Abstract
The balance between Type 1 and Type 2 cytokines is important for the outcome of several infectious diseases. As elderly humans show increased morbidity and mortality from infectious diseases, this study tests if ageing is associated with a change towards Type 2 dominance in T cells. Expression of IFN-γ, and IL-4 was measured in CD4+ and CD8+ T cells by flow cytometry in three groups: young controls (n = 28), 81-year-olds (n = 22), and centenarians (n = 25). The major findings were that the percentage of IFN-γ+ as well as IL-4+ T cells was increased in aged subjects. Furthermore, after adjusting for decreased lymphocyte counts in the elderly, the concentration in the blood of IFN-γ+ and IL-4+ CD8+ T cells was still increased in the 81-year-olds. In centenarians, a shift towards a relative dominance of Type 2 cytokine expression was found within CD8+ T cells. Furthermore, the percentage of T cells with cytokine expression was closely correlated to the in vivo expression of CD95 and CD45RO. In conclusion, we found some evidence for an age-related shift towards a Type 2 cytokine profile.
Keywords: ageing, IFN-γ, IL-4, CD95, Th1/Th2 ratio
INTRODUCTION
Activated T lymphocytes in humans have been separated into T helper 1 (Th1) and T cytotoxic 1 (Tc1) cells, characterized by a cytokine profile dominated by IFN-γ, versus T helper 2 (Th2) and T cytotoxic 2 (Tc2) lymphocytes, characterized by IL-4 production [1]. The balance of Type 1 and Type 2 cytokines is implicated in the regulation of many immune responses, and is thought to be crucial for the outcome of several infectious diseases, e.g. HIV, leismaniasis, and tuberculosis [1]. Elderly humans show increased morbidity and mortality from several infectious diseases [2,3] concomitant with pronounced changes in the function and the phenotype of T lymphocytes and increased circulating levels of autoantibodies [4]. These observations have formed the basis for the hypothesis that ageing involves a shift towards dominance of a Type 2 cytokine response [5,6]. However, data in the area is controversial and mainly based on studies of cytokine levels in culture supernatants measured by ELISA.
Changes in the T lymphocyte phenotype have been suggested to underlie much of the age-related changes in the cytokine network [7,8]. Thus, ageing leads to the replacement of naive T cells expressing CD62L+CD45RA+ on the surface by memory/ activated T cells expressing CD45RO and/or CD95 [9–11]. The initial activation of naive cells results in a limited program of cytokine gene expression including IL-2, whereas previously activated cells exhibit unchanged or decreased potential for IL-2 production as well as enhanced capacity for production of cytokines such as IFN-γ and IL-4 upon polyclonal stimulation in vitro [8]. Accordingly, it is possible that an altered cytokine production in ageing simply reflects increased numbers of activated T lymphocytes.
The purpose of the present study was to test the hypothesis that ageing is associated with a shift in the balance between Type 1 and Type 2 cytokine-producing T lymphocytes towards a relative Type 2 dominance. Furthermore, it was tested if age-related changes in the cytokine profile of T lymphocytes were related to a changed phenotype (increased expression of CD95 and CD45RO in elderly groups). Flow cytometry with the advent of intracellular cytokine staining was used because this method has the advantage of determining the cytokine production on a single cell level. We also wished to investigate if the single Type 1 or Type 2 cytokine expressing cell released a smaller amount of cytokine into the supernatants in the elderly humans. Thus, we investigated to which extent the number of lymphocytes expressing intracellular cytokines correlated with the concentration of cytokines in supernatants following the same in vitro stimulation procedure.
MATERIALS AND METHODS
Subjects
Three different age groups were studied:
28 healthy young volunteers, including 20 women and 8 men, with a mean age of 23 years (range 21–30 years);
22 relatively healthy humans, including 6 women and 16 men, aged 80–81 years. Subjects were chosen out of a cohort who had agreed to have blood samples taken in 1995–96 [12] as a part of a longitudinal study of ageing called the 1914-population in Glostrup [13]. The selection of the 22 subjects in the present study was based on the following criteria: no one suffered from dementia, cancer, acute or chronic inflammatory disorders (e.g. infections, rheumatoid arthritis, polymyalgia rheumatica), or acute illness. Furthermore, no one had a daily intake of systemic corticosteroids, acetyl salicylic acid (>100 mg), and nonsteroid anti-inflammatory drugs. No one showed a skewed biochemical profile in the blood including haemoglobin <6·5 mmol/l, leucocyte number >15 × 109/l, blood glucose >10 mmol/l, sedimentation rate >30, alkaline phosphates > 400 IU/l, alanine aminotransferase >60 IU/l, or carbamide >15 mmol/l;
25 centenarians, including 20 women and 5 men aged 100–103 years who were characterized by multi morbidity and represented the end stage of immunosenescence.
Isolation of blood mononuclear cells (BMNC)
BMNC were isolated by density centrifugation (Lymphoprep Nyegaard, Oslo, Norway) on LeucoSep tubes (Greiner, Frickenhausen, Germany) and washed three times in medium 1640 RPMI (Gibco, NY, USA). Cells were cryopreserved in freezing medium (50% 1640 RPMI, 30% human serum (HS), and 20% DMSO (Bie & Berntsen, Rødovre, Denmark). Cell samples were stored in nitrogen until thawed for analysis. At the day of the assay BMNC were rapidly thawed in a 37°C water bath and washed in RPMI 1640 supplemented with 10% foetal calf serum (FCS), 40 IU/ml Penicillin, 40μg/ml Streptomycin, and 0·12 mg/ml Glutamine. Cells were stained with tryphan blue to check viability.
