Abstract
We have investigated the proliferation rates of T-cell subsets in colorectal carcinomas using immunohistochemistry. It was found that the tumour-infiltrating T cells in contact with the tumour cells have a significantly higher frequency of proliferation than those in the stroma. In particular, the CD8+ intraepithelial lymphocytes (T-IEL) within the tumours have a significantly higher frequency of proliferation in comparison with CD8+ T cells in the stromal compartment or in any normal mucosal lymphoid tissues. It is possible that the proliferation of the CD8+ T-IEL may be driven by self-antigens expressed on the tumour cells. The proportion of CD3+ CD7– T cells is increased within carcinomas compared with the normal colon, and a population of CD57+ T cells was observed which is absent from the normal colon. It is possible that these phenotypes are acquired in situ due to repeated stimulation of the T cells by tumour antigens. Intact colorectal carcinoma explants were cultured, and the presence of tumour-infiltrating T cells analysed after 3 days of culture in isolation from the systemic compartments. CD3+ T cells were proliferating (at a low rate) within the explants after 3 days of culture, indicating that they may be sustained by factors present in the tumour microenvironment.
Keywords: colorectal carcinoma, T cells, CD7, CD57, TCRγδ
INTRODUCTION
It has been known for decades that solid malignancies in humans are associated with an infiltrate of inflammatory cells. A number of studies have found a clear positive correlation between the density of the lymphoid infiltrate and the prognosis of the patient [1–4]. This, together with the partial oligoclonality of carcinoma-infiltrating T cells [5], indicates that local immune responses to the tumours are likely to be occurring, although the precise nature of the response, and the reason for the survival of the tumours despite the anti-tumour response, are not known. It has been shown that in vitro, CD8+ T-IEL are able to spontaneously lyse colon carcinoma cell lines [6]. Around 20% of the stromal, but almost none of the intraepithelial CD8+ T cells express perforin in Dukes’ A carcinomas, decreasing to just 3% in the stroma in Dukes’ C cases [7]. Carcinoma-infiltrating Vδ1+ T cells also lyse colon carcinoma cell lines [8], recognizing MICA and MICB on the tumour cells [9], and it has been shown in mice that Vα14+ NK T cells lyse tumour cells [10]. As CD1, the target for human Vα24+ NK T cells, is expressed on the intestinal epithelium [11], these cells may also respond to colorectal carcinoma cells.
The phenotypes of the infiltrating lymphocytes have been investigated, and the proportions of CD4+, CD8+, TCRαβ+ and TCRγδ+ cells reported vary considerably in different studies [12–19]. However, overall, it appears that CD8+ T cells localize to the epithelium, as in the normal gut. It has also been shown that the numbers of CD56+ and CD57+ NK T cells are increased within colorectal carcinomas compared with the normal gut [20,21].
To increase understanding of the anti-tumour immune response, we have asked which subsets of tumour-infiltrating T cells are stimulated to proliferate in the T-IEL compartment, where T cells are in direct contact with the tumour, and in the stroma. We have also compared the proliferation frequencies observed for each T-cell subset with that observed in areas of maximal T cell stimulation in tonsils and Peyer’s patches. In addition, T cells within intact cultured explants of colorectal carcinomas were studied to determine whether the T cell response is supported by factors in the tumour microenvironment. The explant model described here will also enable future studies of the functional relationship between the stromal and lymphoid elements of the tumour microenvironment.
MATERIALS AND METHODS
Tissues
Fresh surgical bowel specimens were received on the day of surgery. Blocks of carcinomas were removed and either snap frozen and stored in liquid nitrogen, or used immediately for organ culture experiments. Intestinal biopsies were taken during routine endoscopies, snap frozen immediately on removal and stored in liquid nitrogen. Fresh palatine tonsils were received on the day of surgery. Tissue was cut into blocks, snap frozen and stored in liquid nitrogen. Specimen details are shown in Table 1.
Table 1.
