Abstract
The potential role of dendritic cells (DC) in the immunopathology of human immunodeficiency virus 1 (HIV-1) disease remains controversial. This study examines replication of a panel of HIV-1 strains (both laboratory adapted and primary) within DC, in the context of the well-established monocyte–DC and monocyte–macrophage transition. Viral replication was assessed by p24 ELISA assay. All strains of HIV-1 tested replicated in DC. Only CCR5-tropic virus replicated in macrophages. Lipopolysaccharide (LPS) induced DC maturation (as reflected in altered cell phenotype) and at the same time diminished the ability of DC to support HIV-1 replication. In contrast the presence of activated T cells, which had been fixed to prevent them acting as a site for viral replication, enhanced the ability of the DC to support viral replication, as has been reported previously for macrophages. Thus cells that are DC by phenotype, but are not activated, act as the optimum reservoir for HIV-1 replication. If this form of DC is present in peripheral tissues, this will be permissive for amplification of the in vivo viral load at sites where there are few responder cells available, and hence contribute to the persistent immunopathology.
Keywords: activated T cell, dendritic cell, HIV-1, macrophage replication
INTRODUCTION
Dendritic cells (DC) have been implicated in human immunodeficiency virus (HIV-1) infection since very early after the virus was first identified, and this has been incorporated into many of the immunological analyses of the disease [1]. However, the precise nature of this role remains elusive and controversial. The replication of HIV-1 during the course of natural infection has been difficult to monitor, although some studies have reported that virus can be detected within epidermal Langerhans cells [2,3]. Within the lymph node viral capsids are clearly trapped on the follicular DC [4,5], but the interdigitating DC has been difficult to monitor in situ. There are more data on the ability of HIV-1 to replicate within DC infected in vitro. For example, there have been several studies which report that DC infected in vitro are fully competent to support HIV-1 replication [6,7] at least for strains able to use the CCR5 receptor. For these strains the addition of lipopolysaccharide (LPS) (which is used widely as a model for DC maturation) inhibits replication. In parallel with this, passive uptake of these forms of virus into DC may also occur through binding to DC-SIGN, a C-type lectin that is found on the DC surface [8].
In this study we have extended the analysis of the DC/HIV-1 interaction further, using a panel of primary isolates as well as the laboratory-adapted strains used in the previous studies. In addition we have re-examined the role of T cells in promoting replication of HIV-1 within DC. Most previous studies [9] have assumed that within mixed DC–T cell cultures, the T cells amplify HIV-1 replication by serving themselves (i.e. the T cells) as a more susceptible host cell type. However, recent studies from one of our laboratories [10] showed that T cells may promote viral replication within other cells, such as macrophages, by signals mediated via direct T cell–macrophage interaction. These studies were performed specifically under conditions where HIV-1 replication in T cells was excluded by fixing the T cells prior to adding them to the macrophages. We have now extended this work to DC, and shown that in these cells the presence of activated T cells also increases replication. Thus DC ‘activation’ by LPS and T cells result in opposite effects in terms of HIV-1 replication. Our results add strong support to the notion that DC are indeed a source of virus, and to the notion that DC–T cell interaction plays an important role in the immunopathogenesis of HIV-1 associated disease.
MATERIALS AND METHODS
Cell culture conditions
All primary cells were grown in complete medium (CM): RPMI 1640 supplemented with 5% fetal calf serum (FCS), 2 μM l-glutamine, 50 μm 2-mercaptoethanol, 100 U/ml penicillin and 100 μg/ml streptomycin (all from Gibco) unless otherwise stated. Tissue culture plasticware was supplied by Falcon unless otherwise stated.