Surface marker staining on unstimulated BMNC
BMNC were washed in phosphate-buffered saline (PBS; Bie & Berntsen) with 1% FCS. BMNC were incubated with conjugated monoclonal antibodies for 30 min at 4°C in volumes recommended by the manufacturer. Labelled cells were washed twice and analysed by flow cytometry (see details in the next section). The following antibodies were used: ECD-conjugated anti-CD45RO (clone UCHL1, Immunotech, Marseille, France) in combination with Cy5-conjugated anti-CD4 (clone MT310, DAKO, Glostrup, Denmark) or Cy5-linked anti-CD8 (clone DK25, DAKO). FITC-conjugated anti-CD95 (clone DX2, Becton & Dickinson, Oxnard, CA, USA) in combination with ECD-conjugated CD4 (clone SFCI12T4D11, Coulter, Florida, USA) and Cy5-linked anti-CD8 (clone DK25, DAKO). For determination of background staining, cells were incubated with relevant mouse isotype antibodies as negative controls: FITC + rPe + Cy5 mouse IgG1 (clone DAK-GO1, DAKO) and ECD-conjugated mouse IgG2A (clone U7.27, Immunotech). CD4+ and CD8+ T cells are expressed as percentages among total lymphocytes. CD45RO+ cells and CD95+ cells are expressed as percentages within CD4+ or CD8+ T lymphocytes.
Cell stimulation and determination of cytokine-producing cell populations by flow cytometry
BMNC (106 BMNC/ml) were stimulated with Phorbol 12-myristate 13-acetate (PMA) (50 ng/ml) and ionomycin (2 nmol/ml) for 4 h at 37°C in the presence of monensin (2 nmol/ml). Intracellular cytokines were detected in accordance with the method described by Sander et al. [14] and modified by others [15,16]. Stimulated BMNC were harvested, washed in staining buffer and incubated with ECD-conjugated anti-CD3 (clone UCHT1, Immunotech) and Cy5-linked anti-CD8 (clone DK25, DAKO) for 20 min at 4°C. After wash and fixation in 4% paraformaldehyde, cell membranes were made permeable by incubation with 0·1% saponin buffer for 10 min at room temperature. Cells were then incubated with anti-IL4 (clone; MP4-25D2, Pharmingen) in combination with rPe-conjugated anti-IFN-γ (clone; 4S.B3, Pharmingen) in the presence of saponin buffer for 30 min at 4°C, and subsequently washed twice in saponin buffer. Antibodies to cytokines were used at 10 μg/ml. For determination of background staining, cells were incubated with relevant mouse or rat isotype antibodies as negative controls.
Labelled cells were analysed by a flow cytometer (Epics XL-MCL, COULTER, Florida, USA). Winlist software 4.0 (Verity software House, USA) was used for subsequent analysis. Lymphocytes were distinguished from monocytes based on their forward versus right-angle light scatter and controlled by the CD14/CD45 double-stained sample. CD8–CD3+ cells were used as an approximation to CD4+ cells [17,18] because the stimulation with PMA induce down regulation of the CD4 molecule by phosphorylation of cytoplasmatic serine residues via an isoform of protein kinase C [19]. It has been confirmed that in healthy European donors the majority of the CD3+CD8– cells are indeed CD4+ T cells [20]. The percentage of cytokine positive cells is expressed as percentages within CD3+CD8+cells or CD3+CD8–cells. CD3+CD8– cells are designated CD4+ T cells throughout the manuscript. The concentration of cytokine-producing cells was calculated by multiplying the lymphocyte count of each individual with the percentage of CD4+ or CD8+ T cells and the percentage of cytokine-producing cells within the same lymphocyte subset.
Detection of cytokines in culture supernatants
Levels of IFN-γ and IL-4 were measured in culture supernatants by ELISA. PBMC (106 BMNC/ml) were stimulated with PMA (50 ng/ml) and ionomycin (2 nmol/ml) at 37°C in 5% CO2 for 5 h and 48 h, respectively. After centrifugation at 5°C, supernatants were collected and stored at –80°C until analysed by commercially available kits from R & D systems (DY285, and HS400). According to the protocol of the manufacture, detection limits were 31·2–2000 pg/ml for IFN-γ, and 0·13–16 pg/ml for IL-4. When samples showed cytokine levels above the detection limit, new samples were thawed and diluted appropriately. All samples were run as duplicates.
Subjects from all three age groups were included in each immune-assay to ensure that the effect of day-to-day variation would affect groups similarly.
Statistics
Statistical data analysis was performed using systat statistical software 8.0 (systat, Evanston, IL, USA). Initial analysis revealed that the cytokine data was not normally distributed. In order to obtain normal distributions with equal variance across age groups data was ln or log10 transformed and checked by plots of histograms before further statistical analyses. Differences across age groups were tested by a one-way analysis of variance for three independent groups. If a significant effect was found (P < 0·05), pair wise comparisons were performed by a Tukey test. Linear relations between parameters were tested by linear regression analysis. Each independent variable was tested for an interaction with age groups. If an interaction was found (P < 0·05), separate slopes were calculated for the three age groups.
