Abstract
Transplantation tolerance is a dynamic state that involves several homeostatic mechanisms intrinsic to the host. One of these mechanisms is activation-induced T cell death (AICD). However, it is unclear where AICD takes place during alloreactive responses. Since activated T cells can re-enter the thymus, we hypothesized that mature T cells activated by an allograft could be deleted upon re-entry into the thymus. To test this hypothesis, we used wild-type or 2C TCR transgenic mice receiving syngeneic or allogeneic heterotopic, vascularized heart grafts. First, we demonstrated that ex vivo CFSE-labelled T cells re-entered the thymus when transferred into allograft recipients but not when transferred into isograft recipients. Next, we compared the changes in cell subset numbers and incidence of apoptosis in the thymi and spleens of allograft or isograft recipients. Seven days after transplantation, at a time in which all the allografts were undergoing rejection, cells expressing donor-MHC class II molecules had migrated to the thymus and to the spleen. In the thymus of allograft recipients, overall cellularity was significantly reduced by 40% and associated with an increase in the number of double negative (CD4−CD8−) thymocytes and a decrease in double positive (CD4+CD8+) thymocytes, consistent with increased negative selection of thymocytes. Additionally, thymi of allograft recipients showed an increase in the number of recently activated, mature T cells (TCRhi, CD25+, CD44+) and a significant increase in the number of apoptotic cells, especially in the thymic medulla, that involved mature T cells as indicated by the TCRhi, CD44+, CD4 or CD8 single positive phenotype. Spleens of allograft recipients were increased in size and cellularity but did not show any of the changes in cell subsets seen in the thymi. Our data show that after allografting there is an increase in apoptotic cell death that is associated with negative selection of developing thymocytes as well as of alloreactive mature T cells that have re-entered the thymus upon activation in the periphery. This may occur upon migration of graft-derived antigen-presenting cells to the thymus.
Keywords: negative selection, T cell recirculation, thymus, transplantation
Introduction
The establishment of reliable and feasible protocols for the induction of long-term graft tolerance is a priority in the field of transplantation [1]. In the absence of tolerogenic strategies, one has to depend on immunosuppressive therapy to overcome allograft rejection by the recipient's immune system. It is becoming apparent that the induction of transplantation tolerance is a dynamic process that may involve several host-inherent, homeostatic mechanisms operational at different stages after grafting [2–4]. These may include clonal deletion [5,6], induction of T cell anergy or generation of immunoregulatory cells [7–10]. Although some or all of these mechanisms may be operational, as suggested by the reduction of rejection episodes after the first 3 months following transplantation [11], none of them in isolation seems to be sufficient to induce transplantation tolerance in non-manipulated allograft recipients.
Recent evidence indicates that activation-induced cell death (AICD) of alloreactive T cells plays a major role in the induction of transplantation tolerance [5,12–17]. The location where alloreactive T cells are deleted following transplantation is unclear. An obvious place where AICD of T cells may occur is within the graft upon re-stimulation of alloreactive T cells with allogeneic parenchymal cells. This is supported by evidence indicating that AICD of alloreactive T cells does occur within the graft as well as within peripheral lymphoid organs [17–21]. However, although apoptotic bodies can be seen within mononuclear infiltrates in rejecting grafts [22,23], it may be difficult to distinguish between apoptosis affecting mainly parenchymal cells or the infiltrating T cells [24,25]. This suggests that AICD of alloreactive T cells may occur outside the graft, probably in the lymphoid organs of the recipient [7,8].
One attractive location for the deletion of activated T cells is the thymus. Clonal deletion of autoreactive thymocytes occurs during T cell development. Furthermore, clonal deletion of alloreactive thymocytes can be induced upon intrathymic injection of alloantigen, suggesting that this strategy may be useful to induce transplantation tolerance [26]. In addition, graft antigenicity can be displayed in the thymus either through presentation of soluble peptides from peripheral tissues by thymic antigen-presenting cells (APC) [27], or through migration of graft-derived APC into the thymus [11]. Based on this evidence we hypothesized that, following allografting, AICD of alloreactive T cells could occur in the thymus in the form of negative selection not only of alloreactive developing thymocytes but also of mature T cells. Such a hypothesis is consistent with isolated reports of activated mature T cells being able to re-enter the thymus [28]. To test our hypothesis we used a mouse model of heterotopic vascularized heart transplantation into untreated recipients, and examined the changes in thymic and splenic cell populations after syngeneic and allogeneic transplantation. Our results indicate that following allografting, donor-derived APC migrate to the thymus and that this is associated with phenotypic changes in the cellularity of this organ that are compatible with increased negative selection of both alloreactive developing thymocytes and mature activated T cells that have re-entered the thymus. These changes demonstrate in vivo a tolerogenic mechanism that is spontaneously operational after transplantation, and that may explain the development of ‘partial’ tolerance to the graft. However, in the absence of any additional manipulation of the recipient, spontaneous negative selection of alloreactive T cells is generally not sufficient to prevent graft rejection.
Materials and methods
Mice
Male C57BL/6 (H-2b) and BALB/c (H-2d) mice (12 weeks old at the time of use) were obtained from Harlan Sprague–Dawley (Indianapolis, IN, USA), and selected as donors and recipients, respectively. This combination was chosen to provide a strong histocompatibility barrier due to mismatching in both major and minor MHC antigens. Breeding stock of 2C TCR transgenic mice on C57BL/6 background were kindly provided by Dr D. Y. Loh (Nippon Roche Research Center, Kamakura-shi, Japan). 2C TCR transgenic mice carry functionally rearranged TCR transgenes from a cytotoxic T cell clone 2C, which is specific for Ld MHC class I antigen [29]. Mice were housed under conventional conditions at the University Animal Care Facility and cared for according to guidelines of the Canadian Council on Animal Care. At least three mice per group were studied in each of these experiments.
Surgical model
Intra-abdominal heterotopic cardiac transplantation was performed as described previously [30]. Briefly, a median sternotomy was performed in the donor, and the superior vena cava was ligated. The ascending aorta and the pulmonary artery were transected, and all pulmonary veins were ligated en bloc. The donor ascending aorta and the pulmonary artery were anastomosed end to side to the recipient aorta and inferior vena cava, respectively.
Assessment of graft rejection
Heartbeat was monitored by daily abdominal palpation. The degree of pulsation was scored as: A, beating strongly; B, noticeable decline in the intensity of pulsation; or C, complete cessation of cardiac impulses. The graft was removed for histopathological study when cardiac impulses were no longer palpable [31].