Tissue specimens used: palatine tonsils; Peyer’s patch-containing small bowel specimens; colorectal carcinoma specimens
| Patient | Age | Sex | Clinical information |
|---|---|---|---|
| Palatine tonsils | |||
| 1 | 5 | F | |
| 2 | 12 | F | |
| 5 | 5 | M | |
| 9 | 24 | M | |
| 11 | 21 | F | |
| Peyer’s patch-containing small bowel | |||
| a | 72 | M | Ileo-colonic resection for polyps |
| b | 59 | F | Right hemicolectomy for hepatic flexure carcinoma |
| c | 72 | M | Right hemicolectomy for caecal carcinoma |
| d | 22 | F | Endoscopic biopsy, diagnosed normal |
| e | 37 | F | Endoscopic biopsy, diagnosed normal |
| f | 24 | M | Endoscopic biopsy, diagnosed normal |
| Patient | Age | Sex | Location | Dukes’ grade | Differentiation |
|---|---|---|---|---|---|
| Colorectal carcinoma | |||||
| A | 81 | M | Splenic flexure | C | Moderate to poor |
| B | 80 | M | Transverse | B | Moderate to poor |
| C | 81 | F | Sigmoid | B | Moderate |
| D | 74 | M | Right colon | B | Moderate |
| E | 64 | F | Ascending | B | Moderate |
| F | 91 | F | Colon (NOS) | B | Moderate |
| G | 78 | F | Ascending | B | Moderate |
| H | 64 | M | Sigmoid | C | Moderate |
| I | 81 | F | Colon (NOS) | C | Well |
| J | 45 | M | Colon (NOS) | C | Moderate |
| K | 41 | M | Sigmoid | B | Moderate |
| L | 67 | M | Sigmoid | C | Moderate |
| M | 77 | F | Rectal | B | Moderate |
| N | 63 | M | Rectal | C | Moderate |
| O | 86 | M | Caecal | C | Moderate |
| P | 76 | F | Sigmoid | C | Moderate |
| Q | 79 | F | Caecal | C | Poor |
| R | 79 | M | Caecal | B | Moderate |
| S | 79 | F | Sigmoid | B | Moderate |
| T | 66 | F | Sigmoid | C | Moderate |
| U | 70 | F | Rectal | C | Moderate |
| V | 73 | M | Sigmoid | B | Moderate |
| W | 78 | M | Rectosigmoid | C | Moderate |
| X | 77 | M | Caecal | B | Moderate |
Immunohistochemistry
Acetone-fixed 8μm frozen sections of colorectal carcinoma, Peyer’s patches and tonsil were stained using an indirect immunoperoxidase method with a horseradish peroxidase-conjugated rabbit antiserum to mouse Ig (Dako Ltd, High Wycombe, UK) and diaminobenzidine substrate. For double staining, another primary antibody was added and the sections incubated overnight at 2–4°C. These antibodies were detected using biotin-conjugated rabbit anti-mouse monoclonal antibody (Dako Ltd), followed by an avidin–alkaline phosphatase conjugate. Bound alkaline phosphatase was visualized using fast blue substrate. The sources and specificities of primary antibodies used are shown in Table 2. It was not necessary to use isotype-specific antibodies for primary and secondary staining as identification of double-stained cell surfaces was not required.
Table 2.
Primary antibodies used in enzyme-linked immuno-histochemistry
| Antibody specificity | Dilution | Clone | Isotype | Source |
|---|---|---|---|---|
| Vα24 | 1:100 | C15 | IgG1 | Immunotech |
| panTCRγδ | 1:50 | 5.A6.E9 | IgG1 | Endogen |
| CD3 | 1:50 | UCHT1 | IgG1 | Dako Ltd |
| CD4 | 1:50 | MT310 | IgG1 | Dako Ltd |
| CD7 | 1:10 | DK24 | IgG2a | Dako Ltd |
| CD8 | 1:50 | C8/144B | IgG1 | Dako Ltd |
| CD45RO | 1:50 | UCHL1 | IgG2a | Dako Ltd |
| CD57 | 1:1000 | VC1·1 | IgM | Sigma |
| Ki67 | 1:50 | Ki67 | IgG1 | Dako Ltd |
Counting the proportions of different subsets among colorectal carcinoma-infiltrating T cells
The number of cells stained with a subset-specific antibody and the number stained with CD3 were counted in at least 10 fields of view (×40 magnification). IEL and stromal lymphocytes were counted separately. The proportion of T cells with each different phenotype was calculated for both the T-IEL and stromal compartments.
Counting the proliferation rates of different subsets of colorectal-carcinoma infiltrating, Peyer’s patch and tonsillar T cells
The number of cells with subset-specific antibody-stained surfaces, and those with both Ki67+ nuclei and stained surfaces, were counted separately in at least 10 fields of view (×40 magnification) in colorectal carcinomas. The T-IEL and stromal compartments were counted separately. At least six fields of view (×40 magnification) of the Peyer’s patch and tonsillar T cell zones were counted. The proportion of cells with each phenotype that were proliferating was calculated for each microenvironment.
Culture of colorectal carcinoma explants
The method was based on that of Wilson and Macartney [22]. Non-necrotic pieces of tumour were removed aseptically from surgical specimens, placed in a sterile Petri dish and cut into pieces of approximately 1–2 mm in diameter. The pieces were placed on sterile steel grids in organ culture dishes containing 1·5 ml culture medium consisting of 60% Trowells T8 (Gibco BRL, Paisley, UK), 20% NCTC (Gibco BRL), 15% foetal bovine serum (Sigma-Aldrich, Poole, UK), 2%l-glutamine (Sigma-Aldrich), 2% penicillin/streptomycin (10 000 U penicillin and 10 mg streptomycin/ml, Sigma-Aldrich) and 1% 1 m Hepes (Sigma-Aldrich) in the inner compartment, and 1·5 ml sterile water in the outer compartment. The grids were such that the explants were maintained at the liquid/gas interface, with the culture medium being pulled over the explants by surface tension. Explants were cultured in an atmosphere of 95% oxygen at 37°C for 3 days. The explants were snap-frozen in liquid nitrogen for immunohistochemical analysis.