RESULTS
Intracellular expression of cytokines among T cell subsets following PMA and ionomycin stimulation
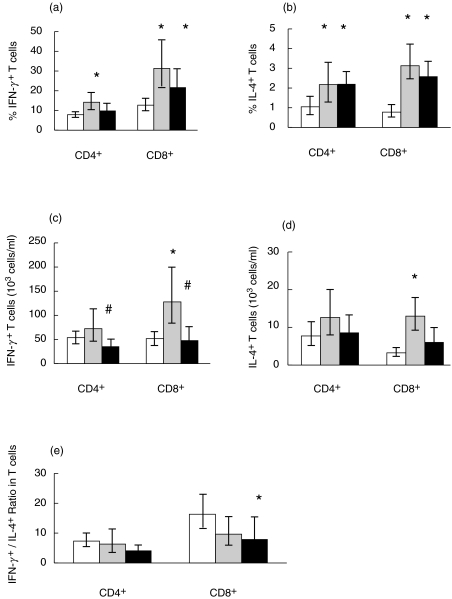
An age-related decline regarding percentages of CD3+ and CD8+ lymphocyte subsets were detected before stimulation, whereas the decline in CD4+ lymphocytes was not significant (P = 0·09) (Table 1). Upon stimulation, both 81-year-olds and centenarians showed increased percentages of IFN-γ and IL-4 expressing cells among CD4+ as well as CD8+ T cells compared to young controls (Fig. 1a,b). No difference was detected in the intracellular expression of these cytokines between the two elderly groups. Accordingly, ageing was associated with increased expression of both Type 1 and Type 2 cytokines among T lymphocyte subsets following PMA + ionomycin stimulation. Gender was left out of the final analyses because it did not influence results. Only a minute number of T lymphocytes was found to coexpress IFN-γ and IL-4 in all age groups (data not shown). Furthermore, it was tested if the percentage of cells expressing IFN-γ and IL-4 were linearly interrelated. IFN-γ expression was positively correlated to IL-4 expression among CD4+ cells (R = 0·45, P ≤ 0·0005, n = 64) as well as CD8+ T cells (R = 0·45, P = ≤ 0·0005, n = 64).
Table 1.
Total lymphocyte counts and percentages of different T-cell subsets in unstimulated lymphocytes
| 21–30 years (n = 26) | 81 years (n = 15) | 100 years (n = 25) | P (anova) | |
|---|---|---|---|---|
| Total lymphocytes(109/l) | 1·97 (1·77–2·20) | 1·77 (1·49–2·10) | 1·40 (1·09–1·79)* | 0·02 |
| CD3+ | 69% (65–72) | 62% (57–68) | 60% (54–67)* | 0·03 |
| CD4+ | 38% (34–42) | 31% (25–37) | 31% (25–37) | 0·09 |
| CD8+ | 21% (19–23) | 22% (18–28) | 15% (12–20)* | 0·02 |
| CD4+CD45RO+ | 41% (37–44) | 67% (58–75)* | 72% (64–80)* | < 0·005 |
| CD8+CD45RO+ | 29% (26–32) | 53% (43–62)* | 47% (40–55)* | < 0·005 |
| CD4+CD95+ | 33% (29–38) | 56% (48–64)* | 62% (54–70)* | < 0·005 |
| CD8+CD95+ | 29% (24–34) | 64% (51–81)* | 65% (58–72)* | < 0·005 |
CD3+, CD4+ and CD8+ cells are expressed as percentages within all lymphocytes. CD45RO+ and CD95+ cells are expressed as percentages within CD4+ or CD8+ T lymphocytes.
Geometric mean and 95%CI are shown. An analysis of variance was carried out. If P < 0·05 a Tukeys test was used for pairwise comparisons.
Denotes a significant difference (P < 0·05) from the young group (pairwise comparisons by Tukeys test).
Fig. 1.
Cytokine expression in T lymphocytes. Cytokine expression among CD4+ and CD8+ T cells was detected by flow cytometry after 4 h of stimulation with PMA and ionomycin. Young n = 24, 81-year-old n = 14, 100-year-old n = 25. (a) The percentage of IFN-γ+ cells among CD4+ and CD8+ lymphocytes. (b) The percentage of IL-4+ cells among CD4+ and CD8+ lymphocytes. (c) The number of IFN-γ producing cells/ml blood. (d) The number of IL-4 producing cells/ml blood. (e) IFN-γ/IL-4 ratio within CD4+ and CD8+ cells. Geometric means and 95% confidence intervals are shown. * denotes a significant difference (P < 0·05) from the young group; # denotes a significant difference (P < 0·05) from the 81-year-old group; □ 21–30 years;  81 years; ▪ 100 years.
81 years; ▪ 100 years.
The lymphocyte number was lower in the two elderly groups compared to the young controls (Table 1). Therefore, the concentration in the blood of cytokine-producing cells was calculated by adjusting for the declining lymphocyte count in the elderly as described in the Material and Methods section. Within the CD4+ T cells, the concentration of IFN-γ producing cells was decreased in the centenarian group compared to the 81-year-olds (Fig. 1c). There was no difference across age groups regarding the number of IL-4 producing CD4+ T cells (Fig. 1d). Within CD8+ T cells, there was a significantly higher concentration in the blood of both IFN-γ and IL-4 producing cells within the 81-year-olds compared to the young group, whereas the centenarian group had lower concentration of IFN-γ expressing CD8+ T cells compared to the 81-year-old group. Within the CD8+ T cells, the IL-4 expression in the centenarian group compared to the young group was only borderline to be significant (P = 0·07) (Fig. 1c,d)
The balance between Type 1 and Type 2 lymphocytes following PMA and ionomycin stimulation
To test the hypothesis that ageing is associated with a shift in the balance between Type 1 and Type 2 T lymphocytes, ratios of cells producing IFN-γ to IL-4 was calculated for CD4+ and CD8+ T cells (Fig. 1e). In the centenarians we found a significantly decreased ratio of IFN-γ/IL-4 among CD8+ T cells, whereas no significant change was found in the ratio among CD4+ T cells (anovaP = 0·07).