Graft histology
At necropsy, tissue samples were harvested and fixed in 10% buffered formaldehyde for haematoxylin phloxine saffron staining. Microscopic sections were examined blindly for evaluation of rejection. Criteria for allograft rejection included the presence of vasculitis, infarction, lymphocytic infiltration and ischaemia [31]. These changes were scored as: 0 = no change; 1 = mild change; 2 = moderate change; 3 = marked change; and 4 = advanced change.
Monoclonal antibodies
The following monoclonal antibodies were used in these experiments: rat monoclonal antibodies against mouse CD4 (PE-labelled H129·19, IgG2a), CD8 (FITC-labelled 53–6·7, IgG2a), CD25 (PE-labelled PC61, IgG1), and CD44 (PE-labelled IM7, IgG2b); Armenian hamster antibodies against mouse CD69 (PE-labelled H1·2F3, IgG) and against the T cell receptor (TCR) (FITC-labelled H57-597, IgG); and mouse antibodies against I-Ab molecules (PE-labelled AF6-120·1, IgG2a) against I-Ad molecules (FITC-labelled 39-10-8, IgG3) (all of them purchased from Pharmingen, San Diego, CA, USA). The monoclonal antibody 1B2 recognizes the 2C clonotypic TCR, and was produced in-house (hybridoma kindly provided by Dr H. Eilson, MIT, Boston, USA).
Blood cortisol levels
Cortisol levels in serum were measured by a commercially available immunoassay (Advia Centaur, Bayer Diagnostics, Etobicoke, ON, Canada). Normal levels for this assay are 119–618 nmol/l.
Cell suspensions
Mice were sacrificed 7 days after receiving heart transplants. Spleen and thymus were removed and placed in 10 ml of complete DMEM medium containing 10% fetal calf serum on ice. Single cell suspensions from these organs were prepared by gentle dislodging and compression with a ridged syringe plunger. Cells were washed (1500 r.p.m., 10 min, 4°C), and the pellets resuspended in 5 ml of cold medium. Erythrocytes in spleen cell suspensions were lysed in lysis buffer (2 ml Tris-buffered ammonium chloride [90 ml of 0·16 m NH4Cl+ in 10 ml of 0·12 m Tris, pH 7·65]) for 2 min at room temperature. Viable splenocytes were subsequently washed (1500 r.p.m., 10 min, 4°C) twice and counted. Cells (1·0 × 106) were used per group for staining in PBS and the appropriate antibody.
Adoptive transfer experiments
To assess thymic re-entry of alloreactive T cells, resting spleen lymphocytes (20 × 106 cells per mouse) were labelled with CFSE (Molecular Probes, Eugene, OR, USA) (final concentration of 10 µm in 10 × 106 cells/ml for 15 min at 37°C) and injected i.v. into mice receiving syngeneic or allogeneic hearts as described above (three mice per group). Four to five days later, thymi from graft recipients were collected and single cell suspensions prepared and examined for fluorescence (FL-1) by flow cytometry.
FACS analysis
Thymocytes and splenocytes were incubated in 100 µl of FITC- or PE-labelled antibodies against different cell surface molecules for 25 min, on ice and in the dark. Cells were subsequently washed twice with PBS and finally resuspended in 500 µl of PBS. Flow cytometric analysis was carried out using CellQuest (Becton Dickinson Co., Mountain View, CA, USA). Selective gating of live lymphocytes/thymocytes on forward light scatter versus side light scatter was performed. Expression of I-Ad and I-Ab was analysed after gating for large cells on forward light scatter versus side light scatter.
In situ apoptotic cell labelling and quantification
Apoptosis was assessed in thymus and spleen frozen sections by the TUNEL technique, as described previously. Briefly, after deparaffinization and rehydration, slides were incubated in proteinase K (15 min at room temperature), washed in deionized water and endogenous peroxidase inactivated with hydrogen peroxide (3%) in methanol (5 min at room temperature). Subsequently, slides were stained according to manufacturer's specifications (ApopTag™, Intergen Co., Purchase, NY, USA). Negative control slides were processed at the same time in which TdT enzyme was excluded. The number of apoptotic cells was determined by light microscope examination at × 400 magnification. For each slide, 10 fields of non-necrotic areas were examined and the stained nuclei were counted. Number of apoptotic nuclei for each group is expressed as mean ± standard deviation of, at least, three mice per group.
Statistics
Experiments were run in triplicate (with at least three mice per group in each), and results expressed as mean ± s.d. Results were analysed using two-way anova and subsequent t-test. Level of significance was set as P <0·05.
Results
Graft survival and histopathology
As we have characterized previously [31], graft rejection in the vascularized, heterotopic heart transplantation model used in our studies occurs between 7 and 10 days after transplant in all the allograft recipients. In contrast, all the syngeneic grafts survive indefinitely (> 100 days). In the series used for the current studies, all heart allografts had histological signs of severe cellular and vascular rejection by POD 7. In contrast, normal histology was found in isografts at that time.
Thymi of allograft recipients show changes compatible with increased negative selection
As reported previously [32–34], 7 days after transplantation we found that the thymi of allograft recipients were significantly reduced in size and had an average of 40% less cellularity compared to isograft controls (28·3 × 106 ± 15·3 × 106 thymocytes/thymus versus 44·5 × 106 ± 22·5 × 106 thymocytes/ thymus, P <0·01) (Fig. 1a). This observation prompted us to examine the cell subsets in the thymi. The major stages of T cell development in the thymus are defined on the basis of expression of co-receptor molecules (CD4 and CD8) [35]. Immature thymocytes are double negative (CD4−CD8−) and they constitute about 5% of all thymocytes. Double negative cells may progress toward a stage characterized by expression of both coreceptors (double positive or CD4+CD8+ thymocytes), which constitute about 80% of all thymocytes. It is during this transition from double negative stage to double positive stage that positive and negative selection occurs. Positively selected thymocytes develop into single positive cells (either CD4+ or CD8+) and these are the cells ready to be exported to the periphery. Negatively selected cells die by apoptosis [36].