Dendritic cell preparation
DC were prepared following a protocol that had been tested previously in our laboratory [11]. PB mononuclear cells were obtained from healthy volunteers (120 ml into a heparinized syringe), or in the form of buffy coats (equivalent to ~500 ml PB, from the National Blood Transfusion Service). This was diluted 1:2 in phosphate buffered saline (PBS), layered over Lymphoprep 1077 density medium (Nycomed) and centrifuged for 30 min at 600 g with the brake off. The interface was recovered, washed in PBS at least three times to remove platelets and resuspended (3–8 × 106 cells/ml) in CM. After 2-h adherence to plastic (37°C, 5% CO2) the non-adherent cells were removed by gentle washing with warm PBS. The residual adherent monocytes were cultured in fresh CM supplemented with 100 ng/ml human recombinant granulocyte–macrophage colony stimulating factor (GM-CSF) and 50 ng/ml human recombinant interleukin 4 (IL-4) (both from Schering-Plough). At different time points up to 7 days (as indicated in the text) the non-adherent cells were harvested and layered on Lymphoprep, then washed as before, and DC further purified from these cells by negative selection. Briefly, cells were resuspended in CM containing 2 μg/ml CD2 MoAb, 2 μg/ml CD3 MoAb, CD19 MoAb (supernatant diluted 1:10), and incubated (4°C, 30 min) to deplete T and B cells. The cells were washed three times with PBS at 4°C and resuspended in 1·5 ml CM. Washed M-450 sheep antimouse-coated immunomagnetic beads (Dynal) were added at 10 μl/106 cells and incubated on a rotator at 4°C for 45 min. Bound cells were removed on a magnet for 10 min and the supernatant containing the DC removed. This strategy was repeated to ensure complete purification. To mature the DC further, 10 ng/ml LPS was added to the DC cultures on day 6, and the cells incubated for a further 48 h. As a result, the 7-day culture of DC in the absence of LPS was extended to 24 h for this part of the study so that the LPS+ and LPS- DC preparations could be harvested together. Cell viability was confirmed by preparing DC, plating them in 96-well flat-bottom plates (100 μl/well of 5 × 105DC/ml volume), and adding 20 ml of filtered 3-(4,5-dimethylthiaazol-2-yl)-2,5-diphenylterazolium bromide (MTT) in PBS to triplicate samples for 4h. The reduction of MTT was stopped by the addition of 100 μl of 10% sodium dodecyl sulphate (SDS) in 0·01 m HCl. Absorbance was measured after a further 18 h incubation in the dark.
For phenotypic analysis DC (104–105) were harvested and resuspended in 50 μl PBS with 10% normal rabbit serum (NRS) (Gibco)/0·1% NaN3 for 15 min at 4°C in 96-well round-bottom plates. The cells were washed, resuspended in 50 μl of primary MoAb in PBS/10% NRS and incubated for a further 30 min on ice. In addition to the MoAbs outlined above, other MoAbs used as primary reagents included CD1a (NA1/34, IgG2a, a gift from Prof. A. McMichael, John Radcliffe Hospital, Oxford, UK), CD4 (Q425, IgG1 and Q4120, IgG1, both gifts from Prof. P.C.L. Beverley, Edward Jenner Institute, Compton, UK), CD14 (UCHM1, IgG2a, a gift from Prof. P.C.L. Beverley), CD40 (MAB89, IgG1, Immunotec, Luton, Bedsfordshire, UK), CD86 (BU63, IgG1, a gift from D. Hardie, Birmingham University, Birmingham, UK) and HLA-DQ (Ia3, IgG1, a gift from Prof. R. Winchester, New York University School of Medicine, NY, USA), HLA-DR (L243, IgG2a, a gift from Prof. P.C.L. Beverley), CD195 (CCR5) (LS100–2D7, IgG1 and 3A9, IgG1, from VIIth HLDA workshop) and CD184 (CXCR4) (12G5, IgG2a, from VIIth HLDA workshop) After primary MoAb binding they were washed twice with PBS/10% NRS to remove any unbound antibody, and incubated for a further 30 min with the secondary antibody, a fluorescein isothiocyanate (FITC) conjugated rabbit antimouse IgG (Dako, Glostrup, Denmark) diluted 1:20 in PBS/10% NRS. Finally, cells were washed twice in PBS, resuspended in 50 μl PBS/0·1% NaN3 and fixed by adding 100 μl PBS/3·7% formaldehyde. Samples were stored at 4°C in the dark and analysed within 5 days of staining. Data were acquired on a Becton-Dickinson FACScan and analysed using WinMDI software.