Correlation between activation markers on resting T lymphocytes and intracellular cytokine expression
It was hypothesized that the increased percentage of cytokine-producing cells in elderly humans following stimulation was related to increased preactivated states. The 81-year-olds and the centenarians showed increased in vivo expression of CD95 and CD45RO compared to the young group within both CD4+ and CD8+ T cells (Table 1). There was no difference between the two elderly groups. There was no significant interaction between age group and CD95 expression in a linear regression model (cytokine expression = Constant + %CD95 + age group + age group ×%CD95). Accordingly, this interaction was left out. Afterwards age group had no significant influence on the cytokine expression in the new linear regression model (cytokine expression = Constant + %CD95 + age group). Thus, age groups were pooled in the final statistical analysis. In this model, percentages of IL-4 and IFN-γ producing cells in response to stimulation were linearly dependent on CD95 expression in both CD4+ and CD8+ T cells in vivo (Table 2). Similarly, expressions of both cytokines were also positively correlated with CD45RO expression within CD4+ T cells (IFN-γ: R = 0·39, n = 58, P = 0·03; IL-4: R = 0·41, n = 59, P = 0·001). However, within the CD8+ subset, CD45RO was correlated to IFN-γ expression in the young and in the 81-year-old group, but not in the centenarians (significant interaction between age and CD45RO, data not shown).
Table 2.
Linear regression analysis of the percentage of cytokine-producing T lymphocytes after PMA + ionomycin stimulation on the percentage of cells expressing CD95 in vivo
| Gate | Dependent variable | Independent variable | Regression coefficient | s.e. | R | n | P (anova) |
|---|---|---|---|---|---|---|---|
| CD4+ | IFN-γ | CD95+ | 0·019 | 0·004 | 0·536 | 58 | < 0·005 |
| IL-4 | 0·017 | 0·005 | 0·407 | 59 | 0·001 | ||
| CD8+ | IFN-γ | CD95+ | 0·014 | 0·004 | 0·430 | 60 | 0·001 |
| IL-4 | 0·018 | 0·005 | 0·405 | 60 | 0·001 |
s.e. = Standard error; R = Pearson’s correlation coefficient. Each independent variable was tested for an interaction with the age group. No interactions were found (P > 0·05), and accordingly age groups were pooled.
Levels of cytokines in BMNC culture supernatants following stimulation
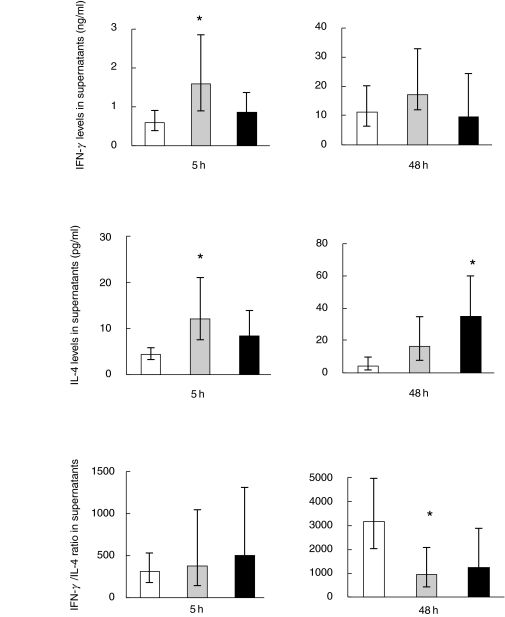
BMNC were stimulated with PMA and ionomycin for 5 h using the same protocol as used for stimulation before detecting intracellular cytokines except for the addition of monensin. Five hours were chosen instead of 4 h to ensure that the intracellular content of cytokines measured by flow cytometry at 4 h had been released to the extracellular supernatant. BMNC were also stimulated for 48 h, which was the time point with maximal cytokine levels in supernatants according to pilot studies. Concentrations of IFN-γ and IL-4 were markedly increased in supernatants from 81-year-old subjects compared to young controls after 5 h of stimulation (Fig. 2). Cytokine concentrations in supernatants from centenarians did not differ significantly from those of the 81-year-old subjects or the young controls. Following 48 h of stimulation, the concentration of IL-4 in supernatants from the centenarian group was elevated compared to the young group; the difference between the young group and the 81-year-olds was only borderline significant (P = 0·07). The production of IFN-γ showed no difference across age groups.
Fig. 2.
Cytokine production in BMNC supernatants following PMA + ionomycin stimulation. Cytokine production was detected by ELISA after stimulation for 5 and 48 h. IFN-γ; Young, n = 23; 81-year-old, n = 10; 100-year-old, n = 18. IL-4; Young, n = 17; 81-year-old, n = 8; 100-year-old, n = 18. Geometric means and 95% confidence intervals are shown. * denotes a significant difference (P < 0·05) from the young group; □ 21–30 years;  81 years; ▪ 100 years.
81 years; ▪ 100 years.