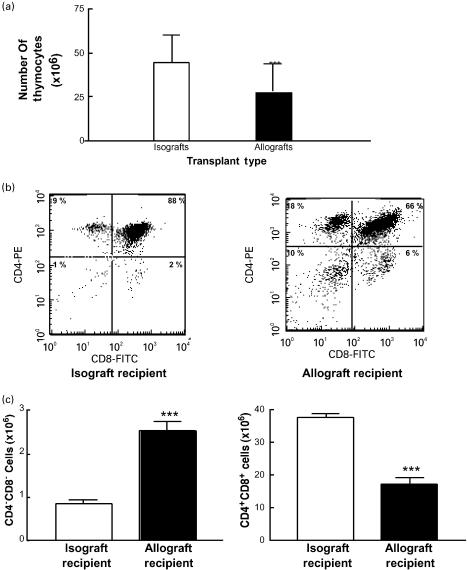
Fig. 1.
Decreased cellularity and increased negative selection in the thymus of allograft recipients. (a) The thymi of allograft recipients house significantly lower numbers of cells. Single cell suspensions from thymi of mice receiving syngeneic (white bars) or allogeneic (black bars) vascularized hearts grafts were prepared and total cell numbers were calculated. The figure represents one experiment with three mice per group (anova: ***P <0·001). (b) Changes in thymocyte subsets after syngeneic or allogeneic vascularized heart transplantation by two colour flow cytometric analysis of CD8 and CD4 expression in thymocyte single cell suspension. Plots are representative of six different mice in each group. (c) Increased numbers of double negative thymocytes and decreased numbers of double positive thymocytes in allograft recipients. Pooled data from thymi of mice receiving syngeneic (white bars) or allogeneic (black bars) heart grafts (n = 5 per group) (***P <0·001).
The decrease in size and cellularity of thymi of allograft recipients could be due to increased negative selection. Under these conditions, one sees an increase in double negative cells and a decrease in double positive thymocytes [37]. When thymocyte subsets in allograft recipients were compared with syngeneic graft recipients, we found that allograft recipients had a significantly higher proportion and number of double negative thymocytes (from 1·9% in isograft recipients to 8·9% in allograft recipients, P <0·01; see Fig. 1b,c). In addition, the thymi of allograft recipients had a significant decrease in the number of double positive thymocytes (from 85·2% in isograft recipients to 61·6% in allograft recipients, P <0·01; Fig. 1b,c). Together, these findings are consistent with the conclusion of increased negative selection in the thymus.
The increase in negative selection observed in the thymus of allograft recipients was not seen in the spleen after allografting, where 75–85% of lymphocytes were B cells and less than 1% were double positive T lymphocytes. These findings also argue against the differences in thymi being non-specific changes due to circulating lymphocytes.
Since stress responses associated with high levels of steroid production can induce specific deletion of double positive thymocytes [38–40], we determined the levels of cortisol at day 7 post-transplantation. We found that pooled cortisol levels were similar in syngeneic and allogeneic graft recipients: 128 nmol/l (n = 3) in syngeneic graft recipients, and 120 nmol/l in allograft recipients (n = 3). Thus, the decreased thymus cellularity and decreased numbers of double positive thymocytes were not due to a glucocorticoid-induced death of double positive cells.
Increased number of mature T cells with ‘activated’ phenotype in the thymi of allograft recipients
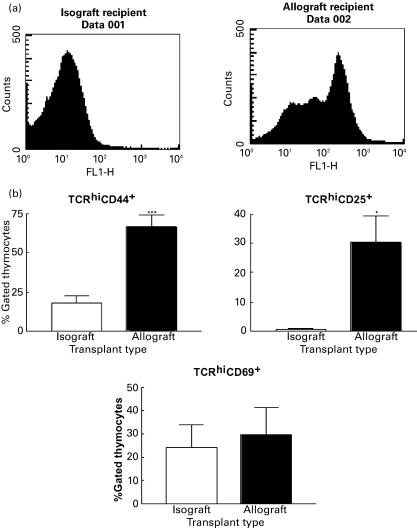
Surprisingly, we found that the thymi of allograft recipients also had a significantly greater proportion of single positive cells compared to syngeneic graft recipients (14·72 ± 1·25% versus 6·48 ± 1·04%, P <0·01). This applied to CD4 single positive cells and to CD8 single positive cells, although the changes were more prominent for the latter subset. The increase in single positive (CD4+CD8− or CD4−CD8+) cells in the thymus could reflect an increase in terminally differentiated thymocytes ready to be exported to the periphery. However, this is unlikely in the context of a significant decrease in the number of double positive thymocytes. Alternatively, the increase in single positive thymocytes may illustrate thymic re-entry of mature activated T cells consistent with a previous report of activated T cells being able to re-enter the thymus [28]. To test this hypothesis, we first examined the capacity of ex vivo, CFSE-labelled alloreactive mature T cells to re-enter the thymus upon adoptive transfer into graft recipients at the time of transplant. As illustrated in Fig. 2a, no significant fluorescence was detected in the thymus of isograft recipients 4–5 days after injection. In contrast, we observed significant fluorescence in the thymus of allograft recipients, demonstrating the re-entry of mature T cells into the thymus of allograft recipients. In addition, the profile of fluorescence with at least three distinctive peaks implied that these cells had gone through three cycles of division, and therefore were activated T cells (Fig. 2a).
Fig. 2.
Allograft recipients have increased numbers of mature T cells activated in the periphery that have re-entered the thymus. (a) Re-entry of activated T cells into the thymus of allograft recipients. Ex vivo CFSE-labelled T cells (20 × 106 cells/mouse) were adoptively transferred into isograft or allograft recipients. Thymi were harvested 4 or 5 days after transplant and examined for fluorescence (FL-1) by flow cytometry. (b) Percentages of cells expressing high levels of TCR on their surface and CD44 (***P <0·001), CD25 (*P <0·05) and CD69. White bars: syngeneic allograft recipients; black bars: allogeneic graft recipients.
Next, we examined the expression of surface molecules that are indicative of mature T cell priming in mature T cells in the thymus of graft recipients [41]. Specifically, we looked at the expression of CD44, CD25 (IL-2 receptor α chain) and CD69 in cells expressing high levels of TCR on the surface. CD44 is the main hyaluronic acid receptor on the cell surface [42]. This receptor mediates lymphocyte migration to lymphoid organs. It is expressed at high levels by 80–90% of fetal thymocytes, most of them in the double negative stage [42]. However, only 5% of adult thymocytes express CD44. Acquisition of CD44 by mature, peripheral T cells is a differentiation event linked to antigenic priming. Therefore, expression of CD44 in high levels is a good marker for primed T cells. We found that the thymi of allograft recipients had a significantly higher percentage of CD44+ cells expressing high levels of TCR compared to syngeneic graft recipients (66·7 ± 11·2% versus 17·5 ± 6·8%;P <0·01) (Fig. 2b), consistent with these cells being single positive mature T cells. In the thymus of allograft recipients, the ratio of CD4+CD44+ cells to CD8+CD44+ cells was skewed towards a CD4+ T cell predominance (data not shown). These changes were not seen in the thymi of isograft recipients. This finding implied that there is an increase in mature and activated T cells (as indicated by the high levels of TCR expression) in the thymi of allograft recipients, compatible with the hypothesis of recirculation of peripherally activated T cells into the thymus.