Macrophage preparation
Monocytes were obtained from adherent PBMC as before and the adherent cells were incubated for 7 days in CM without FCS but with 2% (v/v) AB+ human serum. The resultant adherent macrophages were purified after 7 days by extensive washing with warm media until only firmly adherent cells remained. These cells were de-adhered using PBS (4°C) containing 3 mm ethylene diamine tetra-acetic acid (EDTA), counted and replated immediately at the required number in appropriate plates.
Virus culture and infection
The following strains of HIV-1 were used in this study: BaL NSI CCR5/CCR3 restricted (R5/R3)], RF SI, CXCR-4 restricted (X4)] (AIDS reagent project, NIBSC, Potters Bar, UK) and primary isolates SL-2 NSI, predominantly CCR-5 restricted (R5)], 2044 SI, CXCR-4 restricted (X4)] or 2076 SI, dual tropic, able to use CCR-5, CCR-3 and CXCR-4 (R5/R3/X4)] (generously donated by Paul Clapham, UCL, UK). All viral stocks were grown in phytohaemagglutinin (PHA) and IL-2 stimulated PBMC cultures and TCID50 values predetermined by assessment of p24 production in stimulated PBMC cultures. Cell-free viral preparations were passed through 0·2 μm filters and treated with DNase I prior to use (Sigma, 100 units/ml, 5 mm MgCl2, 30 min at 37°C). The quantity of HIV-1 p24 in supernatants from infected cells was measured using a commercially available p24 ELISA kit (NEK-060 A, NEN) following the manufacturer’s protocol.
HIV-1 studies
Work involving HIV-1 virus was performed in the Category III facility at St George’s Hospital Medical School, following established safety protocols. All cultures were performed in CM (37°C in humidified, 5% CO2 atmosphere). To infect primary cells, they were seeded at 2 × 105 per well in 96-well plates and HIV-1 virus was added to each well (TCID50 of added virus was 1 × 103 per well for all isolates), and the volume made up to 200 μl. After 2 h incubation, the cells were washed three times in PBS. CM (0·2 ml) supplemented with the appropriate cytokines was added to each well and the plates reincubated and supernatants taken at the appropriate time points, stored at –20°C and assayed in batches. Viral replication was confirmed by measuring p24 levels at 24h and 7 days.
HUT78 cells
HUT78 cells were cultured in CM alone or in the presence of activation agents PHA (1 μg/ml) and phorbol myristate acetate (PMA) (5 ng/ml)] for 24 h. Cells were washed three times in PBS, fixed (2h, 4°C, 1% (w/v) paraformaldehyde) washed again three times in PBS and stored at 4°C until used, according to the protocol published previously [10].
RESULTS
The experimental approach used DC that had been prepared with or without LPS treatment. The phenotype of these two populations is illustrated in a representative assay (n = 7) as shown in Fig. 1. The DC were confirmed as HLA-DR+, HLA-DQ+, CD3−, CD4+, CD14 low, CD86+; and also CD1a low, CD18+, CD19−, CD13+, CD87+, CD98+, CD147+ (data not shown). This phenotypic pattern, incorporating the progressive changes in profile (e.g. the starting monocyte CD1a-, CD14 high to dendritic cell CD1a low, CD14 low) has been reported previously [11]. The LPS stimulated DC showed increased levels of HLA-DR, HLA-DQ and CD86, and a further decrease in expression of CD14. Both stimulated and unstimulated DC showed low levels of CXCR4+ (confirmed during the VIIth Human Leukocyte Differentiation Antigen Workshop, data not shown). Unstimulated DC expressed higher levels of CCR5, which was down-regulated following stimulation with LPS. The DC CXCR4 and CCR5 levels were not affected by the presence of T cells during the culture period (data not shown).
Fig. 1.