The balance between Type 1 and Type 2 cytokines in supernatants
The IFN-γ/IL-4 ratio was calculated within supernatants after 5 and 48 h. After 48 h of stimulation, a significant decrease in the T1/T2 ratio was observed in the 81-year-old group compared to the young controls. The difference between the centenarians and the young group was only borderline to be significant (P = 0·07) (Fig. 2).
Quantitatively versus qualitatively cytokine detection in ageing
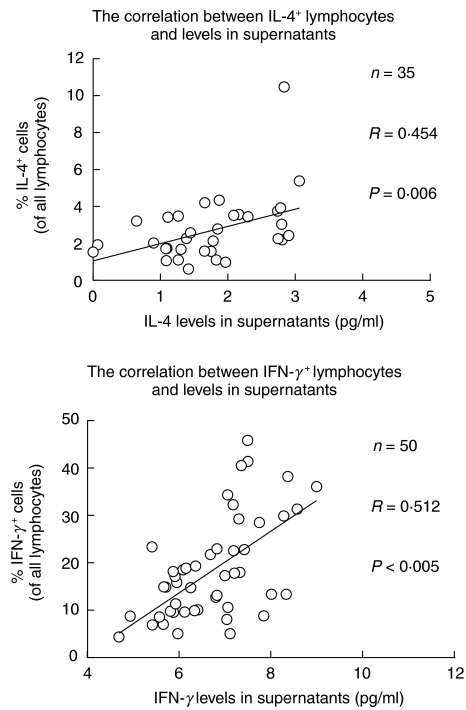
Although T lymphocytes are the major source of the measured cytokines, other lymphocyte subsets, e.g. NK cells and B cells, also contribute to IFN-γ and IL-4 production. Accordingly, the percentage of total cytokine-expressing lymphocytes within a lymphocyte gate based on forward versus right-angle light scatter was calculated. The percentages of all lymphocytes with intracellular expression of either IL-4 or IFN-γ+ cytokines, was increased in both elderly groups. There was no difference between 81-year-olds and centenarians. For both cytokines, the percentage of cytokine-producing cells was correlated with the cytokine level in BMNC supernatants following stimulation for 5 h (Fig. 3), but not after 48 h (data not shown). Age group had no significant effect and was left out in the final analysis.
Fig. 3.
Correlation between the percentage of cytokine-expressing lymphocytes and cytokine levels in culture supernatants. BMNC for intracellular cytokine detection and BMNC for supernatants were stimulated by using exactly the same amounts of PMA + ionomycin. The percentage of all lymphocytes expressing intracellular cytokines was correlated with cytokine levels in supernatants after 5 h. The supernatants after 5 h were ln transformed. Each independent variable was tested for an interaction with age groups. No interactions were found, and accordingly age groups were pooled. Geometric means and 95% confidence intervals are shown.
DISCUSSION
The major findings in the present study were that the percentage of IFN-γ+ cells as well as IL-4+ cells within T lymphocytes was increased in aged subjects after stimulation. However, the total concentration of cytokine-producing CD8+ cells was enhanced only in the 81-year-olds. In the centenarians, a shift in the balance between Type 1 and Type 2 cytokine-producing cells was found among CD8+ cells reflected by a relatively more pronounced increase in IL-4+ cells compared to IFN-γ+ cells. Also, in the 81-year-olds, a shift was found in the balance (the ratio) between levels of IFN-γ and IL-4 in BMNC supernatants after 48 h.
In both young and elderly humans, we found a tight correlation between percentages of lymphocytes expressing intracellular cytokines and the concentration of these cytokines in BMNC supernatants following the same stimulation procedure of lymphocytes in vitro. This finding suggests that cells from elderly humans do not suffer from a quantitatively decline in cytokine production on a per cell basis. Finally, the increased numbers of T lymphocytes producing cytokines upon polyclonal stimulation in vitro was related to increased in vivo activation (increased expression of CD95 and CD45RO) in ageing.
Consistent with the finding of age-related expansions of Type 1 as well as Type 2 lymphocytes in the present study, mice models have demonstrated both increased IFN-γ+ cells and IL-4+ cells among T lymphocytes from old animals [21,22]. These studies did not test if the balance between the number of IFN-γ+ cells and IL-4+ cells were altered. With regard to human studies, Sakata-Kaneko et al. [23] reported increased intracellular expression of IFN-γ, but no age-related difference in IL-4 expression, among CD4+ cells from middle-aged humans (55–65 years). Furthermore, Bandres et al. [24] found a positive correlation between age on one hand and IFN-γ+CD4+ cells and IFN-γ+CD8+ cells, respectively, on the other in 50 humans aged 17–62 years but only minimal levels of IL-4 producing T lymphocytes were detected. These discrepancies with regard to IL-4 may be explained by the fact that we investigated much older age groups in the present study. Studies on neonates, children and adults have demonstrated progressively increases in IFN-γ producing T lymphocytes in ageing [25,26] with a strong correlation to CD45RO expression [25]. This early age-dependent maturation of the immune response has been explained as a result from antigen exposure [25,26]. The present data suggest that such an activation and expansion of Type 1 cell populations continue beyond the phase of young adolescence. Furthermore, to our knowledge the present data is the first to demonstrate that also expansion of Type 2 cell populations occurs at advanced ages and that this expansion includes a shift in the balance between Tc1 and Tc2 cells. Despite a shift in the balance of the cytokine network Type 2 lymphocytes constitute still only a minute part of the total lymphocyte pool compared to Type 1 cells in elderly humans, and it is questionable if it has any physiological and/or clinical relevance. However, cytokines produced in the early stage of T lymphocyte activation determine the subsequent differentiation. Accordingly, the early age-related shift in the balance between Tc1 and Tc2 subsets may be an important regulatory factor of the immune response in vivo. The finding in the present study of an age-related shift in the ratio between production of IFN-γ and IL-4 in BMNC culture supernatants after 48 h stimulation, but not after 5 h, may reflect such a regulation.