Next, we looked at the expression of CD25 by TCRhi-expressing cells. CD25 is the α chain of the IL-2 receptor [35]. CD25 is expressed at low levels by thymocytes in early stages of their development. However, CD25 expression disappears during thymic development and is only re-expressed after T cell activation in the periphery [35,43]. We found that the thymi of allograft recipients had a significant increase in cells expressing a TCRhiCD25+ phenotype that was not apparent in the thymi of isograft recipients (30·4 ± 13·1% versus 0·72 ± 0·06%; P <0·01) (Fig. 2b). In the context of an increased proportion of both single positive cells and TCRhiCD44+ cells in the thymi of allograft recipients, this result further corroborates that these thymi have increased numbers of mature activated T cells, likely upon re-entry from the periphery.
CD69 is a very early activation molecule expressed on the surface of T cells soon after activation [44]. In the thymus, CD69 is expressed predominantly by single positive cells expressing high levels of TCR, implying that CD69 expression in the thymus maybe linked to selection events [35,45]. We found that although allograft recipients had slightly higher numbers of CD69+TCRhi cells in the thymus, the difference did not reach statistical significance when compared with syngeneic graft recipients (Fig. 2b).
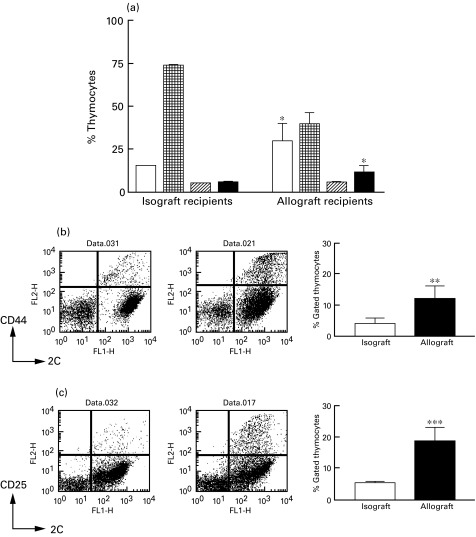
To confirm in vivo that the previous changes within the thymus of allograft recipients involved in part mature alloreactive T cells activated in the periphery, we analysed the changes in thymus populations in 2C TCR transgenic mice of selective MHC background, grafted with syngeneic or allogeneic hearts. In these mice, up to 95% of peripheral T cells express the 2C TCR. This TCR has a well-defined alloreactivity that is sufficient to mount a T cell-dependent graft rejection. The profiles for graft survival in these mice were consistent with the results observed with non-transgenic mice: when 2C TCR transgenic mice were grafted with syngeneic hearts, the grafts survived indefinitely; in contrast, graft rejection occurred after 8 days of 2C TCR transgenic C57Bl/6 mice receiving allogeneic BALB/c hearts. As shown in Fig. 3, 2C TCR transgenic mice that received allografts had similar changes in their thymi as those seen in allograft-receiving wild-type mice including a significant increase in double negative thymocytes and concomitant decrease in double positive thymocytes, compared to littermates receiving syngeneic grafts. These mice also provided the opportunity to confirm that the increase in single positive cells in the thymus following allografting was due in part to recently activated mature T cells re-entering the thymus. This was shown by looking at the expression of activation markers on the alloreactive T cells, i.e. those T cells expressing high levels of the 2C TCR (detected with the clonotypic antibody 1B2). As shown in Fig. 3b,c, we detected significant increases in the number of cells expressing high levels of the 2C TCR and CD44 or CD25 in allograft recipients. Since the 2C TCR-expressing T cells are responsible for most of the alloreactivity in this transplantation model, we concluded that the increased number of mature T cells in the thymus of allograft recipients are alloreactive T cells activated by the graft.
Fig. 3.
Increased negative selection and increased number of activated, alloreactive mature T cells in the thymi of 2C TCR transgenic mice after receiving a vascularized allograft. (a) Changes in thymocyte subsets of 2C TCR transgenic C57Bl/6 mice after syngeneic or allogeneic vascularized heart transplantation by two-colour flow cytometric analysis of CD8 and CD4 expression in thymocyte single cell suspension. Plots are representative of three different mice in each group (*P <0·05). (b) and (c) Increased numbers of mature alloreactive (high 2C TCR-expressing), activated T cells based on surface expression of CD44 (b) and CD25 (c). Pooled data from thymi of mice receiving syngeneic (white bars) or allogeneic (black bars) heart grafts (n = 3 per group) (**P <0·01; ***P <0·001). □, DN; 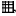 , DP;
, DP;  , CD4+SP; ▪, CD8+SP.
, CD4+SP; ▪, CD8+SP.
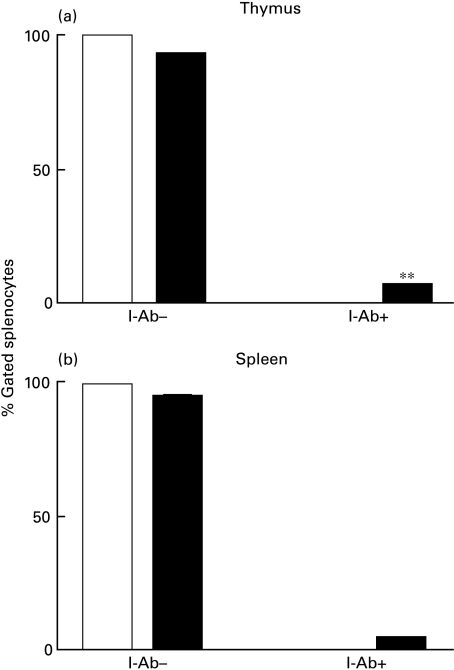
Migration of APC from the allograft to host thymus
Negative selection in the thymus is primarily mediated by bone marrow-derived cells expressing MHC molecules [41,46,47]. Thus, we looked for the presence of allogeneic (I-Ab-expressing) cells in the thymus and spleen of allograft recipients (I-Ad). We found that both thymus and spleen of allograft recipients contained significant numbers of I-Ab expressing cells (Fig. 4a,b). Although passive uptake of CD4 or CD8 by thymocytes can occur at very low levels [48,49], this has not been reported for MHC class II molecules. Thus, detection of allogeneic MHC molecules is more compatible with migration of allogeneic cells to the thymus [50].