Counting tumour cell proliferation rates in cultured colorectal carcinoma explants
Frozen sections of colorectal carcinomas, cultured for 3 days, were stained using an antibody to Ki67, and counterstained with haematoxylin. The number of Ki67+ tumour cell nuclei and the total number of tumour cell nuclei were counted, and the proliferation frequencies calculated.
Counting T cells and T cell proliferation in cultured colorectal carcinoma explants
Frozen sections of seven colorectal carcinomas, cultured for 3 days, were stained using an antibody to CD3 and counterstained with haematoxylin, to identify the tumour cell nuclei. The number of CD3+ T-IEL per 1000 tumour cells was determined.
Sections were also double stained using an antibody to Ki67, followed by an antibody to CD3. The numbers of cells stained with CD3, and the numbers with both Ki67+ nuclei and CD3-stained surfaces, were counted and the proportions of proliferating T cells were determined. The T-IEL and stromal compartments were not counted separately.
Statistical analysis
The Mann–Whitney U-test was used for all statistical comparisons.
RESULTS
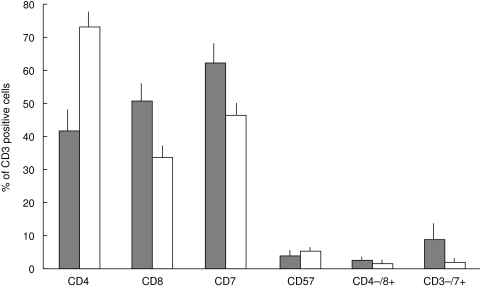
The proportions of tumour-infiltrating T-cell subsets in the IEL and stromal compartments were quantified (Fig. 1). CD4+ cells were found to be significantly concentrated in the stroma (P = 1·1 × 10–3). CD8+ and CD3+ CD7+ cells were significantly concentrated in the IEL (P = 9·8 × 10–3 and 0·026, respectively). CD3+ CD57+ cells were also identified but were found to be equally distributed in the IEL and stroma. In 19 specimens, < 0·1% of the T cells were TCRγδ+. In the remaining specimens (A, D and K), TCRγδ+ cells were present in the stroma (2·2%, 3·4% and 1·1%, respectively) and in A and K, they were also present in the IEL (8·7% and 1·3%, respectively). In each of eight specimens, < 0·1% of the T cells expressed Vα24.
Fig. 1.
A comparison of the proportions of colorectal carcinoma-infiltrating T cells of different subsets in the IEL and stromal compartments. The mean percentages (and standard errors) of T cells expressing various phenotypic markers are compared in colorectal carcinoma-infiltrating IEL ( ) and colorectal carcinoma-infiltrating stromal cells (□).
) and colorectal carcinoma-infiltrating stromal cells (□).
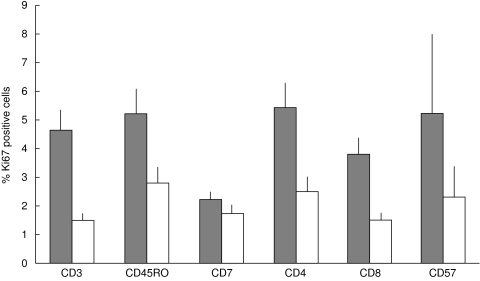
Proliferation of T-cell subsets in the IEL and stromal compartments was identified by co-expression of Ki67 (Fig. 2,Fig. 3a,b). Table 3 shows the number of specimens used to quantify the proliferation of each different T-cell subset in each compartment. The proliferation frequencies of the T-IEL are significantly greater than those of the cells in the stromal compartment in the CD3+ (P = 0·004), CD45RO+ (P = 0·02), CD4+ (P = 0·017) and CD8+ (P = 0·037) subsets. The proliferation frequencies of CD57+ and CD7+ cells in the IEL and stroma were not significantly different (P = 0·46 and 0·59). It was only possible to quantify proliferation of TCRγδ+ cells in the IEL of two specimens: the rates were 7% (patient A) and 0% (patient K). In the stroma, the proliferation frequencies were quantified in three specimens: they were 10·5% (patient A), 0% (patient K) and 0% (patient D).
Fig. 2.
A comparison of the proliferation frequencies of different colorectal carcinoma-infiltrating T-cell subsets. The mean percentages (and standard errors) of proliferating T cells from various subsets were calculated in intraepithelial cells ( ) and in stromal cells (□).
) and in stromal cells (□).