In the present study, cell populations with the ability to produce both IL-4 and IFN-γ were minimal in accordance with reports by Sakato-Kaneko et al. [23]. In contrast, Paganelli et al. reported a shift towards a Th0 response in healthy centenarians. This may be due to that the latter conclusion was based on long-term cultures of CD4+ T cell clones and furthermore, only two centenarians were studied [27].
In contrast to studies of cytokines measured by flow cytometry, the literature regarding IFN-γ and IL-4 production in culture supernatants by ELISA from elderly versus young humans is large and controversial. In accordance with the present study, purified CD4+ as well as CD8+ cells from old humans produced high amounts of IFN-γ whereas only CD8+ cells showed increased production of IL-4 in culture supernatants after stimulation with anti-CD3 and anti-CD28 stimulation for 72 h [28]. Consistently with this, several other studies have reported increased quantitatively production of IFN-γ[29–31] and IL-4 [32] in ageing, but decreased production of IFN-γ[33–36] and IL-4 [33] has also been reported. Contradicting results may result from different time kinetics, different assays and differences in the studied populations. The present study clearly demonstrates the importance of culture duration. Increased IL-10 production has consistently been detected in cultures from elderly humans [35,37] and in animal studies [10,38]. However, IL-10 is not restricted to Type 2 clones in humans as in mice [39].
When the total concentration of cytokine-producing cells was calculated, only the 81-year-old group had higher concentrations of cytokine-producing CD8+ T cells, whereas the centenarians did not differ from the young controls. The difference between the two elderly groups is not easily explained. The 81-year-old individuals are healthy, whereas the Danish centenarians are characterized by multi morbidity. However, we do not know if it should be considered beneficial or detrimental for elderly people to have a high number of cytokine-producing T cells in the blood. Accordingly, the finding of a low number of cytokine-producing cells in the centenarians could in principal be interpreted as either inability to mount a necessary immune response due to multi morbidity or as representing successful ageing.
Recall antigen stimulation will normally induce either a Type 1 or a Type 2 cytokine response [40]. In the present study, we wanted to determine the number of circulating cells with the capacity of being either Type 1 or Type 2 cytokine producers upon stimulation. Therefore, a polyclonal stimulation was chosen in order to investigate the unspecific cytokine profile of the majority of T lymphocytes in humans. However, this response may differ largely from the response to antigens in vivo. Furthermore, BMNC cultures do not take into account that during in vivo activation, the microenvironment is also important for the direction of the T lymphocyte response, e.g. increased circulating levels of IL-12p40 may facilitate a Type 2 cytokine response in vivo [41]. Accordingly, a maximal stimulation in a short-term culture may be the best indicator for the in vivo capacity of cells to express Type 1/Type 2 cytokines.
In conclusion, the proportion of both Type 1 and Type 2 cytokine-producing T lymphocytes is increased with ageing. This phenomenon is closely associated with increased number of activated lymphocytes in vivo. Some evidence is found for a shift towards a relative dominance of Type 2 cytokines, especially among CD8+ T cells, in ageing. The clinical significance remains to be determined, but this shift may play a role in immunosenescence.
Acknowledgments
The excellent assistance of Ruth Rousing and Hanne Willumsen is acknowledged.
REFERENCES
- 1.Mosmann TR, Sad S. The expanding universe of T-cell subsets: Th1, Th2 and more. Immunol Today. 1996;17:138–46. doi: 10.1016/0167-5699(96)80606-2. [DOI] [PubMed] [Google Scholar]
- 2.Crossley KB, Peterson PK. Infections in the elderly. Clin Infect Dis. 1996;22:209–15. doi: 10.1093/clinids/22.2.209. [DOI] [PubMed] [Google Scholar]
- 3.Yoshikawa TT. Perspective aging and infectious diseases: past, present, and future. J Infect Dis. 1997;176:1053–7. doi: 10.1086/516547. [DOI] [PubMed] [Google Scholar]
- 4.LeMaoult J, Szabo P, Weksler ME. Effect of age on humoral immunity, selection of the B-cell repertoire and B-cell development. Immunol Rev. 1997;160:115–26. doi: 10.1111/j.1600-065x.1997.tb01032.x. [DOI] [PubMed] [Google Scholar]
- 5.Shearer GM. Th1/Th2 changes in aging. Mech Ageing Dev. 1997;94:1–5. doi: 10.1016/s0047-6374(96)01849-0. [DOI] [PubMed] [Google Scholar]