Phenotypic analysis of immature and mature DC. DC prepared, purified and either untreated/treated with LPS were examined by flow cytometry as outlined in Materials and methods. The negative (no primary antibody) control is shown in all histograms, and is similar to that for the isotype controls (data not shown). Immature DC are shown as shaded areas and mature DC are shown as a single black line. Results are representative of seven experiments.
The replication of a panel of HIV-1 strains, all added to DC at equivalent titres, in both stimulated and unstimulated DC, is shown in Fig. 2. In every experiment (n = 5), as shown previously Ba-L, a laboratory-adapted strain that is CCR-5 tropic, infected DC efficiently, as judged by synthesis of p24 released into the supernatant. This replication was down-regulated significantly by treatment of DC with LPS. RF, a laboratory-adapted CXCR4 tropic strain, infected DC less effectively, although the viral replication was significantly greater than that seen in unstimulated DC. Unexpectedly, however, SL2, a primary isolate showing CCR5 tropism was very inefficient at infecting either stimulated or unstimulated DC, while in contrast V2044, a primary isolate showing CXCR4 tropism, replicated more efficiently than either RF or SL2. Finally V2076, a primary dual tropic isolate, was the most efficient of the primary isolates tested, comparable to Ba-L. Infection of DC by Ba-L and V2076, but not RF or V2044, was inhibited by MIP-1α (50 ng/ml) (data not shown), confirming the predicted receptor usage by these strains. Examination of the DC cultures by light microscopy showed that in every experiment the DC remained non-adherent throughout the time course (7 days postinfection) and there was no evidence of syncytium formation or of other morphological change. There was also no change in DC viability as measured by MTT reduction (data not shown).
Fig. 2.
HIV-1 infection of DC. Both immature and mature DC from healthy volunteers were exposed to a range of laboratory adapted and primary HIV-1 isolate. Viral replication was monitored by p24 levels in the supernatant from duplicate wells at 7 days after infection. A representative experiment is shown. Both mature and immature DC produced p24, and there was significantly less p24 in the mature than the immature DC in every instance. ▪, Immature DC; □ mature DC.
In order to compare HIV-1 replication in DC with that in MO directly, DC (unstimulated) and MO were prepared from the same donor and infected with four viral strains (Fig. 3). Infection of MO with strains able to use the CCR5 co-receptor (Ba-L or V2076) led to more efficient replication in MO than in DC. However, CXCR4-tropic strains were completely unable to replicate in MO, but showed a low – but none the less significant – level of replication in DC. As has been reported previously [12], no viral replication was observed with any strain if adherent MO were obtained from cultures grown with GM-CSF alone (i.e. without IL-4) (data not shown).
Fig. 3.
HIV-1 infection of DC and MO. Macrophages and immature DC from three healthy volunteers were exposed to a range of laboratory adapted and primary HIV-1 isolates and replication monitored by p24 levels in the supernatant at 7 days after infection. p24 levels were below the level of sensitivity of the assay in some samples (*). The same pattern of replication was seen in all three subjects. Only M-tropic laboratory-adapted virus (BaL) and not T-tropic (RF) appeared to replicate in macrophages. Although the maximum p24 levels were seen with BaL infection of macrophages, p24 was detected in all the supernatants from the immature DC. ▪, Immature DC; □ macrophage.