Fig. 4.
Presence of donor (I-Ab+) MHC class II-expressing cells in the thymus (a) and spleen (b) of allograft recipients. Donor MHC-expressing cells (I-Ab) detection was quantified after background extraction for each sample. A comparison of the quantity of cells expressing an I-Ab phenotype in the thymi of isograft and allograft recipients (**P <0·01). Isograft, white bars. Allograft, black bars.
Increased numbers of apoptotic cells in cortex and medulla of thymi from allograft recipients
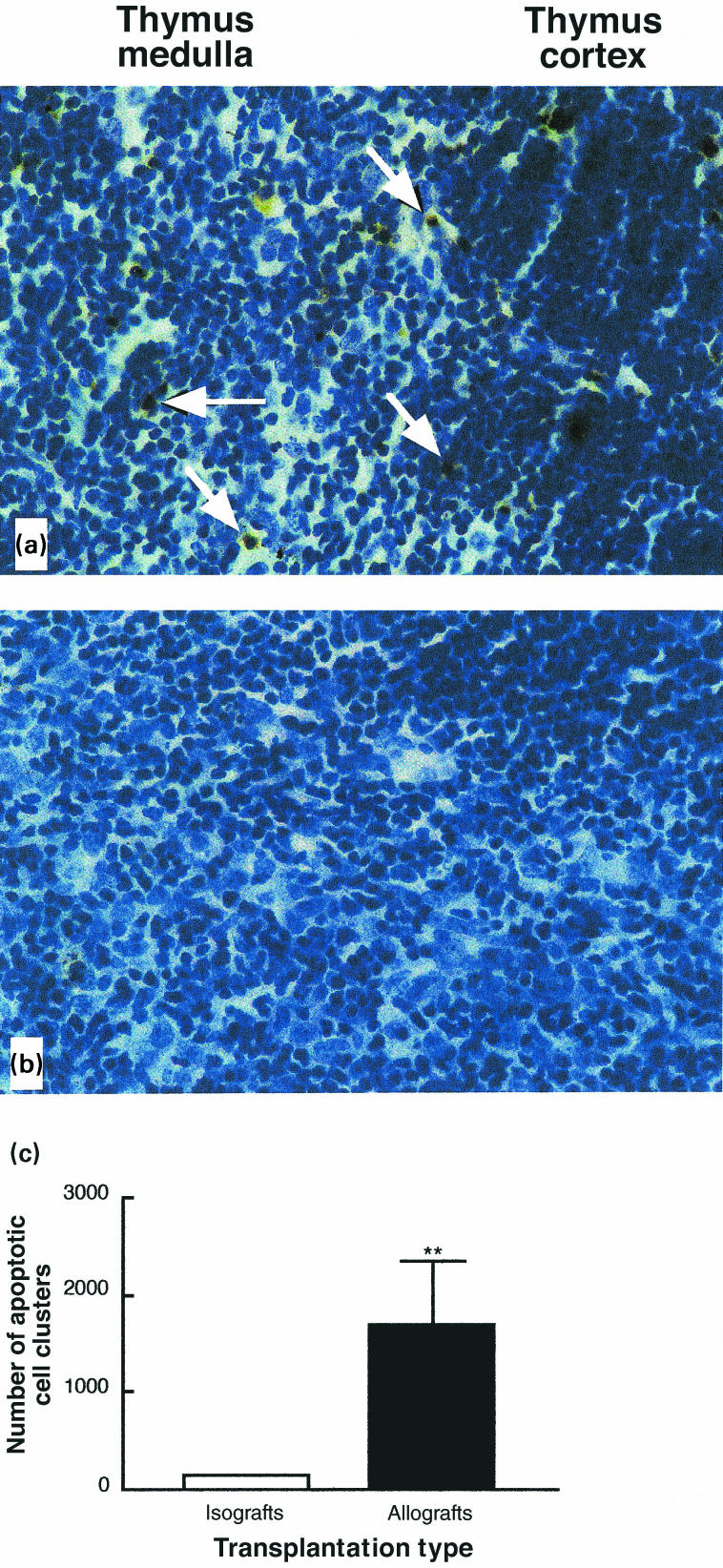
If increased negative selection is taking place in the thymi of allograft recipients, one should see an increase in apoptosis in these thymi [36]. Furthermore, the location of apoptotic cells would also be important as it is indicative of the underlying process leading to cell death [36]. As shown previously, normal thymi have scattered apoptotic cells primarily in the cortex, probably reflecting lack of positive selection. However, if negative selection is increased, there is a significant augmentation in apoptotic cells mostly in the thymic medulla [36]. To assess the incidence and location of apoptosis in the thymi of syngeneic and allogeneic graft recipients, we used standard TUNEL staining of thymic sections. As shown in Fig. 5, we observed a significant increase in the number of apoptotic cells in the thymi of allograft recipients compared to those of syngeneic graft recipients (1692 ± 934 apoptotic cells/section versus 140 ± 9 apoptotic cells/section; P <0·01). This increase was particularly dramatic in the medulla. A quantitative assessment of apoptotic cells is shown in Fig. 6. The phenotypic characterization of single positive, apoptotic cells suggest that they are mature T cells as indicated by the expression of high levels of TCR and CD44. In allograft recipients, this subset of cells represented 12% for TCRhi CD4+, and 4·7% for TCRhi CD8+.
Fig. 5.
Increased apoptosis in the thymic medulla of allograft recipients. Histological samples of thymi of allogeneic (a) and syngeneic (b) transplant recipients. Apoptotic cells, as detected by TUNEL are indicated by white arrows. (c) Quantification of the increased numbers of apoptotic cells in the thymus of allograft recipients as detected by TUNEL (**P < 0·01).
Discussion
Our results with wild-type or TCR transgenic mouse transplant models provide the first in vivo demonstration of increased negative selection in the thymi of vascularized allograft recipients, acting on both developing thymocytes and mature, activated T cells that have re-entered the thymus. Based on this evidence, we propose a model in which activation-induced death of some alloactivated T cells occurs, in part, in the thymus after activation in the periphery. This process may induce some degree of tolerance to the graft, and contribute to the decreased need for immunosuppression with time. However, this spontaneous process is not sufficient to induce graft acceptance since it occurs in mice undergoing graft rejection.