- 6.Rink L, Cakman I, Kirchner H. Altered cytokine production in the elderly. Mech Ageing Dev. 1998;102:199–209. doi: 10.1016/s0047-6374(97)00153-x. [DOI] [PubMed] [Google Scholar]
- 7.Miller RA. The aging immune system: primer and prospectus. Science. 1996;273:70–4. doi: 10.1126/science.273.5271.70. [DOI] [PubMed] [Google Scholar]
- 8.Hobbs M, Va EDN. The role of cytokines in aging. In: Aggarwal BB, Puri RK, editors. Human cytokines: their role in disease and therapy. Cambridge, Massachussets, USA: Blackwell Science; 1996. pp. 1–499. [Google Scholar]
- 9.Bruunsgaard H, Pedersen AN, Schroll M, Skinhoj P, Pedersen BK. Proliferative responses of blood mononuclear cells (BMNC) in a cohort of elderly humans: role of lymphocyte phenotype and cytokine production. Clin Exp Immunol. 2000;119:433–40. doi: 10.1046/j.1365-2249.2000.01146.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cossarizza A, Ortolani C, Paganelli R, et al. CD45 isoforms expression on CD4+ and CD8+ T cells throughout life, from newborns to centenarians: implications for T cell memory. Mech Ageing Dev. 1996;86:173–95. doi: 10.1016/0047-6374(95)01691-0. [DOI] [PubMed] [Google Scholar]
- 11.Fagnoni FF, Vescovini R, Passeri G, et al. Shortage of circulating naive CD8(+) T cells provides new insights on immunodeficiency in aging. Blood. 2000;95:2860–8. [PubMed] [Google Scholar]
- 12.Bruunsgaard H, Pedersen AN, Schroll M, Skinhoj P, Pedersen BK. Impaired production of proinflammatory cytokines in response to lipopolysaccharide (LPS) stimulation in elderly humans. Clin Exp Immunol. 1999;118:235–41. doi: 10.1046/j.1365-2249.1999.01045.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schroll M, Joergensen T, Ingerslev J. The Glostrup Population Studies, 1964–1992. Dan. Med Bull. :204–7. [PubMed] [Google Scholar]
- 14.Sander B, Andersson J, Andersson U. Assessment of cytokines by immunofluorescence and the paraformaldehyde-saponin procedure. Immunol Rev. 1991;119:65–93. doi: 10.1111/j.1600-065x.1991.tb00578.x. [DOI] [PubMed] [Google Scholar]
- 15.Jung T, Schauer U, Heusser C, Neumann C, Rieger C. Detection of intracellular cytokines by flow cytometry. J Immunol Meth. 1993;159:197–207. doi: 10.1016/0022-1759(93)90158-4. [DOI] [PubMed] [Google Scholar]
- 16.Prussin C, Metcalfe DD. Detection of intracytoplasmic cytokine using flow cytometry and directly conjugated anti-cytokine antibodies. J Immunol Meth. 1995;188:117–28. doi: 10.1016/0022-1759(95)00209-x. [DOI] [PubMed] [Google Scholar]
- 17.Ledru E, Lecoeur H, Garcia S, Debord T, Gougeon ML. Differential susceptibility to activation-induced apoptosis among peripheral Th1 subsets: correlation with Bcl-2 expression and consequences for AIDS pathogenesis. J Immunol. 1998;160:3194–206. [PubMed] [Google Scholar]
- 18.Rostaing L, Tkaczuk J, Durand M, et al. Kinetics of intracytoplasmic Th1 and Th2 cytokine production assessed by flow cytometry following in vitro activation of peripheral blood mononuclear cells. Cytometry. 1999;35:318–28. doi: 10.1002/(sici)1097-0320(19990401)35:4<318::aid-cyto4>3.0.co;2-4. 10.1002/(sici)1097-0320(19990401)35:4<318::aid-cyto4>3.3.co;2-w. [DOI] [PubMed] [Google Scholar]
- 19.Pelchen-Matthews A, Parsons IJ, Marsh M. Phorbol Ester-induced downregulation of CD4 is a multistep process involving dissociation from p56lck, increased association with clathrin-coated pits, and altered endosomal sorting. J Exp Med. 1993;178:1209–22. doi: 10.1084/jem.178.4.1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kallas EG, Gibbons DC, Soucier H, Fitzgerald T, Treanor JJ, Evans TG. Detection of intracellular antigen-specific cytokines in human T cell populations. J Infect Dis. 1999;179:1124–31. doi: 10.1086/314702. [DOI] [PubMed] [Google Scholar]
- 21.Wakikawa A, Utsuyama M, Wakabayashi A, Kitagawa M, Hirokawa K. Age-related alteration of cytokine production profile by T cell subsets in mice: a flow cytometric study. Exp Gerontol. 1999;34:231–42. doi: 10.1016/s0531-5565(98)00062-x. [DOI] [PubMed] [Google Scholar]
- 22.Mu YXi, Thoman LM. The age-dependent cytokine production by murine CD8+ T cells as determined by four-color flow cytometry analysis. J Gerontol A Biol Sci Med Sci. 1999;54:B116–23. doi: 10.1093/gerona/54.3.b116. [DOI] [PubMed] [Google Scholar]
- 23.Sakata-Kaneko S, Wakatsuki Y, Matsunaga Y, Usui T, Kita T. Altered Th1/Th2 commitment in human CD4+ T cells with ageing. Clin Exp Immunol. 2000;120:267–73. doi: 10.1046/j.1365-2249.2000.01224.x. 10.1046/j.1365-2249.2000.01224.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bandres E, Merino J, Vazquez B, et al. The increase of IFN-gamma production through aging correlates with the expanded CD8(+high) CD28(–)CD57(+) subpopulation. Clin Immunol. 2000;96:230–5. doi: 10.1006/clim.2000.4894. 10.1006/clim.2000.4894. [DOI] [PubMed] [Google Scholar]