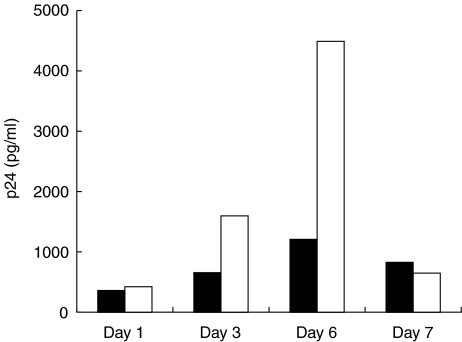
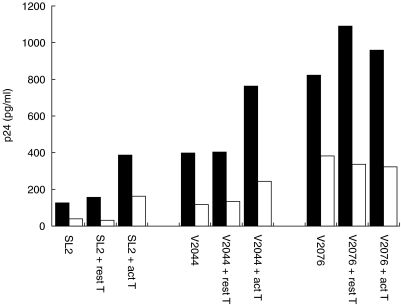
The stimulation of DC by LPS is probably mediated via an interaction with a Toll receptor complex, similar to that seen on monocytes and macrophages, but with lower levels of CD14. In contrast, T cells signal into DC via a variety of other cell surface interactions (such as CD40–CD154 and β2 integrins). The influence of resting and phorbol-ester activated HUT78 cells on HIV-1 replication is shown in Figs 4 and 5. In order to exclude any HIV-1 replication in the T cells, these were fixed prior to addition to the DC cultures. Figure 4 shows the effect of adding the T cells immediately postinfection but at various stages of the differentiation process. The T cells enhanced replication of both Ba-L and RF strains, but this enhancement was only seen using the less mature DC (3–6 days). As reported previously, in MO Ba-L replication was enhanced by T cell co-culture. Figure 5 shows that activated but not resting HUT78 cells also enhanced replication of the primary SL2 and V2044 strains in both stimulated and unstimulated DC. Replication of the dual tropic primary strain V2076 was not changed by T cell co-culture. These data were confirmed using autologous PMA-activated T cells rather than HUT78 cells for co-culture with the DC (data not shown).
Fig. 4.
Effect of T cells on HIV-1 replication in DC. The replication kinetics of HIV-1 in DC as they evolve from monocytes, with or without added T cells, was examined using the same assay as in Figs 2 and 3. A representative experiment (n = 5) using the BaL strain shows that levels of p24 produced increased in the presence of the fixed activated T cells, and with time to day 6 after the initiation of the cultures. ▪, Immature DC; □ immature DC + activated T cells.
Fig. 5.
Effect of T cells on HIV-1 replication in DC. The replication kinetics of HIV-1 in DC alone, with resting T cells and with activated T cells, was examined using the same assay as in Figs 2 and 3. A representative experiment (n = 3) using three primary isolates (SL2, V2044 and V2076) shows that levels of p24 production increased in the presence of the fixed activated T cells. ▪, Immature DC; □ mature DC.
DISCUSSION
This study documents that DC can be infected with HIV-1, and that this can be confirmed by their ability to support viral replication in the absence of significant numbers of any other cell type. The study goes beyond most previous reports, in that a number of primary isolates were tested alongside the well-known laboratory strains, and in that the potential role of T cells in modulating HIV-1 replication in DC has been explored. Although there are quantitative differences between the various strains used i.e. M-tropic (BaL) and primary V2076 infection generate more p24 than the others], the potential for productive infection in these cells is clearly demonstrable. DC show higher levels of p24 production with T-tropic strains (RF, V2044) than macroph-ages, which agrees with our observation that DC express both CXCR4 and CCR5, and might thus be considered as having dual susceptibility.
The results of this study also confirm that LPS-induced activation of DC decreases viral replication in all strains tested. Interaction with LPS is representative of the type of interaction which may drive DC activation and migration in the periphery, i.e. outside lymphoid tissue. The ability to replicate in DC prior to LPS exposure is therefore consistent with the in vivo observation in macaques that SIV infects mucosal DCs [13]. These in vitro DC may also be a model for DC present in the dermis/submucosa, either resident or recently derived from the periphery, which may account for the differences in phenotype between these cells and typical Langerhans cells [14].
In contrast to LPS-induced activation, interaction of DC with activated T cells promotes rather than inhibits viral replication within DC. This observation implies that a simple correlation between co-receptor expression and usage is not the explanation for the viral replication pattern observed here. The disadvantage of the approach used is that the fixed T cell may not have the same dynamic interaction with the DC as an in vivo T cell. However, the approach has been used before and has proven valuable both in our own laboratory [15] and in others e.g 16,17] to clarify molecular events during antigen presenting cell–T cell interaction. For this particular study, the major advantage is that it ensures that there is no T cell contribution to the replication. Furthermore, it also confirms that the effect is mediated by cell–cell contact, rather than by release of soluble mediators from the T cells.