We have found that allograft recipients have a significant decrease in thymus cellularity, confirming previous reports of similar finding following allogeneic transplantation [32–34]. These changes are not due to age-dependent loss of thymic function because they did not occur in age-matched, syngeneic graft recipients or in non-manipulated controls (data not shown). On the contrary, our results indicate that the thymus of adult individuals has the required microenvironment for negative selection. Recent data indicate that the thymus preserves its ability to support T cell development into late stages of life, despite its apparent involution after puberty [51]. During an allo-response, with concomitant migration of allogeneic MHC molecule-expressing cells to the thymus, thymic function may be enhanced.
Assuming a constant input of precursors into the thymus, the decrease in cellularity after allogeneic transplantation may result from increased death rate within the thymus or from increased export of mature T cells. We favour the former since we demonstrate an increase in apoptosis in the thymi of allograft recipients for the first time. Furthermore, the increase in the number of apoptotic cells is particularly significant in the medulla. This is also consistent with our claim of negative selection of mature T cells since re-entry in to the thymus takes place likely at the cortico–medullary junction, and it is in the medulla where most negative selection events have been demonstrated [36].
A critical finding in our experiments has been the detection of increased numbers of mature activated T cells in the thymi after allogeneic stimulation. This observation was associated with thymic re-entry of mature T cells upon activation as shown by adoptive transfer of CFSE-labelled T cells, and consistent with the previous report by Agus et al. [28]. Our claim is particularly supported by the results obtained with a monoclonal T cell mouse transplant model. The significance of recirculation into the thymus of mature, activated T cells is not established. It has been suggested previously that recirculation into the thymus following activation in the periphery may provide an opportunity for re-tolerization of T cells [52]. This could or could not be associated with T cell death [52]. Alternatively, thymic re-entry of mature activated T cells may reflect a fundamental mechanism to regulate T cell homeostasis. In this scenario, the thymus may provide the appropriate environment where activation-induced cell death takes place, as proposed for the gut [53,54], and ensure the efficient removal of unwanted T cells in late stages of immune responses. This could complement previous reports on clonal deletion of alloreactive T cells, but without evidence of where this occurs [13].
Although double positive thymocyte apoptosis induced by steroids may be associated with up-regulation (e.g. CD3, TCR, CD69 and CD25) or down-regulation (e.g. CD4, CD8 and heat stable antigen) of surface molecules [55], the possibility that steroid-mediated stress responses are responsible for the observations reported here has been excluded in our study and in previous studies using similar models [32,54]. First, the levels of cortisol were similar between syngenic and allogeneic graft recipients. Secondly, cell death occurred in the context of an increased proportion of cells expressing high levels of TCR and CD44, the latter being a molecule that is not up-regulated in developing thymocytes undergoing apoptosis [55]. Thirdly, increased cell death by apoptosis was mostly seen in the medulla, and thus affecting primarily single positive T cells and these cells are less sensitive to steroid-induced apoptosis.
One striking finding of this study is the apparent inability of thymic clonal deletion in isolation to induce full tolerance to an allograft in most recipients. This observation is not only applicable to our study but also to previous reports using intrathymic injection of alloantigens that have shown substantial inter-experimental variation [7]. Four different explanations can be offered. First, the kinetics of alloresponse and AICD in the thymus may be very different, the former occurring earlier than the latter. According to this possibility, alloreactive T cells activated in the regional secondary lymphoid organ may go to the graft where they would act as effectors before their deletion in the thymus. A second possibility is that the elements for negative selection are less efficient in the adult thymus. Such an argument is suggested by the observation that fetal thymocytes are more sensitive to tolerization than adult thymocytes [56]. A third potential explanation is that both allograft rejection and thymic deletion of alloreactive T cells take place simultaneously but involve two separate pools of T cells due to recirculation from the spleen into separate compartments. Fourthly, non-T cell-mediated mechanisms may be operational for rejection and not affected by thymic negative selection. This may include NK cell-mediated or antibody-mediated mechanisms. Analysis of the fine antigen specificity of graft rejection in the model used in the current study is beyond the scope of this report and will be the subject of future investigations to provide further insight with regards to this possibility. Finally, an additional argument to be made in human transplantation is that some of the immunosuppressive therapies used in patient management can interfere with negative selection in the thymus. For example, it has been reported that cyclosporin may interfere with the deletion of autoreactive thymocytes [57] and that could also be applicable to AICD in the thymus.
In summary, our study demonstrates that recipients of vascularized allografts have increased negative selection in their thymi. Allograft-induced T cell death in the thymus affects not only developing thymocytes but also mature alloreactive T cells that have re-entered the thymus upon activation. The implications of these findings in clinical transplantation remain to be established. However, this may be one of the mechanisms involved in the development of partial tolerance to allografts.
Acknowledgments
This work was supported by the Kidney Foundation of Canada, the London Health Sciences Centre (Multi-Organ Transplant Program, and Internal Research Fund), and the Medical Research Council of Canada. S. R. was a recipient of a Leukaemia Research Fund of Canada studentship. We thank Dr David Grant for critically reading this manuscript, Dr Bertha Garcia for routine pathology, and the members of the Madrenas Laboratory for helpful comments and criticism.