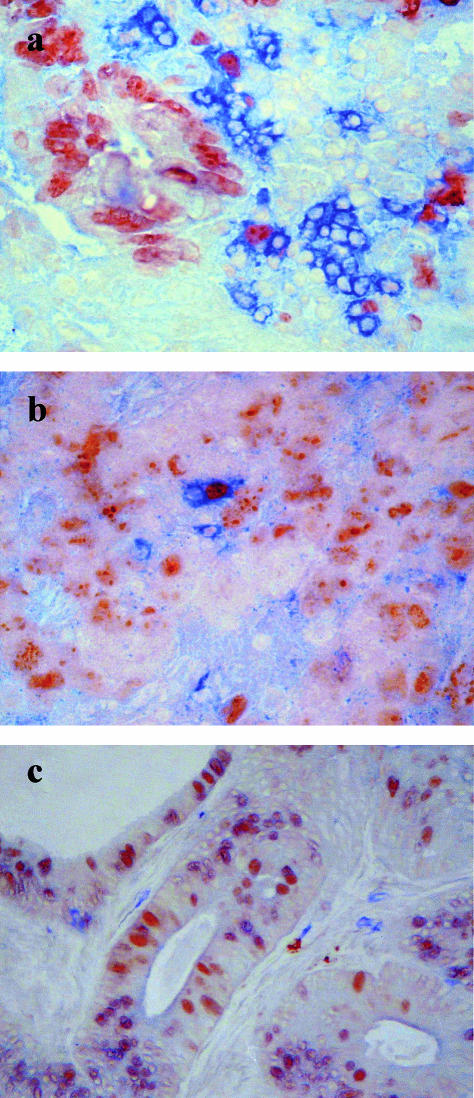
Fig. 3.
(a) Section of colorectal carcinoma from patient A, stained using an antibody to Ki67 (immunoperoxidase: brown), followed by an antibody to CD4 (alkaline phosphatase: blue); (b) section of colorectal carcinoma from patient A, stained using an antibody to Ki67 (immunoperoxidase: brown), followed by an antibody to CD8 (alkaline phosphatase: blue); (c) section of a colorectal carcinoma explant from patient M on day 3 of culture, stained using an antibody to Ki67 (immunoperoxidase: brown), followed by an antibody to CD3 (alkaline phosphatase: blue).
Table 3.
It was not possible to quantify the proliferation frequencies of every T-cell subset in all 22 specimens of colorectal carcinoma. The table shows the number of specimens used to determine the proliferation rates of each subset in the IEL and stromal compartments
| Subset | Number of specimens in which proliferation of IEL was counted | Number of specimens in which proliferation of stromal T cells was counted |
|---|---|---|
| CD3+ | 20 | 22 |
| CD45RO+ | 14 | 14 |
| CD7+ | 13 | 14 |
| CD4+ | 20 | 22 |
| CD8+ | 19 | 22 |
| CD57+ | 7 | 10 |
| TCRγδ+ | 2 | 3 |
Within the IEL compartment, the mean proliferation frequencies of the CD45RO+, CD4+, CD8+ and CD57+ subsets were not significantly different from that of the CD3+ cells as a whole. The decreased proliferation frequency of the CD7+ cells compared with the CD3+ cells in the IEL compartment is significant (P = 0·018). In the stromal compartment, the mean proliferation frequencies of the CD4+, CD45RO+, CD8+ and CD57+ subsets are not significantly different from that of the CD3+ cells.
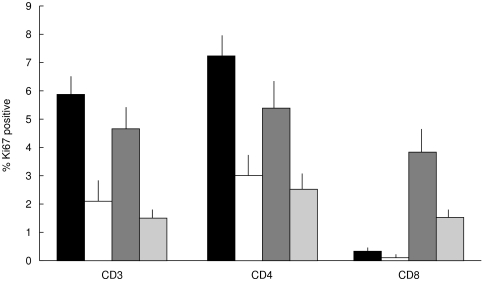
T cells in the IEL and lamina propria compartments of normal gut are known to be generally non-proliferative [23]. This was also observed in the study. The proliferation frequencies of colorectal carcinoma-infiltrating T cells were therefore compared with those observed in areas of lymphoid tissue normally associated with T cell division (Fig. 4). The mean CD3+ cell proliferation frequency is not significantly different in the T-IEL or stromal cells than in Peyer’s patches. The CD8+ cell proliferation frequency is significantly greater in both the T-IEL and stromal compartments than in the tonsils (P = 1 × 10–7 and P = < 10–14) or the Peyer’s patches (P = 6·8 × 10–4 and P = 0·007). The CD4+ cell proliferation frequencies are not significantly different in the T-IEL or stromal cells from those in the Peyer’s patches. It is also interesting to note that the proliferation frequencies of T cells in tonsils is consistently greater than those observed in the Peyer’s patches (Fig. 4).
Fig. 4.