- 25.Chipeta J, Komada Y, Zhang XL, et al. CD4+ and CD8+ cell cytokine profiles in neonates, older children, and adults: increasing T helper type 1 and T cytotoxic type 1 cell populations with age. Cell Immunol. 1998;183:149–56. doi: 10.1006/cimm.1998.1244. [DOI] [PubMed] [Google Scholar]
- 26.Krampera M, Vinante F, Tavecchia L, et al. Progressive polarization towards a T helper/cytotoxic type-1 cytokine pattern during age-dependent maturation of the immune response inversely correlates with CD30 cell expression and serum concentration. Clin Exp Immunol. 1999;117:291–7. doi: 10.1046/j.1365-2249.1999.00977.x. 10.1046/j.1365-2249.1999.00977.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Paganelli R, Scala E, Rosso R, et al. A shift to Th0 cytokine production by CD4+ cells in human longevity: studies on two healthy centenarians. Eur J Immunol. 1996;26:2030–4. doi: 10.1002/eji.1830260910. [DOI] [PubMed] [Google Scholar]
- 28.Yen CJ, Lin SL, Huang KT, Lin RH. Age-associated changes in interferon-gamma and interleukin-4 secretion by purified human CD4+ and CD8+ T cells. J Biomed Sci. 2000;7:317–21. doi: 10.1007/BF02253251. [DOI] [PubMed] [Google Scholar]
- 29.Born J, Uthgenannt D, Dodt C, et al. Cytokine production and lymphocyte subpopulations in aged humans. An assessment during nocturnal sleep. Mech Ageing Dev. 1995;84:113–26. doi: 10.1016/0047-6374(95)01638-4. [DOI] [PubMed] [Google Scholar]
- 30.Chopra RK, Holbrook NJ, Powers DC, McCoy MT, Adler WH, Nagel JE. Interleukin 2, interleukin 2 receptor, and interferon-gamma synthesis and mRNA expression in phorbol myristate acetate and calcium ionophore A23187-stimulated T cells from elderly humans. Clin Immunol Immunopathol. 1989;53:297–308. doi: 10.1016/0090-1229(89)90058-5. [DOI] [PubMed] [Google Scholar]
- 31.Miyaji C, Watanabe H, Toma H, et al. Functional alteration of granulocytes, NK cells, and natural killer T cells in centenarians. Hum Immunol. 2000;61:908–16. doi: 10.1016/s0198-8859(00)00153-1. [DOI] [PubMed] [Google Scholar]
- 32.Nijhuis EW, Remarque EJ, Hinloopen B, et al. Age-related increase in the fraction of CD27–CD4+ T cells and IL-4 production as a feature of CD4+ T cell differentiation in vivo. Clin Exp Immunol. 1994;96:528–34. doi: 10.1111/j.1365-2249.1994.tb06061.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Karanfilov CI, Liu B, Fox CC, Lakshmanan RR, Whisler RL. Age-related defects in Th1 and Th2 cytokine production by human T cells can be dissociated from altered frequencies of CD45RA+ and CD45RO+ T cell subsets. Mech Ageing Dev. 1999;109:97–112. doi: 10.1016/s0047-6374(99)00030-5. [DOI] [PubMed] [Google Scholar]
- 34.Ouyang Q, Cicek G, Westendorp RG, Cools HJ, Klis R, Remarque EJ. Reduced IFN-gamma production in elderly people following in vitro stimulation with influenza vaccine and endotoxin. Mech Ageing Dev. 2001;121:131–7. doi: 10.1016/s0047-6374(00)00204-9. [DOI] [PubMed] [Google Scholar]
- 35.Cakman I, Rohwer J, Schutz RM, Kirchner H, Rink L. Dysregulation between TH1 and TH2 T cell subpopulations in the elderly. Mech Ageing Dev. 1996;87:197–209. doi: 10.1016/0047-6374(96)01708-3. [DOI] [PubMed] [Google Scholar]
- 36.Caruso C, Candore G, Cigna D, et al. Cytokine production pathway in the elderly. Immunol Res. 1996;15:84–90. doi: 10.1007/BF02918286. [DOI] [PubMed] [Google Scholar]
- 37.Castle S, Uyemura K, Wong W, Modlin R, Effros R. Evidence of enhanced type 2 immune response and impaired upregulation of a type 1 response in frail elderly nursing home residents. Mech Ageing Dev. 1997;94:7–16. doi: 10.1016/s0047-6374(96)01821-0. [DOI] [PubMed] [Google Scholar]
- 38.Kurashima C, Utsuyama M. Age-related changes of cytokine production by murine helper T cell subpopulations. Pathobiology. 1997;65:155–62. doi: 10.1159/000164117. [DOI] [PubMed] [Google Scholar]
- 39.Del Prete G, De Carli M, Almerigogna F, Giudizi MG, Biagiotti R, Romagnani S. Human IL-10 is produced by both type 1 helper (Th1) and type 2 helper (Th2) T cell clones and inhibits their antigen-specific proliferation and cytokine production. J Immunol. 1993;150:353–60. [PubMed] [Google Scholar]
- 40.Spellberg B, Edwards JE., Jr Type 1/Type 2 immunity in infectious diseases. Clin Infect Dis. 2001;32:76–102. doi: 10.1086/317537. [DOI] [PubMed] [Google Scholar]
- 41.Rea IM, McNerlan SE, Alexander HD. Total serum IL-12 and IL-12p40, but not IL-12p70, are increased in the serum of older subjects; relationship to CD3(+) and NK subsets. Cytokine. 2000;12:156–9. doi: 10.1006/cyto.1999.0537. [DOI] [PubMed] [Google Scholar]