References
- 1.Krensky AM, Pober JS. Immunological frontiers of transplantation. Immunity. 2001;14:345–6. 10.1016/s1074-7613(01)00114-5. [Google Scholar]
- 2.Davies JD, Martin G, Phillips J, et al. T cell regulation in adult transplantation tolerance. J Immunol. 1996;157:529–33. [PubMed] [Google Scholar]
- 3.Madrenas J, Lazarovits AI. Differential signaling through the T cell receptor: from biochemistry to transplantation tolerance. Histol Histopathol. 1998;13:221–9. doi: 10.14670/HH-13.221. [DOI] [PubMed] [Google Scholar]
- 4.Rossini AA, Greiner DL, Mordes JP. Induction of immunologic tolerance for transplantation. Physiol Rev. 1999;79:99–141. doi: 10.1152/physrev.1999.79.1.99. [DOI] [PubMed] [Google Scholar]
- 5.Li XC, Wells AD, Strom TB, et al. The role of T cell apoptosis in transplantation tolerance. Curr Opin Immunol. 2000;12:522–7. doi: 10.1016/s0952-7915(00)00133-3. [DOI] [PubMed] [Google Scholar]
- 6.Li XC, Strom TB, Turka LA, et al T. cell death and transplantation tolerance. Immunity. 2001;14:407–16. doi: 10.1016/s1074-7613(01)00121-2. [DOI] [PubMed] [Google Scholar]
- 7.Gillanders WE, Arima T, Tu F, et al. Evidence for clonal deletion and clonal anergy after intrathymic antigen injection in a transplantation model. Transplantation. 1997;64:1159–66. doi: 10.1097/00007890-199710270-00014. [DOI] [PubMed] [Google Scholar]
- 8.Ramsdell F, Fowlkes BJ. Clonal deletion versus clonal anergy. the role of the thymus in inducing self tolerance. Science. 1990;248:1342–8. doi: 10.1126/science.1972593. [DOI] [PubMed] [Google Scholar]
- 9.Kabelitz D. Apoptosis, graft rejection, and transplantation tolerance. Transplantation. 1998;65:869–75. doi: 10.1097/00007890-199804150-00003. [DOI] [PubMed] [Google Scholar]
- 10.Waldmann H, Cobbold S. Regulating the response to transplants: a role for CD4+ regulatory cells? Immunity. 2001;14:399–406. doi: 10.1016/s1074-7613(01)00120-0. 10.1016/s1074-7613(01)00120-0. [DOI] [PubMed] [Google Scholar]
- 11.Morris PJ. Kidney transplantationPrinciples and practice, 4th Edn. Philadelphia: W.B. Saunders; 1994. [Google Scholar]
- 12.Wood K, Sachs DH. Chimerism and transplantation tolerance: cause and effect. Immunol Today. 1996;17:584–7. doi: 10.1016/s0167-5699(96)10069-4. [DOI] [PubMed] [Google Scholar]
- 13.Nikolic B, Sykes M. Clonal deletion as a mechanism of transplantation tolerance. J Heart Lung Transplant. 1996;15:1171–8. [PubMed] [Google Scholar]
- 14.Lechler R, Bluestone JA. Transplantation tolerance – putting the pieces together [editorial] Curr Opin Immunol. 1997;9:631–3. doi: 10.1016/s0952-7915(97)80041-6. [DOI] [PubMed] [Google Scholar]
- 15.Li Y, Li XC, Zheng XX, et al. Blocking both signal 1 and signal 2 of T-cell activation prevents apoptosis of alloreactive T cells and induction of peripheral allograft tolerance. Nat Med. 1999;5:1298–302. doi: 10.1038/15256. 10.1038/15256. [DOI] [PubMed] [Google Scholar]
- 16.Wells AD, Li XC, Li Y, et al. Requirement for T-cell apoptosis in the induction of peripheral transplantation tolerance. Nat Med. 1999;5:1303–7. doi: 10.1038/15260. [DOI] [PubMed] [Google Scholar]
- 17.Wekerle T, Kurtz J, Sayegh M, et al. Peripheral deletion after bone marrow transplantation with costimulatory blockade has features of both activation-induced cell death and passive cell death. J Immunol. 2001;166:2311–6. doi: 10.4049/jimmunol.166.4.2311. [DOI] [PubMed] [Google Scholar]
- 18.Wekerle T, Sayegh MH, Hill J, et al. Extrathymic T cell deletion and allogeneic stem cell engraftment induced with costimulatory blockade is followed by central T cell tolerance. J Exp Med. 1998;187:2037–44. doi: 10.1084/jem.187.12.2037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lin MT, Tseng LH, Frangoul H, et al. Increased apoptosis of peripheral blood T cells following allogeneic hematopoietic cell transplantation. Blood. 2000;95:3832–9. [PubMed] [Google Scholar]
- 20.Li W, Lu L, Wang Z, et al. Il-12 antagonism enhances apoptotic death of T cells within hepatic allografts from Flt3 ligand-treated donors and promotes graft acceptance. J Immunol. 2001;166:5619–28. doi: 10.4049/jimmunol.166.9.5619. [DOI] [PubMed] [Google Scholar]
- 21.Meyer D, Thorwarth W, Otto C, et al. Early T-cell inactivation and apoptosis-critical events for tolerance induction after allogeneic liver transplantation. Transplant Proc. 2001;33:256–8. doi: 10.1016/s0041-1345(00)02003-0. [DOI] [PubMed] [Google Scholar]
- 22.Jollow KC, Sundstrom JB, Gravanis MB, et al. Apoptosis of mononuclear cell infiltrates in cardiac allograft biopsy specimens questions studies of biopsy-cultured cells. Transplantation. 1997;63:1482–9. doi: 10.1097/00007890-199705270-00019. [DOI] [PubMed] [Google Scholar]
- 23.Van Hoffen E, Van Wichen DF, Leemans JC, et al. T cell apoptosis in human heart allografts. association with lack of co-stimulation? Am J Pathol. 1998;153:1813–24. doi: 10.1016/S0002-9440(10)65696-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Matsuno T, Nakagawa K, Sasaki H, et al. Apoptosis in acute tubular necrosis and acute renal allograft rejection. Transplant Proc. 1994;26:2170–3. [PubMed] [Google Scholar]
- 25.Matsuno T, Sasaki H, Ishido N, et al. Apoptosis in human kidney allografts. Transplant Proc. 1996;28:1226–7. [PubMed] [Google Scholar]
- 26.Remuzzi G. Cellular basis of long-term organ transplant acceptance: pivotal role of intrathymic clonal deletion and thymic dependence of bone marrow microchimerism-associated tolerance. Am J Kidney Dis. 1998;31:197–212. doi: 10.1053/ajkd.1998.v31.pm9469488. [DOI] [PubMed] [Google Scholar]
- 27.Volkmann A, Zal T, Stockinger B. Antigen-presenting cells in the thymus that can negatively select MHC class II-restricted T cells recognizing a circulating self antigen. J Immunol. 1997;158:693–706. [PubMed] [Google Scholar]