A comparison of the proliferation frequencies of different T-cell subsets in colorectal carcinomas, tonsils and Peyer’s patches. The mean percentages (and standard errors) of proliferating CD3+, CD4+ and CD8+ T cells are compared in tonsillar TCZ (▪), Peyer’s patch TCZ (□), colorectal carcinoma IEL ( ) and colorectal carcinoma stroma (
) and colorectal carcinoma stroma ( ). The CD8+ subset is proliferative in the carcinoma specimens, but not in the tonsils or the Peyer’s patches.
). The CD8+ subset is proliferative in the carcinoma specimens, but not in the tonsils or the Peyer’s patches.
Intact pieces of seven colorectal carcinomas were maintained in culture for 3 days. The proliferation frequencies of the tumour cells (Fig. 5a) and the numbers of CD3+ IEL per 1000 tumour cells (Fig. 5b) were counted on day 0 and day 3 of culture. Viable T cells were apparent in each specimen after culture. Proliferating T cells were identified by co-expression of Ki67 (Fig. 3c), though the frequency observed was reduced in culture to < 1%.
Fig. 5.
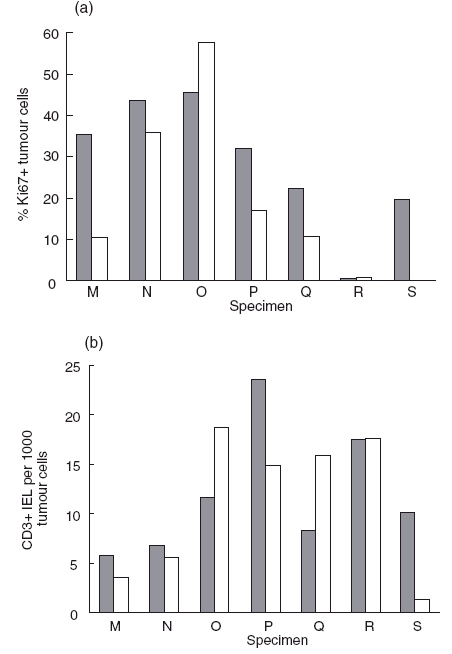
(a) The proportions of Ki67+ tumour cells in colorectal carcinomas before culture ( ) and after 3 days of culture (□); (b) the numbers of CD3+ cells in the IEL compartment were determined on day 0 (
) and after 3 days of culture (□); (b) the numbers of CD3+ cells in the IEL compartment were determined on day 0 ( ) and day 3 (□) of culture of specimens of colorectal carcinomas.
) and day 3 (□) of culture of specimens of colorectal carcinomas.
DISCUSSION
We have observed that the tumour-infiltrating T cells in contact with the tumour have a higher frequency of proliferation than the stromal T cells. This is particularly notable in the CD8+ T-cell subset. The proliferation frequency of the CD8+ IEL is significantly higher than that of stromal CD8+ T cells or of the CD8+ T cells in any area of normal mucosal lymphoid tissue. Proliferating T cells are absent from the normal intestine, but proliferating CD8+ T cells have been observed in untreated coeliac disease [23]. However, this response is likely to be driven by extraneously-derived gliadin antigen [23]. The factors driving the CD8+ T cell proliferation in colorectal carcinomas are not known but are likely to be the tumour cells themselves. As the CD8+ IEL are perforin negative [7], they may have a regulatory role, rather than a cytotoxic function. The CD4+ T cells also proliferate more when in contact with the tumour. This frequency was more comparable with that observed in the CD4+ component of the T-cell zones of the tonsil and the Peyer’s patch. It is also interesting to note that the T cell proliferation frequencies are significantly higher in the tonsils than in the Peyer’s patches of individuals of comparable age.
The percentage of CD3+ T cells that co-express CD7 in colorectal carcinomas has not been previously reported. In this study it was found that just 62% of CD3+ cells in the IEL compartment and 47% in the stromal compartment co-express CD7 on average, significantly more in the IEL than in the stroma. This means that the proportion of CD3+ CD7– T cells is much greater than the 5–15% seen in the peripheral blood of elderly people [24]. This is not altogether surprising, as the CD3+ CD7– subset in the blood has been reported to contain twice as many cells expressing the gut-associated antigen HML-1 than the CD7+ subset [24]. Moreover, CD7– T cells are known to develop preferentially in response to CD2 stimulation [24], to which intestinal T cells are also known to respond strongly [25]. Interestingly, an antibody has been identified that binds strongly to CD7 on IEL but only very weakly to lamina propria T cells or thymocytes, implying that CD7 is structurally different on IEL [26]. The mean rates of proliferation of the CD7+ cells did not differ significantly between the IEL and stromal compartments. This contrasts with the other T-cell subsets studied, which (with the exception of the CD57+ subset) all had significantly higher proliferation frequencies in the IEL than in the stromal compartment. The frequencies were also significantly lower in the CD7+ cells than in the total CD3+ population. This implies that the CD7– IEL are responsible for the increased proliferation frequencies of the other subsets in the IEL, and explains why the proportion of CD7– cells is elevated in this compartment. The CD7– IEL may be proliferating in response to self-antigens on the tumour cells with which they are in intimate contact.