The results are similar to those reported previously with macrophages, although the amplification is less dramatic. These findings may help to explain why exposure to other organisms (e.g. from other concomitant sexually transmitted diseases) can be a co-factor in HIV-1 replication, despite the fact that the microbial product itself may not provide a link. It is generally assumed that genital ulceration due to sexually transmitted diseases increases opportunities for exposure to microvasculature, and hence to infection by HIV-1. This study suggests that another factor is any local T cell activation that may arise in response to organisms which cause such diseases. This activation will itself be a factor in increasing the local DC-derived viral load, and hence contribute to further CD4+ T cell infection and depletion.
Although the data presented in this paper, using fixed T cells, are not strictly representative of immune homeostasis in HIV-1 infection, none the less they do contribute a new dimension as to how the overall dynamic process of HIV-1 infection takes place. The current model of immunopathogenesis holds that DCs at mucosal surfaces are the primary site of infection, spreading virus to lymphoid tissue as a concomitant of their maturation and migration. DC, however, are less efficient than macrophages at supporting CCR5-tropic virus, and thus might not provide a large component of the total primary virus reservoir. However, if DC can be infected by both CXCR-4-tropic and CCR5-tropic primary isolates, then this would ‘compensate’ for the lower level of replication and hence make them equally if not more important than MO in overall contribution to viral persistence. It is also interesting to note that the lack of CCR-5 expression, which has been shown to be highly protective against HIV-1 infection, would reduce or abrogate the MO-derived viral reservoir but would not affect the DC. Thus ‘CCR-5 negative protection’ cannot be reconciled with any simple primary DC model; rather, DC-CXCR4 viral uptake in the absence of DC-CCR5 uptake might lead to replication that generates protective immunity.
The data are most in favour of the alternative to the simple mucosal surface DC model, which is that DC are important not only because of their sentinel role, but also because they act as a dual tropic conduit for the maintenance of the viral reservoir, despite the low level of replication in them. Uptake of virus into a DC from a T cell or macrophage has to be envisaged within the context of the key role of these DC in the activation of CD4+ T cells. If there are activated T cells present, and there is resultant ongoing increased viral replication in DC, then any naive T cell recruited to a DC harbouring virus would be infected immediately and killed, and this would make an important contribution to the rapid disappearance of HIV-1 specific CD4+ T cells following infection.
Acknowledgments
These experiments were performed in accordance with the human experimentation guidelines of UCL Medical School. There are no commercial or other associations that might pose conflict of interest. The research is supported by a UK Medical Research Council PhD studentship (THJM) and MRC project grant G9803142.
REFERENCES
- 1.Patterson S, Knight SC. Susceptibility of human peripheral blood dendritic cells to infection by human immunodeficiency virus. J Gen Virol. 1987;68:1177–81. doi: 10.1099/0022-1317-68-4-1177. [DOI] [PubMed] [Google Scholar]
- 2.Zambruno G, Mori L, Marconi A, et al. Detection of HIV-1 in epidermal Langerhans cells of HIV-infected patients using the polymerase chain reaction. J Invest Dermatol. 1991;96:979–82. doi: 10.1111/1523-1747.ep12476469. [DOI] [PubMed] [Google Scholar]
- 3.Simonitsch I, Geusau A, Chott A, Jurecka W. Cutaneous dendritic cells are main targets in acute HIV-1 infection. Mod Pathol. 2000;13:1232–7. doi: 10.1038/modpathol.3880227. [DOI] [PubMed] [Google Scholar]