- 28.Agus DB, Surh CD, Sprent J. Reentry of T cells to the adult thymus is restricted to activated T cells. J Exp Med. 1991;173:1039–46. doi: 10.1084/jem.173.5.1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sha WC, Nelson CA, Newberry RD, et al. Selective expression of an antigen receptor on CD8-bearing T lymphocytes in transgenic mice. Nature. 1988;335:271–4. doi: 10.1038/335271a0. [DOI] [PubMed] [Google Scholar]
- 30.Corry R, Russell P. New possibilities for organ allografting in the mouse. In: Calne RY, editor. Immunological aspects of transplantation surgery. New York: Wiley; 1973. pp. 279–95. [Google Scholar]
- 31.Zhang Z, Zhu L, Quan D, et al. Pattern of liver, kidney, heart, and intestine allograft rejection in different mouse strain combinations. Transplantation. 1996;62:1267–72. doi: 10.1097/00007890-199611150-00016. [DOI] [PubMed] [Google Scholar]
- 32.Tanaka K, Koga Y, Zhang XY, et al. Extensive apoptosis occurring in the thymus during accelerated rejection of cardiac allografts in presensitized rats. J Immunol. 1993;151:748–58. [PubMed] [Google Scholar]
- 33.Miyanari N, Yamaguchi Y, Hisama N, et al. T-cell responses in the thymus after hepatic transplantation in the rat. Transplant Proc. 1995;27:1571–4. [PubMed] [Google Scholar]
- 34.Yamaguchi Y, Okabe K, Miyanari N, et al. Tumor necrosis factor-beta is associated with thymic apoptosis during acute rejection. Transplantation. 1998;66:894–902. doi: 10.1097/00007890-199810150-00014. [DOI] [PubMed] [Google Scholar]
- 35.Janeway CA, Travers P. ImmunobiologyThe immune system in health and disease. New York: Current Biology Ltd; 1997. [Google Scholar]
- 36.Surh CD, Sprent J. T-cell apoptosis detected in situ during positive and negative selection in the thymus [see comments] Nature. 1994;372:100–3. doi: 10.1038/372100a0. [DOI] [PubMed] [Google Scholar]
- 37.Roitt I, Brostoff J, Male D. Immunology. London: Mosby International Ltd; 1998. [Google Scholar]
- 38.Zheng B, Han S, Zhu Q, et al. Alternative pathways for the selection of antigen-specific peripheral T cells. Nature. 1996;384:263–6. doi: 10.1038/384263a0. [DOI] [PubMed] [Google Scholar]
- 39.Iwata M, Ohoka Y, Kuwata T, et al. Regulation of T cell apoptosis via T cell receptors and steroid receptors. Stem Cells. 1996;14:632–41. doi: 10.1002/stem.140632. [DOI] [PubMed] [Google Scholar]
- 40.Tolosa E, Ashwell JD. Thymus-derived glucocorticoids and the regulation of antigen-specific T-cell development. Neuroimmunomodulation. 1999;6:90–6. doi: 10.1159/000026368. [DOI] [PubMed] [Google Scholar]
- 41.Anderson G, Moore NC, Owen JJ, et al. Cellular interactions in thymocyte development. Annu Rev Immunol. 1996;14:73–99. doi: 10.1146/annurev.immunol.14.1.73. [DOI] [PubMed] [Google Scholar]
- 42.Lesley J, Hyman R, Kincade PW. CD44 and its interaction with extracellular matrix. Adv Immunol. 1993;54:271–335. doi: 10.1016/s0065-2776(08)60537-4. [DOI] [PubMed] [Google Scholar]
- 43.Hanke T, Mitnacht R, Boyd R, et al. Induction of interleukin 2 receptor beta chain expression by self-recognition in the thymus. J Exp Med. 1994;180:1629–36. doi: 10.1084/jem.180.5.1629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Werfel T, Boeker M, Kapp A. Rapid expression of the CD69 antigen on T cells and natural killer cell upon antigenic stimulation of peripheral blood mononuclear cell suspensions. Allergy. 1997;52:465–9. doi: 10.1111/j.1398-9995.1997.tb01031.x. [DOI] [PubMed] [Google Scholar]
- 45.Bendelac A, Matzinger P, Seder RA, et al. Activation events during thymic selection. J Exp Med. 1992;175:731–42. doi: 10.1084/jem.175.3.731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Marrack P, Lo D, Brinster R, et al. The effect of thymus environment on T cell development and tolerance. Cell. 1988;53:627–34. doi: 10.1016/0092-8674(88)90578-8. [DOI] [PubMed] [Google Scholar]
- 47.van Meerwijk JP, Marguerat S, Lees RK, et al. Quantitative impact of thymic clonal deletion on the T cell repertoire. J Exp Med. 1997;185:377–83. doi: 10.1084/jem.185.3.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shores EW, Sharrow SO, Singer A. Presence of CD4 and CD8 determinants on CD4-CD8- murine thymocytes: passive acquisition of CD8 accessory molecules. Eur J Immunol. 1991;21:973–7. doi: 10.1002/eji.1830210417. [DOI] [PubMed] [Google Scholar]
- 49.Michie AM, Carlyle JR, Zuniga-Pflucker JC. Early intrathymic precursor cells acquire a CD4 (low) phenotype. [PubMed]
- 50.Lechler R, Ng WF, Steinman RM. Dendritic cells in transplantation – friend or foe? Immunity. 2001;14:357–68. doi: 10.1016/s1074-7613(01)00116-9. 10.1016/s1074-7613(01)00116-9. [DOI] [PubMed] [Google Scholar]
- 51.Rodewald H. The thymus in the age of retirement. Nature. 1998;396:630–1. doi: 10.1038/25251. [DOI] [PubMed] [Google Scholar]
- 52.Zhao Y, Sergio JJ, Swenson K, et al. Positive and negative selection of functional mouse CD4 cells by porcine MHC in pig thymus grafts. J Immunol. 1997;159:2100–7. [PubMed] [Google Scholar]
- 53.Webb S, Morris C, Sprent J. Extrathymic tolerance of mature T cells: clonal elimination as a consequence of immunity. Cell. 1990;63:1249–56. doi: 10.1016/0092-8674(90)90420-j. [DOI] [PubMed] [Google Scholar]
- 54.Ozcay N, Fryer J, Grant D, et al. Budesonide, a locally acting steroid, prevents graft rejection in a rat model of intestinal transplantation. Transplantation. 1997;63:1220–5. doi: 10.1097/00007890-199705150-00006. [DOI] [PubMed] [Google Scholar]
- 55.Kishimoto H, Surh CD, Sprent J. Upregulation of surface markers on dying thymocytes. J Exp Med. 1995;181:649–55. doi: 10.1084/jem.181.2.649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Luo H, Chen H, Qi S, et al. De novo-developed T cells have compromised response to existing alloantigens: using Ld-specific transgenic 2C T cells as tracers in a mouse heart transplantation model. J Immunol. 1998;161:73–82. [PubMed] [Google Scholar]
- 57.Jenkins MK, Schwartz RH, Pardoll DM. Effects of cyclosporine A on T cell development and clonal deletion. Science. 1988;241:1655–8. doi: 10.1126/science.241.4873.1655. [DOI] [PubMed] [Google Scholar]