The presence of elevated numbers of CD56+ and CD57+ T cells in both the tumours and the peripheral blood of colorectal carcinoma patients has been reported using flow cytometry [20,21]. In this study, CD57+ T cells were observed in 15 out of 18 carcinomas. Despite the large variation between individual tumour specimens, the overall mean proportions in the carcinomas were 4·2% of the CD3+ cells in the IEL and 5·2% in the stromal compartment, which are not significantly different. This is a lower proportion than that found by Okada et al. [20] who reported a mean of around 14% from flow cytometric analysis of 22 carcinomas. CD57+ T cells were virtually absent from the normal gut mucosa (data not shown), consistent with previous reports [27–29]. Despite their unusual phenotype and restriction to the carcinomas, the mean proliferation frequencies of the CD57+ cells did not differ significantly. It has been suggested that the CD57+ T cells are of extrathymic origin [20] because they are also present in the bone marrow and the liver, but not in the thymus. The likely alternative is that CD57 is acquired through differentiation of the highly activated carcinoma-infiltrating T cells. CD57 expression has been induced in vitro on peripheral blood CD57– T cells by co-culture with high concentrations of IL-2 [30,31]. Peripheral blood CD8+ CD57+ T cells, like normal intestinal T cells, have been shown to respond preferentially to CD2 stimulation [32]. The idea that the CD57+ phenotype is acquired within the carcinomas by repeated stimulation is consistent with the high frequency of CD7– T cells found in the carcinomas, and with the increase in the proportion of CD57+ T cells in the peripheral blood that is seen with age [33–37].
TCRγδ+ cells were found to be almost absent from the carcinomas. It was possible to quantify them in just three out of the 22 different specimens, and in only one case did more than 5% of the CD3+ cells express the γδ TCR. This is in agreement with several previous immunohistochemical studies [12,17]. However, a recent flow cytometry study by Groh et al. [9] found that up to 15% of the T cells in MICA + colon carcinomas were TCRγδ+. It is possible that the sensitivity of the immunohistochemical studies is low enough for many of the γδ T cells to be missed, but this is unlikely as TCRγδ+ cells can be clearly seen in tonsils using the same method (data not shown). It may be that the expression of the γδ receptor complex itself is down-regulated in the carcinomas and so can be more easily detected by flow cytometry than by immunohistochemistry. It is known that the expression of other cell-surface molecules, such as CD3ζ, CD3ɛ and CD16ζ, is reduced in colorectal carcinoma-infiltrating T and NK cells [17]. Alternatively, as Groh et al. [9] used a lengthy 12 hour enzymatic digestion to release T cells from the tumours for analysis, some selective death of TCRαβ+ cells may have occurred, resulting in a high proportion of TCRγδ+ cells among the T cells analysed. Regardless of the explanation, it appears that the carcinomas are deficient in functional γδ T cells, suggesting that that an effective MIC-mediated anti-tumour immune response is not occurring in vivo.
The tumour explant model described here showed that colorectal carcinoma-infiltrating T cells are able to survive in intact cultured explants, despite the absence of the factors that originate in vivo from the periphery. The presence of even a small number of dividing T cells after 3 days in culture indicates that they may be responding to factors present in the tumour microenvironment, and that factors derived from the periphery are not absolutely required for proliferation. This model has potential for the future study of the interactions between lymphocytes, stromal cells and tumour cells in colorectal carcinomas, and for testing potential therapeutic strategies.
Acknowledgments
S.G. was supported by the Special Trustees for St Thomas’ hospital (project number G/053/0561). We are grateful to Mr Jourdan, Mr Kmiot and Dr Kyriacou for colorectal carcinoma specimens.
