar]
- 88.Godfrey RD, Gradidge CF. Allergic sensitisation of human lung fragments prevented by saturation of IgE binding sites. Nature. 1976;259:484–6. doi: 10.1038/259484a0. [DOI] [PubMed] [Google Scholar]
- 89.Ottesen EA, Kumaraswami V, Paranjape R, Poindexter RW, Tripathy SP. Naturally occurring blocking antibodies modulate immediate hypersensitivity responses in human filariasis. J Immunol. 1981;127:2014–20. [PubMed] [Google Scholar]
- 90.Ottesen E. Parasite infections and allergic reaction – How each affects the other. In: Weiss EB, Stein M, editors. Bronchial Asthma: Mechanisms and Therapeutics. 2. New York: Little, Brown and Co; 1985. pp. 522–7. [Google Scholar]
- 91.Steel C, Guinea A, McCarthy JS, Ottesen EA. Long-term effect of prenatal exposure to maternal microfilaremia on immune responsiveness to filarial parasite antigens. Lancet. 1994;343:890–3. doi: 10.1016/s0140-6736(94)90009-4. [DOI] [PubMed] [Google Scholar]
- 92.Elson LH, Days A, Calvopina M, Paredes W, Araujo E, Guderian RH, Bradley JE, Nutman TB. In utero exposure to Onchocerca volvulus. relationship to subsequent infection intensity and cellular immune responsiveness. Infect Immun. 1996;64:5061–5. doi: 10.1128/iai.64.12.5061-5065.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Johansson E, Aponno M, Lundberg M, van Hage-Hamsten M. Allergenic cross-reactivity between the nematode Anisakis simplex and the dust mites Acarus siro, Lepidoglyphus destructor, Tyrophagus putrescentiae, and Dermatophagoides pteronyssinus. Allergy. 2001;56:660–6. doi: 10.1034/j.1398-9995.2001.00798.x. 10.1034/j.1398-9995.2001.00798.x. [DOI] [PubMed] [Google Scholar]
- 94.Pascual CY, Crespo JF, San Martin S, Ornia N, Ortega N, Caballero T, Munoz-Pereira M, Martin-Esteban M. Cross-reactivity between IgE-binding proteins from Anisakis, German cockroach, and chironomids. Allergy. 1997;52:514–20. doi: 10.1111/j.1398-9995.1997.tb02594.x. [DOI] [PubMed] [Google Scholar]