- 4.Spiegel H, Herbst H, Niedobitek G, Foss HD, Stein H. Follicular dendritic cells are a major reservoir for human immunodeficiency virus type 1 in lymphoid tissues facilitating infection of CD4+ T-helper cells. Am J Pathol. 1992;140:15–22. [PMC free article] [PubMed] [Google Scholar]
- 5.Fujiwara M, Tsunoda R, Shigeta S, Yokota T, Baba M. Human follicular dendritic cells remain uninfected and capture human immunodeficiency virus type 1 through CD54–CD11a interaction. J Virol. 1999;73:3603–7. doi: 10.1128/jvi.73.5.3603-3607.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Macatonia SE, Patterson S, Knight SC. Suppression of immune responses by dendritic cells infected with HIV. Immunology. 1989;67:285–9. [PMC free article] [PubMed] [Google Scholar]
- 7.Granelli-Piperno A, Finkel V, Delgado E, Steinman RM. Virus replication begins in dendritic cells during the transmission of HIV-1 from mature dendritic cells to T cells. Curr Biol. 1999;14:21–9. doi: 10.1016/s0960-9822(99)80043-8. [DOI] [PubMed] [Google Scholar]
- 8.Geijtenbeek TB, Kwon DS, Torensma R, et al. DC-SIGN, a dendritic cell-specific HIV-1-binding protein that enhances trans-infection of T cells. Cell. 2000;100:587–97. doi: 10.1016/s0092-8674(00)80694-7. [DOI] [PubMed] [Google Scholar]
- 9.Granelli-Piperno A, Delgado E, Finkel V, Paxton W, Steinman RM. Immature dendritic cells selectively replicate macrophage tropic (M-tropic) human immunodeficiency virus type 1, while mature cells efficiently transmit both M- and T-tropic virus to T cells. J Virol. 1998;72:2733–7. doi: 10.1128/jvi.72.4.2733-2737.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shattock RJ, Burger D, Dayer JM, Griffin GE. Enhanced HIV replication in monocytic cells following engagement of adhesion molecules and contact with stimulated T cells. Res Virol. 1996;147:171–9. doi: 10.1016/0923-2516(96)80232-9. [DOI] [PubMed] [Google Scholar]
- 11.Woodhead VE, Binks MH, Chain BM, Katz DR. From sentinel to messenger: an extended phenotypic analysis of the monocyte to dendritic cell transition. Immunology. 1998;94:552–9. doi: 10.1046/j.1365-2567.1998.00547.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kedzierska K, Maerz A, Warby T, et al. Granulocyte-macrophage colony-stimulating factor inhibits HIV-1 replication in monocyte-derived macrophages. AIDS. 2000;14:1739–48. doi: 10.1097/00002030-200008180-00008. 10.1097/00002030-200008180-00008. [DOI] [PubMed] [Google Scholar]
- 13.Hu J, Gardner MB, Miller CJ. Simian immunodeficiency virus rapidly penetrates the cervicovaginal mucosa after intravaginal inoculation and infects intraepithelial dendritic cells. Virology. 2000;74:6087–95. doi: 10.1128/jvi.74.13.6087-6095.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kimber I, Cumberbatch M, Dearman RJ, Bhushan M, Griffiths CE. Cytokines and chemokines in the initiation and regulation of epidermal Langerhans cell mobilization. Br J Dermatol. 2000;142:401–12. doi: 10.1046/j.1365-2133.2000.03349.x. 10.1046/j.1365-2133.2000.03349.x. [DOI] [PubMed] [Google Scholar]
- 15.Woodhead VE, Stonehouse TJ, Binks MH, et al. Novel molecular mechanisms of dendritic cell-induced T cell activation. Int Immunol. 2000;12:1051–61. doi: 10.1093/intimm/12.7.1051. [DOI] [PubMed] [Google Scholar]
- 16.Peguet-Navarro J, Dalbiez-Gauthier C, Dezutter-Dambuyant C, Schmitt D. Dissection of human Langerhans cells’ allostimulatory function: the need for an activation step for full development of accessory function. Eur J Immunol. 1993;23:376–82. doi: 10.1002/eji.1830230212. [DOI] [PubMed] [Google Scholar]
- 17.Yamane H, Kato T, Nariuchi H. Effective stimulation for IL-12 p35 mRNA accumulation and bioactive IL-12 production of antigen-presenting cells interacted with Th cells. J Immunol. 1999;162:6433–41. [PubMed] [Google Scholar]