REFERENCES
- 1.Carlon CA, Fabris G, Arslan-Pagnini C, et al. Prognostic correlations of operable carcinoma of the rectum. Dis Colon Rectum. 1985;28:47–50. doi: 10.1007/BF02553907. [DOI] [PubMed] [Google Scholar]
- 2.Jass JR. Lymphocytic infiltration and survival in rectal cancer. J Clin Pathol. 1986;39:585–9. doi: 10.1136/jcp.39.6.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ropponen KM, Eskilinen MJ, Lipponen PK, et al. Prognostic value of tumour-infiltrating lymphocytes (TILs) in colorectal cancer. J Pathol. 1997;182:318–24. doi: 10.1002/(SICI)1096-9896(199707)182:3<318::AID-PATH862>3.0.CO;2-6. 10.1002/(sici)1096-9896(199707)182:3<318::aid-path862>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 4.Svennevig JL, Lunde OC, Holter J, et al. Lymphoid infiltration and prognosis in colorectal carcinoma. Br J Cancer. 1984;49:375–7. doi: 10.1038/bjc.1984.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ostenstad B, Sioud M, Lea T, et al. Limited heterogeneity in the T-cell receptor V-gene usage in lymphocytes infiltrating human colorectal tumours. Br J Cancer. 1994;69:1078–82. doi: 10.1038/bjc.1994.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Taunk J, Roberts AI, Ebert EC. Spontaneous cytotoxicity of human intraepithelial lymphocytes against epithelial cell tumors. Gastroenterology. 1992;102:69–75. doi: 10.1016/0016-5085(92)91785-3. [DOI] [PubMed] [Google Scholar]
- 7.Nakanishi H, Monden T, Morimoto H, et al. Perforin expression in lymphocytes infiltrated to human colorectal cancer. Br J Cancer. 1991;64:239–42. doi: 10.1038/bjc.1991.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Maeurer MJ, Martin D, Walter W, et al. Human intestinal Vδ1+ T lymphocytes recognize tumor cells of epithelial origin. J Exp Med. 1996;183:1681–96. doi: 10.1084/jem.183.4.1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Groh V, Rhinehart R, Secrist H, et al. Broad tumor-associated expression and recognition by tumor-derived γδ T cells of MICA and MICB. Proc Natl Acad Sci USA. 1999;96:6879–84. doi: 10.1073/pnas.96.12.6879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cui J, Shin T, Kawano T, et al. Requirement for Vα14 NKT cells in IL-12-mediated rejection of tumors. Science. 1997;278:1623–6. doi: 10.1126/science.278.5343.1623. [DOI] [PubMed] [Google Scholar]
- 11.Blumberg RS, Terhorst C, Bleicher P, et al. Expression of a non-polymorphic MHC class I-like molecule, CD1d, by human intestinal epithelial cells. J Immunol. 1991;147:2518–24. [PubMed] [Google Scholar]
- 12.Banner BF, Sonmez-Alpan E, Yousem SA. An immunophenotypic study of the inflammatory cell populations in colon adenomas and carcinomas. Mod Pathol. 1993;6:295–301. [PubMed] [Google Scholar]
- 13.Banner BF, Savas L, Baker S, et al. Characterization of the inflammatory cell populations in normal colon and colonic carcinomas. Virchows Arch B Cell Pathol. 1993;64:213–20. doi: 10.1007/BF02915115. [DOI] [PubMed] [Google Scholar]
- 14.Csiba A, Whitwell HL, Moore M. Distribution of histocompatability and leucocyte differentiation antigens in normal human colon and in benign and malignant neoplasms. Br J Cancer. 1984;50:699–709. doi: 10.1038/bjc.1984.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hakansson L, Adell G, Boeryd B, et al. Infiltration of mononuclear inflammatory cells into primary colorectal carcinomas: an immunohistological analysis. Br J Cancer. 1997;75:374–80. doi: 10.1038/bjc.1997.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lennard TWJ, Warford A, Taylor RMR, et al. In situ subpopulations of lymphocytes in human colorectal carcinomas. Invasion Metastasis. 1984;4S1:60–6. [PubMed] [Google Scholar]
- 17.Matsuda S, Yamane T, Hamaji M. CD4- and TCRαβ-positive T lymphocytes predominantly infiltrated into well-moderately differentiated colon adenocarcinoma tissues. Jpn J Clin Oncol. 1998;28:97–103. doi: 10.1093/jjco/28.2.97. [DOI] [PubMed] [Google Scholar]
- 18.Norazmi MN, Hohmann AW, Skinner JM, et al. Density and phenotype of tumour-associated mononuclear cells in colonic carcinomas determined by computer-assisted video image analysis. Immunol. 1990;69:282–6. [PMC free article] [PubMed] [Google Scholar]
- 19.Umpleby HC, Heinemann D, Symes MO, et al. Expression of histocompatability antigens and characterization of mononuclear cell infiltrates in normal and neoplastic colorectal tissues of humans. J Natl Cancer Inst. 1985;74:1161–8. [PubMed] [Google Scholar]
- 20.Okada T, Iiai T, Kawachi Y, et al. Origin of CD57+ T cells which increase at tumour sites in patients with colorectal cancer. Clin Exp Immunol. 1995;102:159–66. doi: 10.1111/j.1365-2249.1995.tb06650.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Takii Y, Hashimoto S, Hai T, Watanabe H, Hatakeyama K, Abo T. Increase in the proportion of granulated CD56+ T cells in patients with malignancy. Clin Exp Immunol. 1994;97:522–7. doi: 10.1111/j.1365-2249.1994.tb06120.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wilson NW, Macartney JC. Organ culture of human gastric mucosa and gastric cancers. 1. Morphological aspects. J Pathol. 1986;150:127–34. doi: 10.1002/path.1711500207. [DOI] [PubMed] [Google Scholar]
- 23.Halstensen TS, Brandtzaeg P. Activated T lymphocytes in the celiac lesion: non-proliferative activation (CD25) of CD4+ alpha/beta cells in the lamina propria but proliferation (Ki67) of alpha/beta and gamma/ delta cells in the epithelium. Eur J Immunol. 1993;23:505–10. doi: 10.1002/eji.1830230231. [DOI] [PubMed] [Google Scholar]
- 24.Reinhold U, Abken H. CD4+CD7– T cells: a separate subpopulation of memory T cells? J Clin Immunol. 1997;17:265–71. doi: 10.1023/a:1027318530127. [DOI] [PubMed] [Google Scholar]
- 25.Ebert EC. Proliferative responses of human intraepithelial lymphocytes to various T-cell stimuli. Gastroenterology. 1989;97:1372–81. doi: 10.1016/0016-5085(89)90379-x. [DOI] [PubMed] [Google Scholar]
- 26.Russell GJ, Parker CM, Cepek KL, et al. Evidence for a structural difference in the CD7 polypeptide on human thymocyte and intraepithelial lymphocytes defined by a new monoclonal antibody, 3D9. Cell Immunol. 1994;154:153–65. doi: 10.1006/cimm.1994.1065. [DOI] [PubMed] [Google Scholar]
- 27.Gibson PR, Jewell DP. Local immune mechanisms in inflammatory bowel disease and colorectal carcinoma. Natural killer cells and their activity. Gastroenterology. 1986;90:12–9. doi: 10.1016/0016-5085(86)90068-5. [DOI] [PubMed] [Google Scholar]
- 28.Hirata I, Berrebi G, Austin LL, et al. Immunohistological characterization of intraepithelial and lamina propria lymphocytes in control ileum and colon and in inflammatory bowel disease. Dig Dis Sci. 1986;31:593–603. doi: 10.1007/BF01318690. [DOI] [PubMed] [Google Scholar]
- 29.Oberhuber GP, Püspök A, Peck-Radosavlevic M, et al. Aberrant esophageal HLA-DR expression in a high percentage of patients with Crohn’s disease. Am J Surg Pathol. 1999;23:970–6. doi: 10.1097/00000478-199908000-00016. 10.1097/00000478-199908000-00016. [DOI] [PubMed] [Google Scholar]
- 30.Brinkmann V, Kristofic C. Massive production of Th2 cytokines by human CD4+ effector T cells transiently expressing the natural killer marker CD57/HNK1. Immunol. 1997;91:541–7. doi: 10.1046/j.1365-2567.1997.00298.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dupuy d'Angeac A, Monier S, Pilling D, et al. CD57+ T lymphocytes are derived from CD57– precursors by differentiation occurring in late immune responses. Eur J Immunol. 1994;24:1503–11. doi: 10.1002/eji.1830240707. [DOI] [PubMed] [Google Scholar]
- 32.Rüthlein J, James SP, Strober W. Role of CD2 in activation and cytotoxic function of CD8/Leu-7-positive T cells. J Immunol. 1988;141:1791–7. [PubMed] [Google Scholar]
- 33.Abo T, Cooper MD, Balch CM. Postnatal expansion of the natural killer and killer cell population in humans identified by the monoclonal HNK-1 antibody. J Exp Med. 1982;155:321–6. doi: 10.1084/jem.155.1.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ligthart GJ, van Vlokhoven PC, Schuit HRE, et al. The expanded null cell compartment in ageing: increase in the number of natural killer cells and changes in T cell and NK-cell subsets in human blood. Immunol. 1986;59:353–7. [PMC free article] [PubMed] [Google Scholar]
- 35.Malinowski K, Rapaport FT. Effects of ageing upon the expression of differentiation and class II MHC antigens on the surface of T lymphocytes from normal human subjects. Cell Immunol. 1995;162:68–73. doi: 10.1006/cimm.1995.1052. 10.1006/cimm.1995.1052. [DOI] [PubMed] [Google Scholar]
- 36.Merino J, Martinez-Gonzalez MA, Rubio M, et al. Progressive decrease of CD8high+ CD28+ CD57– cells with ageing. Clin Exp Immunol. 1998;112:48–51. doi: 10.1046/j.1365-2249.1998.00551.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Miyaji C, Watanabe H, Minagawa N, et al. Numerical and functional characteristics of lymphocyte subsets in centenarians. J Clin Immunol. 1997;17:420–9. doi: 10.1023/a:1027324626199. [DOI] [PubMed] [Google Scholar]
- 38.Matsuda M, Petersson M, Lenkei R, et al. Alterations in the signal-transducing molecules of T cells and NK cells in colorectal tumor-infiltrating, gut mucosal and peripheral lymphocytes: correlation with the stage of the disease. Int J Cancer. 1995;61:765–72. doi: 10.1002/ijc.2910610605. [DOI] [PubMed] [Google Scholar]